Abstract
Human endothelial cells (ECs) enhance human immunodeficiency virus (HIV) replication within CD4+ memory T cells by 50,000-fold in a Nef-dependent manner. Here, we report that EC-mediated HIV type 1 replication is also dependent on an intact vpr gene. Moreover, we demonstrate that despite a requirement for engaging major histocompatibility complex (MHC) class II molecules and costimulators, EC-stimulated virus-producing cells (p24high T cells) do not proliferate, nor are they arrested in the cell cycle. Rather, they are minimally activated, sometimes expressing CD69 but not CD25, HLA-DR, VLA-1, or effector cytokines. Blocking antibodies to interleukin 2 (IL-2), IL-6, IL-7, or tumor necrosis factor do not inhibit viral replication. Cyclosporine effectively inhibits viral replication, as does disruption of the NFAT binding site in the viral long terminal repeat. Furthermore, in the presence of ECs, suboptimal T-cell receptor (TCR) stimulation with phytohemagglutinin L supports efficient viral replication, and suboptimal stimulation with toxic shock syndrome toxin 1 leads to viral replication selectively in the TCR-stimulated, Vβ2-expressing T cells. Collectively, these data indicate that ECs provide signals that promote Nef- and Vpr-dependent HIV replication in memory T cells that have been minimally activated through their TCRs. Our studies suggest a mechanism for HIV replication in vivo within the reservoir of circulating memory CD4+ T cells that persist despite antiretroviral therapy and further suggest that maintenance of immunological memory by MHC class II-expressing ECs via TCR signaling may contribute to HIV rebound following cessation of antiretroviral therapy.
We have recently reported that human endothelial cells (ECs) markedly enhance human immunodeficiency virus (HIV) replication up to 50,000-fold within previously nonactivated memory CD4+ T cells that are allogeneic to the ECs (i.e., from a different donor) both in vitro and in vivo in a manner that requires an intact nef gene (9). In contrast, Nef does not greatly influence viral replication in T cells cocultured with allogeneic B cells, macrophages, fibroblasts, or dendritic cells. Fluorescence-activated cell sorter (FACS) analysis performed on CD4+ T cells cocultured with ECs for 6 or 9 days demonstrated that a discrete subset of T cells (between 5 and 20% of the population) expressed very high levels of intracellular viral proteins and was therefore likely to be responsible for the production of cell-free virus. In EC-T-cell cultures, viral replication depended upon endothelial expression of major histocompatibility complex (MHC) class II molecules (principally HLA-DR), the target molecules recognized by the T-cell receptor (TCR) for antigen of CD4+ T cells, as well as on CD2, the target of the EC costimulatory molecule LFA-3 (CD58). Furthermore, only CD45RO+ memory T cells supported viral replication, consistent with the capacity of ECs to selectively present antigen to memory cells (reference 14 and unpublished data). Moreover, since ECs lacking MHC class II molecules could exert this Nef-dependent effect in three cell cultures (involving B cells and ECs allogeneic to the T cells), our data suggested that ECs also provide a unique signal that augments Nef-dependent viral replication. In other words, Nef-dependent enhanced HIV replication required signals for T-cell activation, as well as additional (and as yet undefined) EC-specific signals.
Since activated CD4+ T cells are a primary source of HIV production in vitro (4, 44) and in vivo (21, 46, 65), it is reasonable to hypothesize that ECs support viral replication in TCR-activated T cells. The productively infected cells observed on days 6 and 9 of culture, i.e., the p24high T cells, could represent the progeny of the cells that had been fully activated and proliferated in response to high-affinity TCR binding to nonself (allogeneic) MHC molecules. The response of mature CD4+ T cells to allogeneic cells is predominantly a cross-reaction of TCRs that were selected during thymic development to recognize peptides derived from foreign proteins bound to self allelic forms of MHC class II molecules with complexes consisting of peptides bound to nonself allelic forms of MHC class II molecules displayed on the surface of the allogeneic cell (MHC alleles are the most diverse genes in the human population). The response to nonself MHC molecules is otherwise identical to activation by foreign proteins (presented as peptides bound to self MHC molecules), depending upon the same intracellular signaling pathways and showing the same requirements for costimulation (41). By limiting dilution analysis, it has been observed that such direct recognition of nonself MHC class II molecules on ECs can lead to the proliferation of between 0.1 and 1% of the input peripheral blood T cells, the precise frequency depending on the MHC alleles of the EC and T-cell donors (43). However, these activated cells expand to 10% or more of the total by 6 days, as measured by carboxy-fluorescein diacetate succinimidyl ester (CFSE) dilution assays (which measure cellular proliferation). It is possible that it is these cells that represent the high virus producers (p24high), which is underscored by the ability of HIV proteins to augment T-cell activation (3, 16). Alternatively, the p24high T cells may represent a population of input cells, larger than the predicted fully activated population (0.1 to 1%), whose TCRs bind to EC MHC class II molecules with moderate to low affinity. While insufficient to support T-cell proliferation, these lower-affinity signals may still be sufficient to activate some specific T-cell functions, including the expression of T-cell activation markers, the elaboration of effector cytokines, and perhaps HIV replication.
Although these are appealing explanations, viral replication may instead, or in addition, be taking place in unactivated or minimally activated T cells. There is growing evidence for circumstances in which T-cell activation and productive viral infection may be uncoupled. In the early and late stages of HIV infection in vivo, most of the cells that express viral RNA do not express markers of cellular activation, and these cells persist despite antiretroviral therapy (ART) (70). Furthermore, resting memory T cells in the gut-associated lymphoid tissue (GALT) are specifically depleted in the initial weeks of simian immunodeficiency virus (SIV) infection, which is thought to establish conditions that eventually lead to progression to AIDS (31, 40). In vitro, infection of thymic stromal histocultures by HIV also leads to replication in cells lacking activation markers (13). High levels of cytokines from highly or moderately activated T cells or ECs could also cause productive infection with minimal activation, thereby enhancing HIV replication in cells that are not receiving a TCR signal (54, 61). Alternatively, low-affinity binding of the TCR with the EC MHC class II molecules may approximate homeostatic signals provided by engagement of the TCR with self MHC class II-peptide complexes. Such signals are not strong enough to induce effector T-cell functions, but they are thought to be necessary for maintenance of CD4+ T-cell memory function (39, 46).
Here, we report that HIV production in this in vitro model requires Vpr, as well as Nef, viral gene products that modulate T-cell signaling pathways (28). Furthermore, despite the requirement for endothelial MHC class II molecules and costimulation, viral replication is largely confined to T-cell populations that do not proliferate and that show minimal evidence of activation. Finally, we present evidence to indicate that viral replication within these cells depends upon suboptimal TCR stimulation and TCR-dependent activation of the transcription factor NFAT, but not cytokine signals.
MATERIALS AND METHODS
Isolation and culture of human cells.
All human cells and tissues were obtained under protocols approved by the Yale Human Investigations Committee. Peripheral blood mononuclear cells (PBMCs) were isolated from HIV-seronegative donors by density gradient centrifugation of leukapheresis products by using lymphocyte separation medium (Gibco BRL, Grand Island, N.Y.). CD4+ T cells were isolated from PBMCs by positive selection using Dynabeads (Dynal, Lake Success, N.Y.). The selected population obtained by this procedure was routinely >97% CD4+ CD3+ by flow cytometry (data not shown). The cells were subsequently purified by using further negative selection as described previously (12). Activated CD4+ T cells were removed by incubating them with an anti-HLA-DR antibody at a concentration of 5 μg/ml (LB3.1; a gift of J. Strominger, Harvard University, Cambridge, MA) for 20 min, washing them twice, and depleting them by using magnetic beads conjugated to goat anti-mouse antibody (Dynal).
Human umbilical vein endothelial cells were isolated from umbilical cords by collagenase digestion and maintained in culture by using conditions and reagents as previously described (48). Such cultures are uniformly CD31+ and von Willebrand factor+ and lack CD45. When indicated, the cells were treated with 50 ng/ml of gamma interferon (IFN-γ) (Biosource, Camarillo, CA) per ml for 3 days prior to coculture with allogeneic CD4+ T cells. After pretreatment with IFN-γ, the ECs were uniformly HLA-DR positive (data not shown).
Disruption of the NFAT binding sites in the HIV long terminal repeats (LTRs).
The NFAT− clones were created by site-directed mutagenesis using the Quick Change Site Directed Mutagenesis kit (Stratagene, La Jolla, CA) with p83-2 and p83-10 plasmid DNAs (containing 5′ and 3′ NL4-3 sequences, respectively) as templates. Individual clones were subjected to fluorescence sequencing (http://info.med.yale.edu/wmkeck) to ensure that the exact desired sequence was used in our experiments.
Generation of virus stocks.
To generate infectious pNL4-3 (wild type) or derivative HIV (Nef− or NFAT−), wild-type or mutated p83-2 and p83-10 plasmid DNAs were digested with EcoRI (New England Biolabs, Beverly, MA), column purified (QIAGEN, Valencia, CA), and combined with an equal amount of similarly prepared EcoRI-digested complementary plasmid DNA. The two plasmids were fused by using T4 DNA ligase (New England Biolabs), and the DNA was introduced into the permissive cell line CEMx174 by DEAE-dextran transfection. The transfected cells were cultured in complete medium consisting of RPMI 1640 (Gibco BRL) supplemented with 10% fetal bovine serum (Gibco BRL), penicillin-streptomycin, and l-glutamine (Gibco BRL). HIV lacking Vpr (Vpr−) was described previously (22) and was obtained from the AIDS Research and Reference Reagent Program (Rockville, MD). For the generation of HIV stocks, culture supernatants containing virus were harvested daily beginning 48 h after the first observation of cytopathic effect and stored at −70°C until experimental use.
Viral infection.
Approximately 1.0 × 106 primary quiescent CD4+ T cells were cocultured with 1.5 × 105 ECs in C24 well microculture plates (BD Falcon, Bedford, MA). Alternatively, CD4+ T cells were pretreated with PHA-L (5 μg/ml; Fisher Chemical, Fairlawn, NJ) and interleukin 2 (IL-2) (25 IU; Biological Resources Branch, National Cancer Institute, Frederick, MD) for 3 days prior to infection. The cultures were infected with HIV at a multiplicity of infection of 0.05, and cultures containing ECs and T cells were infected within 1 h of coculture. In each case, the infections were carried out in triplicate. After overnight incubation with HIV, nonadherent cells and the culture supernatant were subjected to centrifugation. The supernatant was then removed, the cells were washed with phosphate-buffered saline (PBS) to remove residual virus, and the cells were resuspended with ECs in fresh complete medium. At 3-day intervals, the media were changed; supernatants were aliquoted and stored at −70°C. Where indicated, the cultures were treated with cyclosporine or ethanol vehicle or with rapamycin or dimethyl sulfoxide vehicle (Sigma-Aldrich, St. Louis, MO). These agents were added at the onset of coculture and replaced with every medium exchange.
The growth kinetics of HIV under various experimental conditions were assayed by the concentration of HIV p24 in the culture supernatants as assessed by enzyme-linked immunosorbent assay (ELISA; Coulter, Hialeah, FL).
Immunostaining and flow cytometric analyses.
T cells were harvested from the EC-T-cell cocultures as described previously (9). The production of intracellular p24 was assessed by using the fluorescein isothiocyanate (FITC)-conjugated or phycoerythrin (PE)-conjugated anti-p24 monoclonal antibody KC-57 (Coulter). The expression of T-cell surface markers was assessed by using PE-conjugated anti-CD4, anti-CD25, anti-CD69, anti-CD49a (VLA-1), anti-HLA-DR, or immunoglobulin G (IgG) isotype control (Beckman Coulter, Fullerton, CA). For FACS analysis of the Vβ2+ population, the cells were stained first with a monoclonal antibody recognizing Vβ2 (MBP2D5) (Beckman Coulter) for 1 h. The cells were then washed and stained with a PE-conjugated goat anti-mouse IgG antibody (Beckman Coulter). For intracellular cytokine staining of T cells, monensin (2 μM) was added for the last 8 h of coculture. For pharmacologically stimulated T cells, 10 ng/ml phorbol myristate acetate (PMA) plus 1 μM ionomycin in the presence of 2 μM monensin were added to replicate cultures for the last 6 h. The cells were then washed, fixed with 4% paraformaldehyde, permeabilized with 0.1% saponin, and labeled with anti-cytokine antibody as described previously (34). Intracellular cytokines were assessed by using PE-conjugated anti-IFN-γ, FITC-conjugated anti-IL-4, and FITC-conjugated anti-IL-2 (BD Pharmingen). Extensive fixation was necessary to abolish infectivity of the cells prior to FACS analyses. The absolute intensity of fluorescent staining of fixed cells for various antigens varied among experiments, but the relative intensities among different subpopulations were preserved. We attribute this to effects of the fixative on masking antigenic epitopes.
For proliferation assays, the cells were labeled with 250 nM CFSE (Molecular Probes, Eugene, OR) for 15 min prior to coculture with ECs as described previously (9). The data were acquired with a FACSCalibur flow cytometer and analyzed with CellQuest software (BD Biosciences).
For analysis of DNA content, the cells were fixed with 70% ethanol overnight. The cells were incubated with FITC-conjugated anti-p24gag monoclonal antibody KC-57 for 30 min in the dark. After several washes in PBS, the cells were incubated in PBS containing RNase A (100 μg/ml; Sigma-Aldrich) for a minimum of 1 h. Propidium iodide (PI) (50 μg/ml) was added 20 min before FACS analyses.
Superarray RNA analyses of T-cell mRNA.
CD4low cells were isolated by magnetic-bead depletion 9 days postinfection in the Nef+ virus-infected cultures. In brief, the cells were incubated with 5 to 15 μg/ml of α-CD4 antibody (R&D Systems, Minneapolis, MN) for 20 min with continuous agitation at 4°C. The cells were then washed two times to remove unconjugated antibody, and the CD4+ cells were depleted using magnetic beads conjugated to goat anti-mouse antibody (Dynal). T cells from the same donor were labeled with CFSE prior to EC coculture in the uninfected cultures. After 9 days of coculture, CD4+ T cells were harvested, and the CFSEhigh cells were isolated by FACS using the FACSVantage SE (BD Biosciences).
RNAs from these samples were isolated using QiaShredder and an RNEasy MiniPrep kit (QIAGEN). RNA was reverse transcribed using the Ampho-Labeling kit (Superarray, Frederick, MD), and the cDNA was labeled with biotin-16-dUTP (Roche). Cytokine expression was assessed using a cDNA GEArray Human Common Cytokine Microarray (Superarray, Frederick, MD).
Blocking antibodies.
Mouse monoclonal antibodies reactive with human cytokines included anti-bone morphogenic protein 6 (BMP6), anti-IL-2, anti-IL-6, anti-tumor necrosis factor (TNF), IgG1 isotype control, and IgG2b isotype control (R&D Systems). The antibodies against IL-7 and its isotype control were rat monoclonal antibodies (BD Pharmingen). The antibodies were used at 10 times the 50% neutralizing dose; the final concentration of the antibodies ranged from 5 to 10 μg/ml. The antibodies were added at the time of infection, and these antibodies were included in every medium change (every 3 days) and were therefore present throughout the duration of the cocultures.
Polyclonal stimulation of EC-T-cell cocultures.
For polyclonal stimulation, phytohemagglutinin L (PHA-L; Sigma), toxic shock syndrome toxin 1 (TSST-1; Toxin Technologies, Sarasota, FL), or vehicle was added to EC-T-cell cocultures prior to infection. After 24 h, the supernatant was harvested so that IL-2 expression could be assayed by ELISA (eBioscience, San Diego, CA). At the time of medium exchange (24 h postinfection), PHA-L and TSST-1 were again added so that they were present throughout the duration of the coculture.
RESULTS
ECs enhance Nef+ virus replication in minimally activated CD4+ T cells.
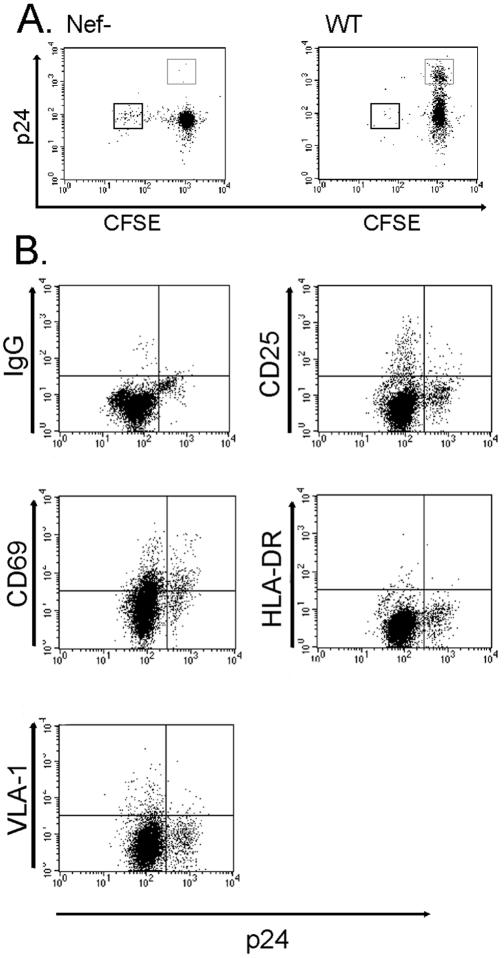
To determine whether the p24high T cells generated in EC-T-cell cocultures represented the population that had proliferated, we labeled CD4+ T cells with CFSE prior to placing them in coculture with allogeneic MHC class II+ ECs and infecting them with HIV. In EC-T-cell cocultures infected with a nef-deficient strain of virus (Nef−) (Fig. 1A), a CFSElow population accumulates, comprising the cells that have proliferated repetitively. This response to IFN-γ-pretreated allogeneic ECs is indistinguishable from that of non-CD4+-infected cells (data not shown). However, in EC-T-cell cocultures infected with wild-type virus, we observed a novel population of p24high cells that remained CFSEhigh, indicating that they had not proliferated (Fig. 1A). The frequency of the p24high CFSEhigh population ranged between 5 and 20% of the starting population, which is substantially higher than the number of input T cells that can proliferate in response to allogeneic ECs (0.1 to 1.0%) prior to expansion. Therefore, even if this entire population became p24high, without expansion via proliferation, they could not account for the majority of the total p24high population. An unexpected finding was that in cultures infected by wild-type virus, but not by Nef− virus, there was a conspicuous absence of CFSElow cells. We suspect that wild-type virus either inhibited T-cell proliferation or, more likely, mediated the death of infected T cells. We did not investigate this issue further in the present study. In the remainder of this study, we focused on the characterization of the p24high CFSEhigh virus producers that emerge when wild-type infected T cells are cocultured with ECs.
FIG. 1.
Characterization of HIV type 1-infected CD4+ T cells. (A) FACS analyses of CFSE-labeled CD4+ T cells cocultured with ECs, infected with either Nef− or wild-type (WT) HIV, and harvested on day 9 postinfection. The thin-walled box identifies the CFSEhigh p24high cells, and the thick-walled box identifies the CFSElow p24low cells. Note the expansion of CFSEhigh p24high cells in the wild-type-infected cultures. (B) FACS analyses of wild-type HIV-infected T cells after 9 days of coculture with ECs stained with antibodies against IgG isotype control, CD25, CD69, HLA-DR, and VLA-1. Note that p24high cells express CD69 but not other markers of activation. The data in panels A and B are from one of three experiments with similar results.
We initially examined the activation status of the high-virus-producing T cells. We analyzed the p24high cells for surface expression of early, intermediate, and late activation markers. A few hours after TCR engagement, T cells express the early activation markers CD25, a component of the high-affinity IL-2 receptor, and CD69, a molecule with unknown function. After 24 h of activation, human T cells will stably upregulate MHC class II molecules (e.g., HLA-DR) (15), and after a week will stably upregulate the β1 integrin, VLA-1 (20). FACS analysis revealed that on day 9 postinfection, the p24high cells did not express CD25, HLA-DR, or VLA-1, although some did express the activation marker CD69 (Fig. 1B). These data are consistent with minimal (and perhaps a very early stage of) activation.
Cytokine synthesis, which can be measured by intracellular staining, is another hallmark of CD4+ T-cell activation (29). We therefore proceeded to examine the cytokines expressed by the high virus producers. We harvested the cells 9 days after infection and performed intracellular cytokine staining for effector cytokines, IL-2, IL-4, and IFN-γ, using monensin to enhance the signal by blocking secretion. FACS analysis revealed that the virally infected cells did not appear to be producing cytokines in the presence of MHC class II-expressing ECs (Table 1). Intracellular cytokine staining of replicate cultures showed that these same cells are capable of producing IL-2 and IFN-γ in response to pharmacological stimulation by PMA plus ionomycin (Table 1). EC-stimulated cells after 9 days of culture do not produce IL-4 with or without PMA and ionomycin. The absence of intracellular cytokine staining is thus also suggestive of minimal T-cell activation.
TABLE 1.
The p24high cells in EC-T-cell cocutures do not spontaneously produce cytokines
| Cytokine | % Positivea
|
|
|---|---|---|
| Monensin alone | Monensin, PMA, ionomycin | |
| IL-2 | 1.7 ± 1.4 | 73.5 ± 0.4 |
| IL-4 | 1.5 ± 0.4 | 2.6 ± 0.3 |
| IFN-γ | 4.4 ± 2.8 | 32.0 ± 15.6 |
Percentage of the p24high cells that were positive for each intracellular cytokine. These data are from one of three experiments with similar results.
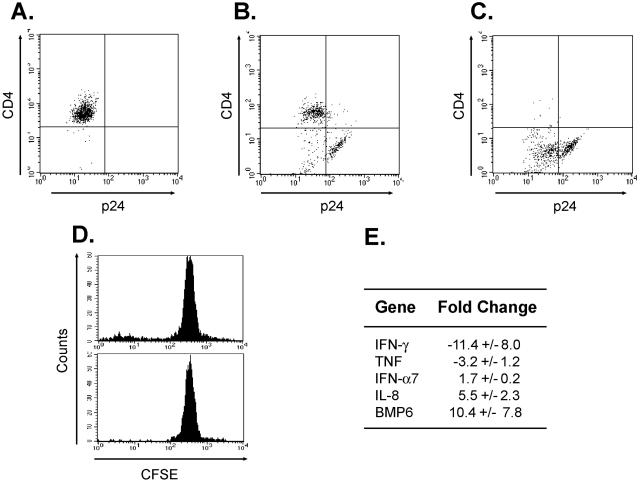
We also characterized the profile of cytokines produced by these cells at the mRNA level. To do so, we took advantage of the well-described abilities of Nef, Vpu, and Env to downregulate surface CD4 in order to isolate a population enriched in virus producers (2, 7, 62, 66). Uninfected T cells are uniformly CD4high p24low (Fig. 2A). In contrast, in the HIV-infected cocultures, we found three populations, a CD4high p24low population, a CD4low p24low population, and a CD4low p24high cell population (Fig. 2B). In other words, the p24high cells are almost exclusively CD4low, presumably due to high levels of expression of HIV protein. By selecting the cells based on CD4 expression, we enriched for a population of the high virus producers (Fig. 2C). In parallel, we isolated the nonproliferating CFSEhigh cells from an uninfected replicate culture by cell sorting (Fig. 2D). We thus collected RNAs from CD4low infected cells and CFSEhigh uninfected cells from replicate EC-T-cell cocultures and analyzed them by using Human Common Cytokine Superarrays. In general, the virally infected cells closely resembled the unactivated T-cell population (data not shown). After normalization to GAPDH (glyceraldehyde-3-phosphate dehydrogenase), these cells actually expressed lower levels of IFN-γ RNA than unactivated T cells isolated from the same donor (Fig. 2E). However, consistent with being HIV infected, the p24high T cells produced more IFN-α7 and IL-8 RNA, the latter presumably due to the effects of Tat (45). The p24high cells also consistently expressed more BMP6 RNA, the function of which is unknown. Despite these few differences, the results from the Superarray analyses generally confirm the lack of T-cell activation.
FIG. 2.
Changes in gene expression induced by HIV infection. FACS analyses after 9 days of EC-T-cell coculture of (A) uninfected CD4+ cells, (B) wild-type HIV-infected T cells without selection, and (C) wild-type HIV-infected T cells following depletion of CD4high cells. (D) FACS histogram of replicate uninfected cultures without sorting (top) and after sorting (bottom) for the CFSEhigh population. (E) Human Common Cytokine Superarray Analyses of RNAs isolated from CD4low cells from wild-type virus-infected EC-T-cell cocultures compared with unactivated CD4+ T cells from the same donor. Cytokine expression levels were normalized to GAPDH. Depicted are genes that displayed a >1.5-fold change in expression in CD4low cells (presumably infected) versus CFSEhigh cells (from uninfected cultures). The data in panels A to E are from one of three experiments with similar results.
High virus producers are not arrested in G2 by Vpr.
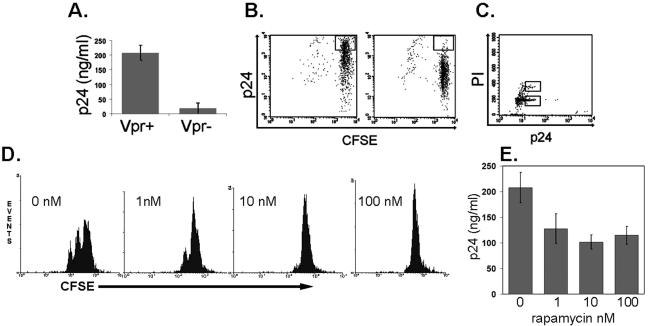
Since virus-producing cells were not dividing, we tested whether an antiproliferative HIV protein could be responsible for causing cell cycle arrest of the host cell. Specifically, it has been reported that CD4+ T cells that have been productively infected with wild-type virus are nonproliferating despite strong stimulation (4), whereas CD4+ T cells infected with a vpr-deficient strain (Vpr−) that were similarly stimulated underwent multiple cell divisions (44). Vpr can arrest infected cells in G2 (19, 23, 49), which is advantageous to the virus because G2 is the phase of the cell cycle that is optimal for viral replication (18). Strikingly, Vpr contributes strongly to viral replication in EC-stimulated T cells, since its absence results in a 10- to 20-fold reduction in cell-free virus (Fig. 3A). The number of p24high CFSEhigh T cells is also drastically reduced in EC-T-cell cocultures infected with Vpr− virus (Fig. 3B), consistent with reduced cell-free virus levels. Because the p24high population is essentially absent in the Vpr−-infected cultures, it was not possible to determine if Vpr actually caused growth arrest. To determine whether the cells infected with wild-type virus were, in fact, arrested in a particular stage of the cell cycle, we stained the T cells with propidium iodide. FACS analysis revealed that the p24high cells are not arrested in G2 or in any other specific stage of the cell cycle but instead represent a heterogeneous population with most of the p24high cells in G0/G1 (Fig. 3C). Rapamycin inhibits mTOR, which is required for the progression of IL-2-stimulated cells from G1 to S (1). To further test if progression beyond G1 is required for enhanced HIV production, we added rapamycin at concentrations that were sufficient to inhibit or completely block proliferation of polyclonally activated T cells (Fig. 3D). We observed that addition of rapamycin reduced viral replication (though not nearly to the extent of deletion of vpr) (Fig. 3E) in our in vitro model using previously resting T cells. Our cumulative observations suggest that the necessity for Vpr for high levels of viral replication in p24high CFSEhigh cells cannot be explained by its ability to induce cell cycle arrest.
FIG. 3.
Characterization of the role of Vpr in enhanced HIV production. (A) Replication of wild-type versus Vpr− HIV in EC-T-cell cocultures 9 days postinfection as assessed by p24 measured in the supernatant by ELISA. (B) CFSE staining of infected cells harvested on day 9 postinfection. The box identifies the CFSEhigh p24high cells, which are fewer in cultures infected by Vpr− virus (right). (C) PI staining of infected cells harvested on day 9 postinfection. The lower box identifies cells in the G0/G1 state, and the upper box identifies the cells in the G2 state of the cell cycle. Note that the majority of cells are in G0/G1. (D) FACS analyses of CFSE-labeled CD4+ T cells treated with PHA-L and rapamycin. Note that the cells remain CFSEhigh. (E) Replication of wild-type HIV in EC-T-cell cocultures treated with rapamycin or vehicle (dimethyl sulfoxide, 0 nM rapamycin) 9 days postinfection as assessed by p24 measured in the supernatant by ELISA. The data in panels A to E are from one of three experiments with similar results. The error bars indicate standard deviations.
TCRs and not cytokine signals provide minimal activation for viral replication.
In our previous study, we showed that the ability of ECs to enhance viral replication in cocultured CD4+ T cells requires endothelial expression of MHC class II and costimulators, yet our new data revealed that the p24high T cells showed little evidence of T-cell activation (9). Two models can explain viral replication in minimally activated T cells. In the bystander model, the T cells that are p24high do not directly recognize MHC class II on the EC surface. Instead, the signals that promote viral replication in these nonproliferating cells, i.e., cytokines, are provided in trans by other T cells which are highly or moderately activated. In the suboptimal-signaling model, the T cells that are p24high directly recognize MHC class II on the EC surface but receive signals that are insufficient to fully activate the cells to proliferate and/or elaborate cytokines. Such signals may be provided by EC MHC class II molecules to cells bearing TCRs that interact with low affinity.
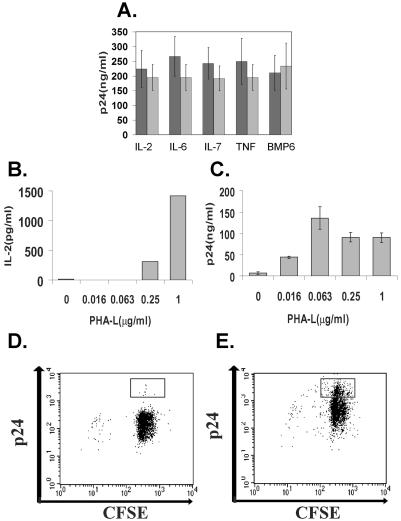
Although activated T cells appeared to have died off by day 9 postinfection, we did detect cytokines, such as IL-2, in the medium on day 1 postinfection at levels that were similar to those found in uninfected EC-T-cell cocultures (9). Therefore, to test whether the bystander model was operative in our in vitro system, we added blocking antibodies to IL-2, IL-6, IL-7, TNF, or BMP-6, which have been identified as cytokines that can support TCR-independent viral replication (54, 61, 63), to EC-T-cell cocultures. These treatments did not inhibit viral replication (Fig. 4A), results that are inconsistent with the bystander model.
FIG. 4.
TCRs and not cytokine signals provide minimal activation necessary for HIV replication. (A) Replication of wild-type HIV in EC-T-cell cocultures 9 days postinfection treated with the indicated blocking antibodies (dark bars) or isotype controls (light bars) as assessed by p24 measured in the supernatant by ELISA. (B) IL-2 measured in the supernatant of infected T cells after 1 day of coculture with ECs in the presence of various doses of PHA-L or vehicle alone. (C) Cell-free virus measured in the supernatant of infected EC-T-cell cocultures on day 7 postinfection as assessed by p24 measured in the supernatant by ELISA. (D and E) FACS analyses of CD4+ T cells cocultured with untreated ECs in the presence of (D) 0 μg/ml and (E) 0.063 μg/ml of PHA-L. The boxes identify the CFSEhigh p24high cells. Note the expansion of CFSEhigh p24high cells in the wild-type-infected cultures in the presence of 0.063 μg/ml of PHA-L. The data in panels A to E are from one of three experiments with similar results. The error bars indicate standard deviations.
To test whether the suboptimal-signaling model was operative, we cocultured CD4+ T cells with untreated ECs, added suboptimal doses of PHA-L, and infected them with wild-type virus. Under such conditions, ECs continue to provide costimulation, but the TCR-mediated signals are mediated primarily through PHA-L rather than MHC recognition. PHA-L provides a dose-dependent increase in the IL-2 produced (Fig. 4B) and dose-dependent proliferation (data not shown). Strikingly, suboptimal doses of PHA-L that are insufficient to induce detectable cytokine production still support efficient viral replication (Fig. 4C). We next assessed the ability of suboptimal stimulation with PHA-L to induce a CFSEhigh p24high population. To do so, we labeled the T cells with CFSE prior to adding them to EC cultures and infecting them. On day 7 postinfection, the T cells were harvested and counterstained for p24. We found that cultures activated with suboptimal stimulation, i.e., 0.0625 μg/ml of PHA-L, displayed negligible T-cell proliferation, but compared to no PHA-L, they show a significant increase in the number of p24high CFSEhigh cells (Fig. 4D and E). These data support the idea that a suboptimal TCR signal promotes HIV replication in T cells cocultured with ECs.
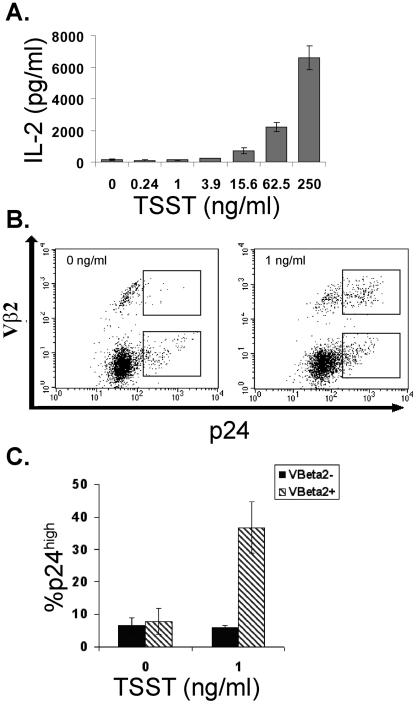
To determine more directly whether the p24high cells were receiving MHC class II signals from ECs, we used a different type of polyclonal stimulator, namely, the superantigen TSST-1. TSST-1 binds to a conserved site on MHC class II molecules and to a site on the Vβ2 chain of the TCR (17). Therefore, like PHA-L, TSST-1 can activate T cells primarily by simulating TCR-MHC interactions. However, unlike PHA-L, TSST-1 requires expression of endothelial MHC class II, and more importantly, TSST-1-mediated activation, unlike PHA-L-mediated activation, is restricted to those T cells that express TCRs containing the Vβ2 receptor chain. We found that TSST-1, like PHA-L, provided a dose-dependent increase in cytokine production (Fig. 5A). If the suboptimal-signaling model were true, TSST-1 should selectively increase viral replication among the cells that express Vβ2. To test this, we cocultured MHC class II-expressing ECs with unselected, resting CD4+ T cells in the presence of increasing doses of TSST-1. On day 7 postinfection, we harvested the T cells and stained them for extracellular Vβ2 and intracellular p24. In the absence of TSST-1 (Fig. 5B), the p24high cells, like the input cells, were predominantly Vβ2−. In the presence of suboptimal TSST-1, we found that the p24high cells were enriched selectively in the Vβ2+ population (Fig. 5C). TSST-1 did not change the proportion of Vβ2− cells that were p24high. In contrast, suboptimal TSST-1 increased the proportion of Vβ2+ cells that were p24high by sevenfold (Fig. 5C). These observations directly confirm a role for suboptimal TCR signaling in promoting HIV replication.
FIG. 5.
TSST-1 selectively leads to viral replication in Vβ2+ T cells. (A) IL-2 measured in the supernatant of infected T cells after 1 day of coculture with ECs in the presence of increasing doses of TSST-1 or vehicle alone. (B) FACS analyses of CD4+ T cells cocultured with IFN-γ-pretreated ECs in the presence of 0 ng/ml or 1 ng/ml of TSST-1. The upper boxes identify the Vβ2+ p24high cells, and the lower boxes identify the Vβ2− p24high cells. Note the expansion on the Vβ2+ p24high cells in the presence of 1 ng/ml TSST-1. The data in panels A and B are from one of three experiments with similar results. (C) Proportions of p24high cells among the Vβ2+ and the Vβ2− populations in infected EC-T cell cocultures stimulated with 0 or 1 ng/ml of TSST-1. Shown are the means from three independent experiments ± standard error.
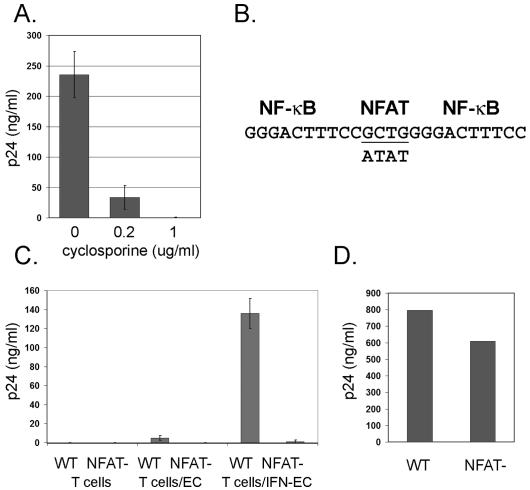
Finally, we investigated signals downstream of the TCR that could underlie the EC effect. Cyclosporine inhibits the TCR-dependent activation of calcineurin. Therefore, to assess a role for calcineurin in our in vitro model, we added cyclosporine to EC-T-cell cocultures. Since previous studies had shown that ECs confer cyclosporine resistance on CD4+ T cells (24), we chose relatively high doses of cyclosporine, namely, 0.2 μg/ml and 1.0 μg/ml. Such concentrations are consistent with doses previously used to examine the impact of calcineurin on HIV replication with minimal effect on cyclophilin A incorporation into virions (11, 26). The addition of cyclosporine significantly inhibited viral replication (Fig. 6A). The major target of cyclosporine is thought to be calcineurin-dependent activation of NFAT. To directly test whether an NFAT-dependent mechanism underlies the EC effect, we disrupted the NFAT binding site in the HIV LTR (26, 27) (Fig. 6B) and tested if this affected the capacity of ECs to enhance HIV production. The recombinant HIV (NFAT−) replicated to only very low levels in comparison to the wild-type derivative strain in previously resting CD4+ T cells cocultured with IFN-γ-treated ECs (Fig. 6C). To ensure that this phenotype was not due to inherently poor NFAT− replicative capacity, it was also grown in CD4+ T cells pretreated with PHA and IL-2. Under these conditions, NFAT− virus replicated similarly to wild-type virus (Fig. 6D).
FIG. 6.
Characterization of the role of NFAT in HIV production. (A) HIV release on day 9 postinfection (representing the peak) from EC-T-cell cocultures treated with cyclosporine or vehicle (ethanol). The data are from one of three experiments with similar results. (B) Schematic diagram of the region of the HIV LTR that contains the NF-κB and NFAT binding sites. The underlined nucleotides are required for NFAT-LTR interactions. The nucleotides on the lower line indicate the loci that differ between the NFAT− strain and the wild-type derivative strain (upper line). (C) HIV released from T cells on day 9 postinfection (representing the peak). T cells represents T cells alone, T cells/EC represents T cells cocultured with untreated ECs, and T cells/IFN-EC represents T cells cocultured with ECs pretreated with IFN-γ. The data are from one of two experiments with similar results. (D) HIV released from CD4+ T cells isolated from PBMCs and pretreated with PHA and IL-2 on day 8 postinfection (representing the peak). Similar results were obtained using the cell line CEMx174 (not shown). HIV release was assessed by p24 measured in the supernatant by ELISA. The error bars indicate standard deviations.
DISCUSSION
In the present study, we made the unexpected finding that HIV replication in EC-T-cell cocultures actually occurs exclusively in memory CD4+ T cells that are only minimally activated. We cannot rule out the possibility that highly or moderately activated cells contributed to viral replication but died before they could be detected. However, on day 9 postinfection, i.e., the peak of viral replication, the cells that are producing the virus (the p24high T cells) are neither proliferating nor arrested at a specific stage of the cell cycle. Furthermore, other than CD69 expression, the p24high T cells do not express activation markers or effector cytokines. In our system, the virally infected T cells are identified by FACS analysis, which can occur only after they express sufficient intracellular viral proteins. Therefore, we cannot rule out the possibility that the cells had previously demonstrated markers of activation, i.e., surface markers and effector cytokines, but had subsequently downregulated them. This seems unlikely, since we examined the cells for early, intermediate, and late activation markers, and we found that the p24high T cells did not express CD25, HLA-DR, or VLA-1 and only small amounts of CD69. Our results using T cells activated by ECs stand in striking contrast to previous studies, which used similar FACS-based techniques to show that viral replication under other conditions is restricted to highly activated T cells (4, 44).
In a fully quiescent cell, HIV exists as incomplete or complete cDNA, neither of which is stable long term, and both are essentially transcriptionally silent (58, 68, 71). Therefore, even in cells that demonstrate limited markers of activation, viral replication appears to require the activation of specific signaling pathways. In the current study, we tested two possible means by which MHC molecule- and costimulation-dependent signals could support viral replication in EC-stimulated T cells but not proliferation or cytokine production from these same T cells. In the bystander model, the high virus producers receive selective activation via a factor, i.e., a cytokine produced by other CD4+ T cells that are highly or moderately activated. This possibility is supported by a previous study, which showed that in autologous dendritic-cell-T-cell cocultures and superantigen, productive HIV replication occurs not only in the superantigen-activated T cells but also in the bystander cells, which did not receive direct TCR activation and were therefore stimulated via cytokines (51). However, in contrast to the previous report on dendritic-cell-T-cell cocultures, we found no direct evidence of highly activated T cells in the EC-T-cell cocultures at the time of peak virus production. In fact, there was a striking absence of the expected CFSElow population, i.e., the population that proliferated in wild-type-infected cultures. In addition, rapamycin, an inhibitor of cytokine-dependent mTOR activation, only minimally inhibits viral replication, and the addition of blocking antibodies to IL-2, IL-6, IL-7, and TNF, which have been identified as cytokines that can support TCR-independent viral replication (54, 61, 63), had no effect on viral replication in EC-T-cell cocultures. Our data suggest that the bystander model does not explain why the p24high T cells in cocultures with ECs appear minimally activated.
An alternative to the bystander model is the suboptimal-signaling model. There is a hierarchical activation of T-cell functions, and different functions are activated sequentially with increasing TCR stimulus. For example, T-cell proliferation requires higher stimulation than does cytokine production (5). In the suboptimal-signaling model, the high virus producers directly recognize endothelial MHC class II molecules but do not receive a signal sufficiently strong to drive the cell to proliferation or to cytokine production. Our results have led us to favor this model. The experiments with PHA-L demonstrate that suboptimal TCR-mediated stimulation is sufficient for efficient virus replication; furthermore, the experiments with TSST-1 demonstrate that viral replication is restricted to T cells whose TCRs are engaged by endothelial MHC class II molecules. We have also examined the effects of cyclosporine, an inhibitor of the TCR-activated enzyme calcineurin. Although this drug has several nonspecific effects (52, 60), its major action is blocking the calcineurin-dependent activation of the transcription factor NFAT. We have demonstrated that cyclosporine, in contrast to rapamycin, effectively inhibits viral replication. More directly, we also showed that the alteration of the NFAT binding site in the HIV LTR blocks the EC effect on HIV replication.
Studies with altered peptide ligands (APLs) have shown that suboptimal T-cell activation preserves some aspects of full T-cell activation but not others (56). Suboptimal T-cell activation with APLs induces only partial TCR-ζ phosphorylation, which in turn leads to the inefficient recruitment of Src family kinases. The biochemical consequence of this is the selective activation of downstream targets. For example, APL-induced calcium signaling, which is reduced and shorter in duration than agonist peptide-induced calcium signaling, can contribute to the selective activation of NF-κB or of NFAT (30, 57). In contrast to the IL-2 promoter, where NFAT binds as a heterodimer with AP-1, NFAT binds in the LTR enhancer as a homodimer (47). Therefore, while IL-2 expression requires both NFAT activation and AP-1 activation (35), HIV replication can occur with the selective activation of NFAT (26). Independent studies have shown that selective NFAT activation produces a phenotype similar to that of EC stimulation in HIV-infected T cells. NFAT-driven HIV production leads to preferential infection of memory T cells (50), and viral replication occurs without T-cell proliferation, activation marker expression, or cytokine expression (26). Lastly, selective NFAT activation, like HIV replication in our system, can be augmented by EC-specific factors. Wnt, produced by ECs, can inhibit glycogen synthase kinase-3β (GSK-3β), which in turn inhibits the nuclear export of NFAT (42).
A primary role for NFAT in EC-stimulated memory T cells may explain the important roles of Nef and Vpr in our model system. Nef is expressed even before the virus is integrated (67). In addition, Nef can interact directly with T-cell signaling molecules, including Lck, Vav, Pak2, and PKC-θ (37), and in response to TCR-derived signals, Nef has been shown to potentiate specific TCR-dependent signaling pathways, including those involving ERK (53), MAPK (37), PI-3-kinase (32), and Ca2+ (38). The rise in intracellular Ca2+ is the mechanism that leads to calcineurin and NFAT activation. The dependence of the EC effect on an intact nef gene is reminiscent of the effect that HIV-infected macrophages have on the infectivity of resting CD4+ T cells (59). However, the effect of Nef is operative in the macrophages and not in the resting T cells, whereas in the EC system, the effect of Nef is operative in the resting T cells and not in the ECs. We have shown that ECs, but not other antigen-presenting cells, greatly enhance Nef-dependent viral replication in resting T cells (9). Combined with the findings from this study, we suggest that the primary role of Nef is to serve as a signaling molecule that specifically modulates EC-specific, TCR-dependent signals, notably NFAT. Thus, Nef may play multiple roles in the establishment of an HIV-favorable microenvironment in peripheral tissue.
Vpr, a viral protein that is necessary for viral pathogenesis in vivo (33), contributes to viral replication in EC-T-cell cocultures in a manner that is independent of its ability to induce G2 arrest. Although we have not determined if the requirement for Vpr occurs prior to or subsequent to integration, we hypothesize that Vpr's ability to promote HIV transcription may underlie its contribution to EC-stimulated viral replication. In support of this, Vpr, a nuclear protein, has been shown to alter signal transduction pathways via direct modulation of transcription factor activity in the nucleus (25, 28), and Vpr-mediated enhancement of HIV transcription is most pronounced under conditions of suboptimal activation. Vpr's ability to activate cyclic AMP responsive element binding factor (CREB) is evident only under conditions that lead to the serine phosphorylation of CREB but not to CREB-dependent gene expression (28). In addition, Vpr's ability to potentiate LTR transcription driven by Sp1, a transcription factor that is active in resting CD4+ T cells (64), is attenuated both by NF-κB activation and by progression of the cell into G2. Moreover, Vpr has been shown to synergize with Nef in augmenting calcineurin-dependent NFAT activation (36-38), which we postulate contributes to the EC effect.
The role of minimal TCR signaling by ECs fits into a general pattern in which HIV subverts normal immune function. CD4+ T cells require TCR-dependent signals for maintenance of immunological memory (39, 55). In humans, MHC class II molecules are displayed on vascular ECs (8). Therefore, as human memory cells circulate through the blood, ECs are well situated to provide the suboptimal TCR signals required for maintenance of immunological memory. We propose that HIV exploits these signals for virus production. Specifically, we hypothesize that EC-expressed MHC class II molecules, perhaps containing self peptides, provide nonredundant signals that enhance virus replication in circulating, latently infected memory T cells.
Our findings have implications for the course of HIV infection in patients. During HIV and SIV infection, cells with limited markers of T-cell activation are productively infected (70). Furthermore, in the initial weeks of SIV infection, memory T cells are greatly depleted in multiple tissues (which suggests that high-affinity MHC class II-TCR interactions are not required for efficient HIV production in human tissue) and resting memory T cells are specifically depleted in the GALT (31, 40). The loss of these cells (which results directly from viral infection and not via a bystander mechanism) is thought to establish conditions that eventually result in progression to AIDS. Ex vivo analysis of infected patients has shown that resting memory cells represent the T-cell compartment that harbors the highest copy number of viral DNA (6). Furthermore, latently infected memory cells are thought to contribute to the viremic “blips” in patients on ART, as well as to breakthrough virus in patients whose ART regimens are changed and rebound virus in patients who discontinue ART (10, 69). All of these phenomena may involve EC effects on infected memory T cells of the kind described in the present report.
In summary, we have described a novel manner in which vascular ECs can boost HIV replication in minimally activated CD4+ T cells. This response depends upon recognition of molecules on the ECs and involves the viral genes nef and vpr and TCR-dependent activation of NFAT. These findings illustrate yet another way in which HIV exploits the human immune system and may contribute to rebound following discontinuance of ART in HIV-infected patients and the depletion of resting memory T cells in the GALT in the initial weeks of infection.
Acknowledgments
This work was supported by NIH grant R01 HL51014 and American Cancer Society institutional research grant IRG 58-012-42. J.C. and P.W. were supported by NIH training grants GM07205 and T32CA09085, respectively; J.W. was supported by the National Science Foundation Graduate Research Fellowship Program.
REFERENCES
- 1.Abraham, R. T., and G. J. Wiederrecht. 1996. Immunopharmacology of rapamycin. Annu. Rev. Immunol. 14:483-510. [DOI] [PubMed] [Google Scholar]
- 2.Aiken, C., J. Konner, N. R. Landau, M. E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76:853-864. [DOI] [PubMed] [Google Scholar]
- 3.Alexander, L., Z. Du, M. Rosenzweig, J. U. Jung, and R. C. Desrosiers. 1997. A role for natural simian immunodeficiency virus and human immunodeficiency virus type 1 nef alleles in lymphocyte activation. J. Virol. 71:6094-6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahbouhi, B., A. Landay, and L. Al-Harthi. 2004. Dynamics of cytokine expression in HIV productively infected primary CD4+ T cells. Blood 103:4581-4587. [DOI] [PubMed] [Google Scholar]
- 5.Bielekova, B., and R. Martin. 2001. Antigen-specific immunomodulation via altered peptide ligands. J. Mol. Med. 79:552-565. [DOI] [PubMed] [Google Scholar]
- 6.Brenchley, J. M., B. J. Hill, D. R. Ambrozak, D. A. Price, F. J. Guenaga, J. P. Casazza, J. Kuruppu, J. Yazdani, S. A. Migueles, M. Connors, M. Roederer, D. C. Douek, and R. A. Koup. 2004. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J. Virol. 78:1160-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buonocore, L., and J. K. Rose. 1990. Prevention of HIV-1 glycoprotein transport by soluble CD4 retained in the endoplasmic reticulum. Nature 345:625-628. [DOI] [PubMed] [Google Scholar]
- 8.Choi, J., D. R. Enis, K. P. Koh, S. L. Shiao, and J. S. Pober. 2004. T lymphocyte-endothelial cell interactions. Annu. Rev. Immunol. 22:683-709. [DOI] [PubMed] [Google Scholar]
- 9.Choi, J., J. Walker, S. Boichuk, N. Kirkiles-Smith, N. Torpey, J. S. Pober, and L. Alexander. 2005. Human endothelial cells enhance human immunodeficiency virus type 1 replication in CD4+ T cells in a Nef-dependent manner in vitro and in vivo. J. Virol. 79:264-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chun, T. W., R. T. Davey, Jr., M. Ostrowski, J. Shawn Justement, D. Engel, J. I. Mullins, and A. S. Fauci. 2000. Relationship between pre-existing viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nat. Med. 6:757-761. [DOI] [PubMed] [Google Scholar]
- 11.Cron, R. Q., S. R. Bartz, A. Clausell, S. J. Bort, S. J. Klebanoff, and D. B. Lewis. 2000. NFAT1 enhances HIV-1 gene expression in primary human CD4 T cells. Clin. Immunol. 94:179-191. [DOI] [PubMed] [Google Scholar]
- 12.Dengler, T. J., and J. S. Pober. 2000. Human vascular endothelial cells stimulate memory but not naive CD8+ T cells to differentiate into CTL retaining an early activation phenotype. J. Immunol. 164:5146-5155. [DOI] [PubMed] [Google Scholar]
- 13.Eckstein, D. A., M. L. Penn, Y. D. Korin, D. D. Scripture-Adams, J. A. Zack, J. F. Kreisberg, M. Roederer, M. P. Sherman, P. S. Chin, and M. A. Goldsmith. 2001. HIV-1 actively replicates in naive CD4+ T cells residing within human lymphoid tissues. Immunity 15:671-682. [DOI] [PubMed] [Google Scholar]
- 14.Epperson, D. E., and J. S. Pober. 1994. Antigen-presenting function of human endothelial cells. Direct activation of resting CD8 T cells. J. Immunol. 153:5402-5412. [PubMed] [Google Scholar]
- 15.Evans, R. L., T. J. Faldetta, R. E. Humphreys, D. M. Pratt, E. J. Yunis, and S. F. Schlossman. 1978. Peripheral human T cells sensitized in mixed leukocyte culture synthesize and express Ia-like antigens. J. Exp. Med. 148:1440-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fortin, J. F., C. Barat, Y. Beausejour, B. Barbeau, and M. J. Tremblay. 2004. Hyper-responsiveness to stimulation of human immunodeficiency virus-infected CD4+ T cells requires Nef and Tat virus gene products and results from higher NFAT, NF-κB, and AP-1 induction. J. Biol. Chem. 279:39520-39531. [DOI] [PubMed] [Google Scholar]
- 17.Fraser, J., V. Arcus, P. Kong, E. Baker, and T. Proft. 2000. Superantigens—powerful modifiers of the immune system. Mol. Med. Today 6:125-132. [DOI] [PubMed] [Google Scholar]
- 18.Goh, W. C., M. E. Rogel, C. M. Kinsey, S. F. Michael, P. N. Fultz, M. A. Nowak, B. H. Hahn, and M. Emerman. 1998. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat. Med. 4:65-71. [DOI] [PubMed] [Google Scholar]
- 19.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 69:6705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemler, M. E., J. G. Jacobson, M. B. Brenner, D. Mann, and J. L. Strominger. 1985. VLA-1: a T cell surface antigen which defines a novel late stage of human T cell activation. Eur. J. Immunol. 15:502-508. [DOI] [PubMed] [Google Scholar]
- 21.Ho, D. D., A. U. Neumann, A. S. Perelson, W. Chen, J. M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373:123-126. [DOI] [PubMed] [Google Scholar]
- 22.Jamieson, B. D., and J. A. Zack. 1998. In vivo pathogenesis of a human immunodeficiency virus type 1 reporter virus. J. Virol. 72:6520-6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jowett, J. B., V. Planelles, B. Poon, N. P. Shah, M. L. Chen, and I. S. Chen. 1995. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J. Virol. 69:6304-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karmann, K., J. S. Pober, and C. C. Hughes. 1994. Endothelial cell-induced resistance to cyclosporin A in human peripheral blood T cells requires contact-dependent interactions involving CD2 but not CD28. J. Immunol. 153:3929-3937. [PubMed] [Google Scholar]
- 25.Kino, T., A. Gragerov, O. Slobodskaya, M. Tsopanomichalou, G. P. Chrousos, and G. N. Pavlakis. 2002. Human immunodeficiency virus type 1 (HIV-1) accessory protein Vpr induces transcription of the HIV-1 and glucocorticoid-responsive promoters by binding directly to p300/CBP coactivators. J. Virol. 76:9724-9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinoshita, S., B. K. Chen, H. Kaneshima, and G. P. Nolan. 1998. Host control of HIV-1 parasitism in T cells by the nuclear factor of activated T cells. Cell 95:595-604. [DOI] [PubMed] [Google Scholar]
- 27.Kinoshita, S., L. Su, M. Amano, L. A. Timmerman, H. Kaneshima, and G. P. Nolan. 1997. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity 6:235-244. [DOI] [PubMed] [Google Scholar]
- 28.Lahti, A. L., A. Manninen, and K. Saksela. 2003. Regulation of T cell activation by HIV-1 accessory proteins: Vpr acts via distinct mechanisms to cooperate with Nef in NFAT-directed gene expression and to promote transactivation by CREB. Virology 310:190-196. [DOI] [PubMed] [Google Scholar]
- 29.Laskay, T., U. Andersson, J. Andersson, R. Kiessling, and M. DeLey. 1986. An immunofluorescent method for identifying individual IFN-gamma-producing lymphocytes. J. Immunol. Methods 95:1-7. [DOI] [PubMed] [Google Scholar]
- 30.Lewis, R. S. 2003. Calcium oscillations in T-cells: mechanisms and consequences for gene expression. Biochem. Soc. Trans. 31:925-929. [DOI] [PubMed] [Google Scholar]
- 31.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148-1152. [DOI] [PubMed] [Google Scholar]
- 32.Linnemann, T., Y. H. Zheng, R. Mandic, and B. M. Peterlin. 2002. Interaction between Nef and phosphatidylinositol-3-kinase leads to activation of p21-activated kinase and increased production of HIV. Virology 294:246-255. [DOI] [PubMed] [Google Scholar]
- 33.Lum, J. J., O. J. Cohen, Z. Nie, J. G. Weaver, T. S. Gomez, X. J. Yao, D. Lynch, A. A. Pilon, N. Hawley, J. E. Kim, Z. Chen, M. Montpetit, J. Sanchez-Dardon, E. A. Cohen, and A. D. Badley. 2003. Vpr R77Q is associated with long-term nonprogressive HIV infection and impaired induction of apoptosis. J. Clin. Investig. 111:1547-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma, W., and J. S. Pober. 1998. Human endothelial cells effectively costimulate cytokine production by, but not differentiation of, naive CD4+ T cells. J. Immunol. 161:2158-2167. [PubMed] [Google Scholar]
- 35.Macian, F., C. Garcia-Rodriguez, and A. Rao. 2000. Gene expression elicited by NFAT in the presence or absence of cooperative recruitment of Fos and Jun. EMBO J. 19:4783-4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manninen, A., P. Huotari, M. Hiipakka, G. H. Renkema, and K. Saksela. 2001. Activation of NFAT-dependent gene expression by Nef: conservation among divergent Nef alleles, dependence on SH3 binding and membrane association, and cooperation with protein kinase C-theta. J. Virol. 75:3034-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manninen, A., G. H. Renkema, and K. Saksela. 2000. Synergistic activation of NFAT by HIV-1 nef and the Ras/MAPK pathway. J. Biol. Chem. 275:16513-16517. [DOI] [PubMed] [Google Scholar]
- 38.Manninen, A., and K. Saksela. 2002. HIV-1 Nef interacts with inositol trisphosphate receptor to activate calcium signaling in T cells. J. Exp. Med. 195:1023-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marrack, P., and J. Kappler. 2004. Control of T cell viability. Annu. Rev. Immunol. 22:765-787. [DOI] [PubMed] [Google Scholar]
- 40.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093-1097. [DOI] [PubMed] [Google Scholar]
- 41.Mestas, J., and C. C. Hughes. 2001. Endothelial cell costimulation of T cell activation through CD58-CD2 interactions involves lipid raft aggregation. J. Immunol. 167:4378-4385. [DOI] [PubMed] [Google Scholar]
- 42.Murphy, L. L., and C. C. Hughes. 2002. Endothelial cells stimulate T cell NFAT nuclear translocation in the presence of cyclosporin A: involvement of the wnt/glycogen synthase kinase-3 beta pathway. J. Immunol. 169:3717-3725. [DOI] [PubMed] [Google Scholar]
- 43.Murray, A. G., P. Libby, and J. S. Pober. 1995. Human vascular smooth muscle cells poorly co-stimulate and actively inhibit allogeneic CD4+ T cell proliferation in vitro. J. Immunol. 154:151-161. [PubMed] [Google Scholar]
- 44.Oswald-Richter, K., S. M. Grill, M. Leelawong, and D. Unutmaz. 2004. HIV infection of primary human T cells is determined by tunable thresholds of T cell activation. Eur. J. Immunol. 34:1705-1714. [DOI] [PubMed] [Google Scholar]
- 45.Ott, M., J. L. Lovett, L. Mueller, and E. Verdin. 1998. Superinduction of IL-8 in T cells by HIV-1 Tat protein is mediated through NF-κB factors. J. Immunol. 160:2872-2880. [PubMed] [Google Scholar]
- 46.Perelson, A. S. 2002. Modelling viral and immune system dynamics. Nat. Rev. Immunol. 2:28-36. [DOI] [PubMed] [Google Scholar]
- 47.Pessler, F., and R. Q. Cron. 2004. Reciprocal regulation of the nuclear factor of activated T cells and HIV-1. Genes Immun. 5:158-167. [DOI] [PubMed] [Google Scholar]
- 48.Pober, J. S., M. P. Bevilacqua, D. L. Mendrick, L. A. Lapierre, W. Fiers, and M. A. Gimbrone, Jr. 1986. Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J. Immunol. 136:1680-1687. [PubMed] [Google Scholar]
- 49.Re, F., D. Braaten, E. K. Franke, and J. Luban. 1995. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J. Virol. 69:6859-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robichaud, G. A., B. Barbeau, J. F. Fortin, D. M. Rothstein, and M. J. Tremblay. 2002. Nuclear factor of activated T cells is a driving force for preferential productive HIV-1 infection of CD45RO-expressing CD4+ T cells. J. Biol. Chem. 277:23733-23741. [DOI] [PubMed] [Google Scholar]
- 51.Scales, D., H. Ni, F. Shaheen, J. Capodici, G. Cannon, and D. Weissman. 2001. Nonproliferating bystander CD4+ T cells lacking activation markers support HIV replication during immune activation. J. Immunol. 166:6437-6443. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt, A., L. Hennighausen, and U. Siebenlist. 1990. Inducible nuclear factor binding to the kappa B elements of the human immunodeficiency virus enhancer in T cells can be blocked by cyclosporin A in a signal-dependent manner. J. Virol. 64:4037-4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schrager, J. A., V. Der Minassian, and J. W. Marsh. 2002. HIV Nef increases T cell ERK MAP kinase activity. J. Biol. Chem. 277:6137-6142. [DOI] [PubMed] [Google Scholar]
- 54.Scripture-Adams, D. D., D. G. Brooks, Y. D. Korin, and J. A. Zack. 2002. Interleukin-7 induces expression of latent human immunodeficiency virus type 1 with minimal effects on T-cell phenotype. J. Virol. 76:13077-13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seddon, B., and R. Zamoyska. 2003. Regulation of peripheral T-cell homeostasis by receptor signalling. Curr. Opin. Immunol. 15:321-324. [DOI] [PubMed] [Google Scholar]
- 56.Sloan-Lancaster, J., and P. M. Allen. 1996. Altered peptide ligand-induced partial T cell activation: molecular mechanisms and role in T cell biology. Annu. Rev. Immunol. 14:1-27. [DOI] [PubMed] [Google Scholar]
- 57.Sloan-Lancaster, J., T. H. Steinberg, and P. M. Allen. 1997. Selective loss of the calcium ion signaling pathway in T cells maturing toward a T helper 2 phenotype. J. Immunol. 159:1160-1168. [PubMed] [Google Scholar]
- 58.Stevenson, M., T. L. Stanwick, M. P. Dempsey, and C. A. Lamonica. 1990. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 9:1551-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swingler, S., B. Brichacek, J. M. Jacque, C. Ulich, J. Zhou, and M. Stevenson. 2003. HIV-1 Nef intersects the macrophage CD40L signalling pathway to promote resting-cell infection. Nature 424:213-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thali, M., A. Bukovsky, E. Kondo, B. Rosenwirth, C. T. Walsh, J. Sodroski, and H. G. Gottlinger. 1994. Functional association of cyclophilin A with HIV-1 virions. Nature 372:363-365. [DOI] [PubMed] [Google Scholar]
- 61.Unutmaz, D., V. N. KewalRamani, S. Marmon, and D. R. Littman. 1999. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J. Exp. Med. 189:1735-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vincent, M. J., N. U. Raja, and M. A. Jabbar. 1993. Human immunodeficiency virus type 1 Vpu protein induces degradation of chimeric envelope glycoproteins bearing the cytoplasmic and anchor domains of CD4: role of the cytoplasmic domain in Vpu-induced degradation in the endoplasmic reticulum. J. Virol. 67:5538-5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang, F. X., Y. Xu, J. Sullivan, E. Souder, E. G. Argyris, E. A. Acheampong, J. Fisher, M. Sierra, M. M. Thomson, R. Najera, I. Frank, J. Kulkosky, R. J. Pomerantz, and G. Nunnari. 2005. IL-7 is a potent and proviral strain-specific inducer of latent HIV-1 cellular reservoirs of infected individuals on virally suppressive HAART. J. Clin. Investig. 115:128-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang, L., S. Mukherjee, F. Jia, O. Narayan, and L. J. Zhao. 1995. Interaction of virion protein Vpr of human immunodeficiency virus type 1 with cellular transcription factor Sp1 and trans-activation of viral long terminal repeat. J. Biol. Chem. 270:25564-25569. [DOI] [PubMed] [Google Scholar]
- 65.Wei, X., S. K. Ghosh, M. E. Taylor, V. A. Johnson, E. A. Emini, P. Deutsch, J. D. Lifson, S. Bonhoeffer, M. A. Nowak, B. H. Hahn, et al. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373:117-122. [DOI] [PubMed] [Google Scholar]
- 66.Willey, R. L., F. Maldarelli, M. A. Martin, and K. Strebel. 1992. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J. Virol. 66:7193-7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu, Y., and J. W. Marsh. 2001. Selective transcription and modulation of resting T cell activity by preintegrated HIV DNA. Science 293:1503-1506. [DOI] [PubMed] [Google Scholar]
- 68.Zack, J. A., S. J. Arrigo, S. R. Weitsman, A. S. Go, A. Haislip, and I. S. Chen. 1990. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell 61:213-222. [DOI] [PubMed] [Google Scholar]
- 69.Zhang, L., C. Chung, B. S. Hu, T. He, Y. Guo, A. J. Kim, E. Skulsky, X. Jin, A. Hurley, B. Ramratnam, M. Markowitz, and D. D. Ho. 2000. Genetic characterization of rebounding HIV-1 after cessation of highly active antiretroviral therapy. J. Clin. Investig. 106:839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang, Z., T. Schuler, M. Zupancic, S. Wietgrefe, K. A. Staskus, K. A. Reimann, T. A. Reinhart, M. Rogan, W. Cavert, C. J. Miller, R. S. Veazey, D. Notermans, S. Little, S. A. Danner, D. D. Richman, D. Havlir, J. Wong, H. L. Jordan, T. W. Schacker, P. Racz, K. Tenner-Racz, N. L. Letvin, S. Wolinsky, and A. T. Haase. 1999. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 286:1353-1357. [DOI] [PubMed] [Google Scholar]
- 71.Zhou, Y., H. Zhang, J. D. Siliciano, and R. F. Siliciano. 2005. Kinetics of human immunodeficiency virus type 1 decay following entry into resting CD4+ T cells. J. Virol. 79:2199-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]