Abstract
Alphaherpesviruses are parasites of the peripheral nervous system in their natural hosts. After the initial infection of peripheral tissues such as mucosal cells, these neurotropic viruses will invade the peripheral nervous system that innervates the site of infection via long-distance axonal transport of the viral genome. In natural hosts, a latent and a nonproductive infection is usually established in the neuronal cell bodies. Upon reactivation, the newly replicated genome will be assembled into capsids and transported back to the site of entry, where a localized infection of the epithelial or mucosal cells will produce infectious virions that can infect naïve hosts. In this paper, we describe an in vitro method for studying neuron-to-cell spread of alphaherpesviruses using a compartmented culture system. Using pseudorabies virus as a model, we infected neuron cell bodies grown in Teflon chambers and observed spread of infection to nonneuronal cells plated in a different compartment. The cells are in contact with the neurons via axons that penetrate the Teflon barrier. We demonstrate that wild-type neuron-to-cell spread requires intact axons and the presence of gE, gI, and Us9 proteins, but does not require gD. We also provide ultrastructural evidence showing that capsids enclosed within vesicles can be found along the entire length of the axon during viral egress.
All alphaherpesviruses, including the human pathogens herpes simplex virus and varicella-zoster virus or the animal herpesviruses such as bovine herpes virus type 1 and pseudorabies virus (PRV) are pantropic viruses. However, in their natural hosts, alphaherpesviruses are parasites of the peripheral nervous system and they rarely invade the central nervous system. Generally, the infection initiates at peripheral tissues such as the mucosal epithelial layer and subsequently spreads into the peripheral nervous system that innervates the primary site of infection. Following long-distance movement to the neuronal cell bodies in axons, the viral genome enters the nucleus, where it remains latent. When a latent infection is reactivated days, months, or even years after the initial infection, the newly replicated genomes will be assembled into capsids and transported back to the original site of infection, where infectious particles are produced that can spread to other hosts (20). This long-distance, bidirectional movement of the viral genome in axons utilizes the microtubule-based fast axonal transport machinery (54-56, 59).
New technology using fluorescent fusion proteins in conjunction with time-lapse microscopy has allowed us to visualize the axonal localization of viral proteins at various stages of infection. However, the molecular mechanisms of directional transmission of infection from nonneuronal epithelial cells to peripheral nervous system neurons, from peripheral nervous system neurons to central nervous system neurons, and from peripheral nervous system neurons to nonneuronal cells have been difficult to study simply because of the lack of facile in vitro systems that recapitulate the complicated biology of transneuronal infection. For example, we and others discovered that anterograde spread of infection of PRV and bovine herpesvirus type 1 in animal models is regulated by expression of at least three viral membrane proteins, gE, gI, and Us9 (1, 6, 10, 13, 14, 57, 62). However, the kinetics of spread as well as the variability and extent of the defect have not been studied in detail for any viral mutant. In the context of in vivo transneuronal spread, anterograde spread is defined as transfer of infection from the infected presynaptic cell to the uninfected postsynaptic cell and retrograde spread is from the infected postsynaptic cell to an uninfected presynaptic cell. Thus, spread of infection occurs not only between neurons, but also between neurons and the nonneuronal cells in which they innervate.
The first description of culturing dissociated neurons in compartmented chambers was published in 1977 by R. B. Campenot and the chambers were subsequently known as Campenot chambers (8). These compartmented chambers consist of a Teflon ring that is sealed to the surface of a tissue culture dish with silicone grease. Neuron cell bodies that were cultured in one compartment extended axons to a different compartment by penetrating the silicone grease barrier. Despite its obvious importance, the technology has not been used extensively to study spread of infection. The earliest studies of alphaherpesvirus infection in these compartmented chambers focused on virus entry at growth cones (38, 65, 66). Later, Lycke and colleagues studied viral egress of herpes simplex virus type 1 in infected neurons that were cultured in the compartmented chambers (36). More recently, transneuronal spread of herpes simplex virus type 1 has also been studied in a dual-chamber system using human fetal neurons from the dorsal root ganglia and skin explants (27, 42, 43, 49).
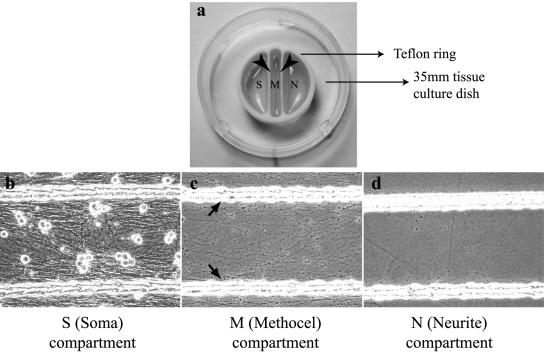
In this report, we describe an improved method of culturing peripheral nervous system neurons that enables analysis of transneuronal spread by standard virology, cell biology, and imaging methods. We define spread of infection from the neuron soma to target cells connected to distal axons as neuron-to-cell spread of infection. We demonstrate that this in vitro system offers technical advantages over its predecessors. Furthermore, data collected not only recapitulates, but also extends what had previously been observable only after animal infection. Unlike the original Campenot chambers, we have employed a Teflon tripartite ring with a sealed central compartment that permits extensive axonal penetration across the barriers, but effectively eliminates leaks and mechanical flaws of sealing ensuring nearly 100% reliability. Neuron cell bodies are always cultured in the soma (S) compartment, while the axons penetrate through the silicone grease barrier and emerge in the central methocel (M) compartment and then in the neurite (N) compartment, where detector cells can be plated (Fig. 1).
FIG. 1.
Trichamber neuron culture system. The trichamber system consists of a Teflon ring outlined with a thin strip of silicone grease and seated inside a 35-mm tissue culture dish (a). The solid arrowheads indicate the central barriers where the axons have to penetrate underneath. The S (Soma)-compartment contains neuron cell bodies which have been cultured for 2 weeks (b) while the M (Methocel)- and N (Neurite)-compartments have an extensive network of axons (c and d). The solid arrows indicate the parallel grooves etched on the surface of the tissue culture dish (c). Note that the neurites grow between the grooves and not in the grooves.
Using the trichamber system to study neuron-to-cell spread of PRV, we describe seven key observations. First, in the absence of any nonneuronal cells, we do not detect any infectious virions released in the medium late during infection from the N-compartment despite extensive infection of the cell bodies in the S-compartment. Second, plating a variety of permissive, nonneuronal cells in the presence of penetrating axons in the N-compartment results in massive neuron-to-cell spread that is entirely dependent on axonal integrity. Third, neuron-to-cell spread of PRV does not require gD, a viral glycoprotein required for infection by extracellular particles. Fourth, gE, gI, and Us9 viral proteins are necessary for wild-type neuron-to-cell spread. Fifth, individual gE, gI, and Us9 null mutants have additive kinetic defects in neuron-to-cell spread. Sixth, the attenuated vaccine strain PRV Bartha has a defect in infection and spread to cell bodies after entry at axons. Finally, capsids enclosed within vesicles can be found in both proximal as well as distal regions of the axons.
MATERIALS AND METHODS
Cells and virus strains.
The swine kidney epithelial cell line (PK15) and the Madin-Darby bovine kidney cell line (MDBK) were purchased from the American Type Culture Collection (CCL-22). The primary rat embryonic fibroblast (REF) cells were established by N. Ray (Princeton University). The primary pig embryonic fibroblasts isolated from swine lungs were provided by Clinton Jones (University of Nebraska-Lincoln). All nonneuronal cells were cultured in Dulbecco's modified Eagle medium supplemented with 10% of fetal bovine serum and 1% penicillin/streptomycin. All PRV stocks were produced in the PK15 cell line. PRV stocks used in this report include PRV Becker, a virulent isolate (51) and PRV Bartha, an attenuated vaccine strain (33).
PRV mutants in the PRV Becker background are PRV 758 (gE null) (12a), PRV 160 (Us9 null) (5), PRV 98 (gI null) (62), PRV BaBe (Becker with deletion in Us region) (10), and PRV 158 (Bartha with Becker Us region) (37). In addition, PRV GS442 (gD null mutant where the green fluorescence protein gene replaces the gD open reading frame; provided by G. Smith (Northwestern University) was grown in a gD complementing cell line (47).
Antibodies and fluorescent dyes.
Antibodies used in this report includes mouse monoclonal antibodies specific for the PRV envelope protein gB (M2) (24) and the PRV major capsid protein VP5 (IN13) (gift from H. Rziha; Federal Research Center for Virus Diseases in Animals, Tubingen, Germany). The lipid-based dye 1-dilinoleyl-3, 3, 3′,3′-tetramethylindocarbocyanine-4-chlorobenzenesulfonate (FAST DiI; Molecular Probes) was used to label neuronal membranes. All secondary Alexa fluorophores and the Hoechst nuclear dye were also purchased from Molecular Probes.
Neuronal cultures.
Detailed protocols for dissecting and culturing neurons are found in Ch'ng et al. (12). Briefly, sympathetic neurons from the superior cervical ganglia were dissected from E15.5 to E16.5 pregnant Sprague-Dawley rats (Hilltop Labs Inc., Pennsylvania) and incubated in 250 μg/ml of trypsin (Worthington Biochemicals) for 10 min. 1 mg/ml of trypsin inhibitor (Sigma Aldrich) was added to neutralize the trypsin for 3 min and then removed and replaced with neuron culture medium. Prior to plating, the ganglia were triturated into dissociated neurons using a fire-polished Pasteur pipette and then plated in the S-compartment of the Teflon ring placed within a 35-mm plastic tissue culture dish coated with 500 μg/ml of poly-dl-ornithine (Sigma Aldrich) diluted in borate buffer and 10 μg/ml of natural mouse laminin (Invitrogen). The neuron culture medium consists of Dulbecco's modified Eagle medium (Invitrogen) and Ham's F12 (Invitrogen) in a 1:1 ratio. The serum-free medium was supplemented with 10 mg/ml of bovine serum albumin (Sigma Aldrich), 4.6 mg/ml glucose (J. T. Baker), 100 μg/ml of holotransferrin (Sigma Aldrich), 16 μg/ml of putrescine (Sigma Aldrich), 10 μg/ml of insulin (Sigma Aldrich), 2 mM of l-glutamine (Invitrogen); 50 μg/ml or units of penicillin and streptomycin (Invitrogen), 30 nM of selenium (Sigma Aldrich); 20 nM of progesterone (Sigma Aldrich) and 100 ng/ml of nerve growth factor 2.5S (Invitrogen). Two days postplating, the neuronal cultures are treated with 1 μM of an antimitotic drug called cytosine β-d-arabinofuranoside (Sigma-Aldrich) to eliminate any nonneuronal cells. The neuron culture medium was replaced every three days and cultures were kept in a humidified, CO2 regulated 37°C incubator. All experimental protocols related to animal use have been approved by The Institutional Animal Care and Use Committee of the Princeton University Research Board under protocol number 1453-AR2 in accordance with the regulations of the American Association for Accreditation of Laboratory Animal Care and those in the Animal Welfare Act (Public Law 99-198).
Trichamber culture system.
All Teflon rings were purchased from Tyler Research (Alberta, Canada) and protocols were modified from previously published reports for Campenot chambers (8). Briefly, all the tools and reagents including the Teflon rings and silicone grease-loaded syringe (Dow Corning) were sterilized via autoclaving prior to assembly. A 10-ml disposable syringe attached to a truncated 18-gauge hypodermic needle was filled with silicone grease. Next, the interior surface of the 35-mm tissue culture dishes was etched with a pin rake creating a series of 16 evenly spaced grooves. These dishes were then coated with 500 μg/ml of poly-dl-ornithine (Sigma) followed by 10 μg/ml of natural mouse laminin (Invitrogen) and then washed and dried briefly.
Using the silicone grease-loaded syringe, a thin, continuous strip of silicone grease was applied over the entire bottom surface of a Teflon ring. Next, a 50-μl drop of neuron medium in 1% methocel (serum free) was placed in the center of each tissue culture dish covering the etched grooves. This prevents the seal from being entirely devoid of moisture, which is needed for axon penetration. Finally, the silicone grease-coated ring was gently seated on the tissue culture dish such that the etched grooves span all three compartments allowing the silicone grease to form a watertight seal between each compartment. Neuron medium was then placed in all three compartments immediately after the chamber was assembled. Once the superior cervical ganglia neurons were dissected and dissociated, approximately one half of a single ganglion (which ultimately resulted in about 5,000 to 6,000 cell bodies) was plated into one of the side compartments, which we define as the S (soma)-compartment (Fig. 1). Neuron cultures were maintained according to established protocols stated in the previous section.
Assaying neuron-to-cell spread of infection.
Neurons were cultured for 2 weeks in the trichamber system with frequent medium changes. After 2 weeks, axon penetration in to the M- and N-compartments was assessed visually and only cultures with comparable axon densities were used for experiments. After axons had penetrated the N-compartment (2 weeks postplating), nonneuronal cells permissive for PRV infection were plated in the N-compartment in neuron medium supplemented with 1% fetal bovine serum to amplify any transmission of infection from the neuronal cell bodies. Unless otherwise stated, PK15 cells were routinely used as detector cells. The plated cells were allowed to attach and interact with the axons for 24 h prior to further manipulation. Next, neuron medium containing 1% methocel was placed in the M-compartment. After 30 min, the neuronal cell bodies in the S-compartment were infected with sufficient virus to infect essentially all cells (approximately 105 PFU) for 1 h. We routinely use a high multiplicity of infection (MOI) to infect all neurons. Direct calculations show that the MOI is 100 to 200. However, it is likely that the MOI is considerably less since virions have a tendency to bind to poly-dl-ornithine and laminin that coats the surface of the dish (55). In addition, virions also bind to cellular debris and membranes on the dish.
Viral inoculum was diluted in neuron medium. After 1 h, the viral inoculum was removed and replaced with neuron medium. The chambers were incubated in a humidified 37°C incubator until the appropriate time when the production of infectious virus in S- and N-compartments was determined. Unless otherwise stated, both intracellular and extracellular virions in the S- and N-compartments were carefully harvested by scraping the bottom of the dish using the pointed end of a gel-loading tip. The cells and medium were then pooled, freeze-thawed, and titered on PK15 cells.
Studying axon-mediated infection of neuronal cell bodies.
Briefly, neurons were grown and cultured as described in the previous section. However, no detector cells were plated in the N-compartment. Neuron medium made with 1% methocel was added to the M-compartment and allowed to incubate for 30 min prior to infection. A high-MOI viral inoculum (about 105 PFU) was added to N-compartment and incubated for 1 h to allow virus entry. After the 1-hour incubation, the viral inoculum was removed and replaced with neuron medium. At the appropriate time after infection, both intra and extracellular virions were harvested from the S-compartment and titered on PK15 cells.
Indirect immunofluorescence and confocal microscopy.
The trichamber system was assembled on the surface of a flexible thermoplastic fluoropolymer film known as Aclar (EM Sciences). Aclar is biochemically inert and exhibits no detectable autofluorescence. Just like setting up the trichamber system on plastic tissue culture dishes, the Aclar was etched with a pin rake, coated with poly-dl-ornithine and laminin, and the Teflon tripartite ring was assembled and seated on the surface of the Aclar and the entire apparatus was placed in a 35-mm tissue culture dish. All subsequent neuron culture and viral infection protocols are similar to those described in previous sections. After infection of S- or N-compartments and after the appropriate time, all three compartments were washed once with phosphate-buffered saline and fixed with 3.2% paraformaldehyde for 10 min. The fixative was then removed and the cells were washed three more times with phosphate-buffered saline. At this stage, the Teflon ring was carefully removed and the remaining silicone grease on the Aclar was gently cleared without disrupting the fixed cells.
The Aclar surface was then incubated in phosphate-buffered saline containing 3% bovine serum albumin and 0.5% saponin for 10 min before the addition of primary antibodies for 1 h. After 1 h, the primary antibodies were removed and the sample was washed three times with phosphate-buffered saline containing 3% bovine serum albumin and 0.5% saponin. Next, secondary antibodies were added to the sample and incubated for 1 h. After 1 h, the secondary antibodies were removed and the sample was washed 3 times with phosphate-buffered saline containing 3% bovine serum albumin and 0.5% saponin. The sample on Aclar was then mounted on a glass slide using Aqua poly/mount (Polysciences) and a coverslip was placed on top of the sample. The Aqua poly/mount (Polysciences) was air dried for 24 h prior to imaging.
Electron microscopy.
The trichamber apparatus was assembled on Aclar (EM Sciences) as described above. Cell bodies in the S-compartment were infected at high MOI as described above and after 14 h, the chambers were washed twice with phosphate-buffered saline, fixed with 2% glutaraldehyde in 0.2 M sodium cacodylate buffer (pH 7.2) for 4 h, and postfixed with 1% osmium tetroxide in sodium veronal buffer for one hour on ice. Samples were then rinsed with sodium veronal buffer four times and incubated with 0.25% toluidine blue in 0.2 M cacodylate buffer (pH 7.2) for 1 h; the staining solution was then removed with four rinses of sodium veronal buffer (pH 7.2), followed by four rinses with 0.05 M sodium maleate buffer (pH 5.1). Overnight incubation with 2% uranyl acetate in 0.05 M sodium maleate buffer was done in the dark followed by four rinses with 0.05 M sodium maleate buffer (pH 5.1). The fixed samples were then dehydrated with ethyl alcohol, embedded in Epon resin (EM Sciences) and cut into 70-μm sections using a Reichert Ultracut E ultramicrotome. Sections were obtained from the S-, M-, and N-compartments and examined using a Leo 912AB transmission electron microscope operated at 80 kV.
RESULTS
Basics of the trichamber culture system.
The key components of the trichamber system are shown in Fig. 1. We label the three compartments S (soma, where neuronal cell bodies are plated), M (middle, methocel barrier), and N (where terminal neurites emerge at least 10 mm distant from the cell bodies). After 2 weeks in culture, these neurites are readily labeled with axonal markers such as the microtubule-associated protein Tau and phosphorylated neurofilament H (58) (data not shown). The plastic surface of the 35-mm tissue culture dish is etched precisely with 16 parallel grooves that help guide axons to penetrate the barrier. The tripartite Teflon ring is then sealed on to the tissue culture dish with a thin ribbon of silicone grease such that the etched grooves run perpendicular to Teflon barriers. The three compartments are filled with neuron medium and dissociated embryonic superior cervical ganglia neurons are plated in the S-compartment. After 2 weeks, a dense axonal network is present in the S-compartment and a fraction of these axons will extend through the first barrier into the M-compartment and then through the second barrier into the N-compartment. Axon growth is very robust and typically, axons will first appear in the N-compartment after 10 to 12 days in culture.
For accurate comparison of infection data, the axonal densities in the N-compartment are ranked and only chambers with equivalent densities are used in experiments. The cell bodies in the S-compartment can then be infected with virus. However, a crucial step prior to infection of the cell bodies in the S-compartment is the addition of neuron medium with 1% methocel to the M-compartment. The presence of the highly viscous cellulose polymer blocks diffusion of the rare virions that diffuse though the grooves which intersect the S-, M-, and N-compartments. This effectively eliminates any spurious, non-axon-mediated neuron-to-cell spread of infection.
To assess intercompartmental leakage, we cultured PK15 cells in both the S- and N-compartments of 16 separate dishes. After allowing the cells to settle and attach to the surface, we replaced the neuron medium in the M-compartment with methocel. Next, we infected the PK15 cells in each S-compartment with PRV 180, a strain that expresses the fusion protein mRFP-VP26 (monomeric red fluorescent protein fused to VP26) (17). Cells in the S- and N-compartments were imaged at 24 and 48 h postinfection and infected cells were assayed by the emission of red fluorescence from mRFP-VP26. Contents of both S- and N-compartments were also titered for infectious virus. In all 16 experiments, only PK15 cells in the S-compartment were infected, producing more than 109 PFU/ml (data not shown). None of the PK15 cells in the N-compartments were infected as deduced by lack of mRFP signal and absence of any infectious particles.
Neurons infected with PRV do not retract their axons early during infection.
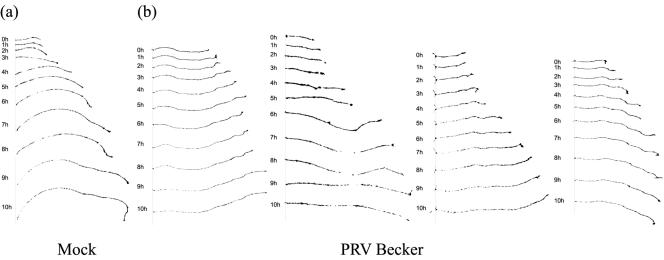
Our goal was to study how the infection is transmitted from neurons to detector cells. For spread of infection to occur, axons and the growth cones must be intact and not degraded early during infection. We randomly selected and tracked the growth of individual axons during infection by PRV Becker. Briefly, neuronal cell bodies in the S-compartment were cultured for 2.5 weeks before being mock infected or infected at a high MOI with PRV Becker. At this high concentration of virus, all neuron cell bodies were infected (data not shown). Prior to infection, several sets of axons in the N- compartment were randomly selected and phase contrast images were collected. At every hour postinfection for 10 h, another image was taken to chart the growth of the axons. Our results indicate that at least up to 10 h postinfection, growth cones do not collapse and the axons do not retract. In fact, the majority of the axons monitored grew at rates similar to the mock-infected samples (Fig. 2).
FIG. 2.
Neurons infected with PRV Becker do not retract their neurites early during infection. Cultured neurons in the S-compartment were mock infected (a) and infected with PRV Becker (b) at very high MOI for 10 h. At each hour, phase contrast images were taken using a Nikon Eclipse TE epifluorescence microscope to chart the growth of several randomly selected axons from the N-compartment. Images were processed and axons traced using Scion Image software.
No infectious virions are released from axons in the N-compartment during late stages of infection at the cell bodies.
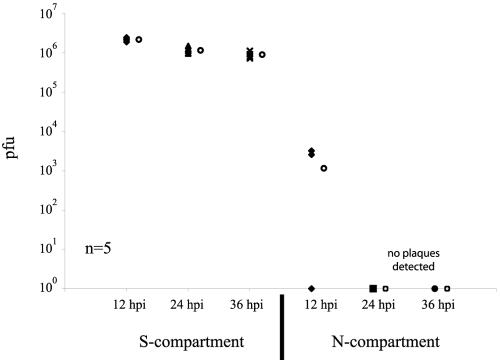
A set of five chambers were assembled as described in the Materials and Methods. After 2 weeks, the axons penetrated the N-compartment and the cell bodies in the S-compartment were subsequently infected at high MOI with PRV Becker. After 12, 24, and 36 h postinfection, the entire contents of the S-compartment and only the neuron medium in the N-compartment were removed and titered. The average titer in the S-compartment was 2 × 106 PFU/ml, but no infectious virions were detected in the N-compartment late during infection (Fig. 3). Interestingly, at 12 h postinfection, we detected a small number of infectious particles in the N-compartment of two chambers. This observation could not be duplicated with other chambers tested at later time points. We surmise that segments of the infected axons were accidentally harvested during the collection of medium in the N-compartment.
FIG. 3.
Infectious particles are not released from axon terminals late during infection. Cultured neurons in the S-compartment were infected with PRV Becker at high MOI. The medium in the N-compartment as well as the total content of the S-compartment were harvested at various time points postinfection (0, 12, 24, and 36 hours postinfection) and titered on PK15 cells. Five chambers were used for each time point. The standard deviations are: S-compartment (12 h, ±2.0 × 105; 24 h, ±2.2 × 105; 36 h, ±1.5 105); N-compartment (12 h, ±1.6 × 103). The hollow circle beside each data set represents the average value for that particular set of data.
We were also concerned that free particles had a high affinity for the polyornithine/laminin-coated surface and hence escaped detection when the neuron medium was collected. Accordingly, we tested this possibility by adding various concentrations of infectious virus to coated chambers in the absence of any cultured cells and incubated them for various times. We added 50 PFU, 500 PFU and 5,000 PFU into each chamber and incubated the dishes for 0, 12, 24, and 36 h. We were able to recover essentially all the infectious units (within a factor of 2) even up to 36 h of incubation (data not shown). Thus, our limit of detection in the titer assays is less than 50 PFU. However, due to the nature of our titering assay, we are unable to rule out the possibility that rare virions might be released from axons.
Neuron-to-cell spread of infection occurs from cell bodies to detector cells in the N-compartment.
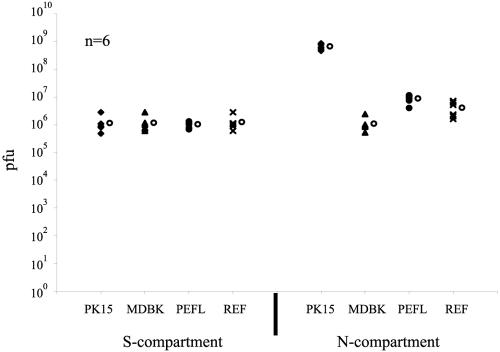
To detect rare infectious particles that might be released from axons, we plated various cell lines in the N-compartment. We started this experiment by plating only PK15 cells and it was only after our initial observations that we extended this experiment to other cell types. These permissive cells are capable of amplifying the presence of any infectious virions. After 2 weeks of growth, when axons from neuronal cell bodies in the S-compartment had penetrated into the N-compartment, we plated the various cell types from different species, different tissues and at different states (primary cells or transformed cell lines) in the N-compartment. The cell lines were: pig kidney epithelial cells (PK15), Madin-Darby bovine kidney cells (MDBK), primary rat embryonic fibroblasts (REF), and primary pig embryonic fibroblasts from the lungs (PEFL). Once complete monolayers were formed in the N-compartment after 24 h postplating, neurons in the S-compartment were infected at a high MOI with PRV Becker. At 48 h postinfection, infected cells and medium were harvested from both S- and N-compartments and titered on PK15 cells. First, the presence of other cell types in the N-compartment has no effect on PRV replication and release of virus from the cell bodies in the S-compartment. Second, we were surprised to see that infection spread from the S-compartment to all cell types tested (Fig. 4). Since we had not detected any significant release of infectious particles from axons without detector cells, we suspected that neuron-to-cell spread of infection is occurring via axons or growth cones.
FIG. 4.
Neuron-to-cell spread of infection is not cell type specific. PK15 cells, Madin-Darby bovine kidney cells, pig embryonic fibroblasts, and rat embryonic fibroblasts were plated in the N-compartment. Neurons in the S-compartment were then infected with PRV Becker at high MOI for 48 h. Infected cells were then harvested from both the S- and N-compartments and titered on PK15 cells. Six chambers were used for each type of infection. The standard deviations are: N-compartment (PK15 cells, ±1.6 × 108, MDBK cells, ±6.6 × 105, pig embryonic fibroblasts, ±2.8 × 106, rat embryonic fibroblasts, ±2.4 × 106); S-compartment (PK15 cells, ±8.3 × 105, MDBK cells, ±8.2 × 105, pig embryonic fibroblasts, ±2.7 × 105, rat embryonic fibroblasts, ±7.8 × 105). The open circle beside each data set represents the average value for that particular set of data.
Spread of infection from infected cell bodies to detector cells requires intact axons.
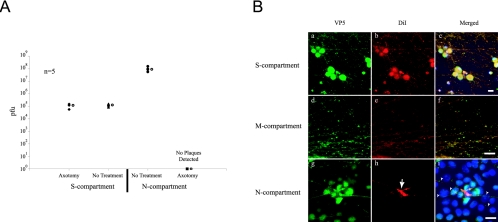
To confirm that axons are required for neuron-to-cell spread, we plated PK15 cells as detector cells in the N-compartment and allowed them to form a monolayer in the presence of axons. At 24 h postplating, the neuron cell bodies in the S-compartment were infected with PRV 180. For half the samples, we physically severed the axons in the M-compartment with a scalpel upon removal of virus inoculum after 1 hour of incubation. The remaining samples were left untreated. After 24 h postinfection, contents from both the S- and N-compartments were harvested and titered. In addition, we monitored mRFP-VP26 expression in the PK15 cells as an indicator of infection (data not shown). Our results indicate that transmission of infection absolutely requires the presence of intact axons. Spread of infection is completely blocked by physically severing axons from their cell bodies (Fig. 5A). We also conclude that transmission of infection does not occur through passive leakage by diffusion of the extracellular virus particles across the M-compartment.
FIG. 5.
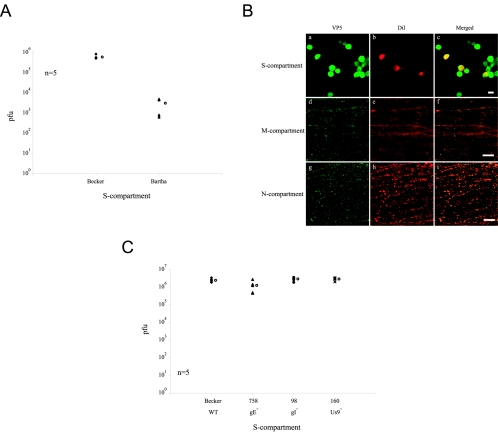
Neuron-to-cell spread of infection requires axons. A: Neurons were infected with PRV 180 and either left untreated as a control or axotomized to remove all axons in the M-compartment. Infected cells were harvested at 24 h postinfection from S- and N-compartments and titered on PK15 cells. Five chambers were used for each type of infection and treatment. The open circle beside each data set represents the average value for that particular set of data. The standard deviations are: N-compartment (not axotomized, ±4.1 × 107); S-compartment (not axotomized, ±3.8 × 104, axotomized, ±2.6 × 104). B: Immunofluorescence experiment on PRV Becker-infected neurons in the trichamber system. Culture samples in the S- (a to c), M- (d to f), and N-compartments (g to i) were labeled with antibodies against VP5 (a, d, and g), DiI (b, e, and h) and the nuclear dye Hoechst shown in the merged image (c, f, and i). Scale bar: 20 μm.
Next, we visualized the penetration of axons into the N-compartment containing PK15 cells using DiI, a fluorescent lipophilic dye. DiI spreads quickly through neuronal membranes and labels neuritic extensions and axons from cell bodies with bright red fluorescence. We infected neurons in the S-compartment with PRV Becker. At 14 h postinfection, we fixed, processed, and labeled neurons and PK15 cells with antibodies against the capsid protein VP5 to identify the PRV infected cells via immunofluorescence. In addition, we added DiI to the S-compartment to visualize the axons. We observed that DiI rapidly incorporates into all lipid membranes within the cell body and is transported along the axons all the way to the growth cones in the N-compartment.
As expected, all the neuron cell bodies in the S-compartment were labeled with VP5 (Fig. 5B, a to c). The DiI labeling enables visualization of an extensive and unorganized neuritic network in the S-compartment and a much more organized, parallel bundle of axons in the M-compartment (Fig. 5B, d to f). As expected, many PK15 cells in the N-compartment were infected and labeled with antibodies to VP5 (Fig. 5B, g to i). The majority of infected PK15 cells were located close to the central barrier where the axons first enter the compartment. Interestingly, infected PK15 cells were often in clusters and tightly associated with dense patches of DiI-labeled membranes. These DiI patches were at apparent termini as well as along axons (Fig. 5B, h, solid arrow). We believe that these hot spots for DiI labeling either are varicosities, sites of contact with detector cells, growth cones, or contact sites with the plastic surface (Fig. 5B, i, solid arrowheads). We also observed infected PK15 cells in other regions within the N-compartment where DiI-labeled axons are not present. As these infected cells appeared later after infection was established in the PK15 cells, spread of infection presumably occurred by extracellular virions released in the medium from the initially infected PK15 cells that were in contact with axons.
Neuron-to-cell transmission of infection to PK15 detector cells does not require gD.
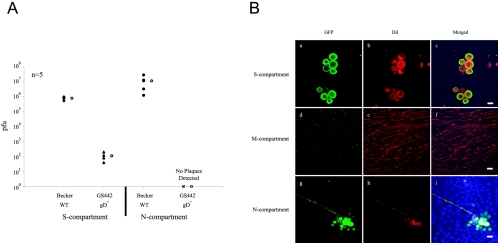
Next, we tested if neuron-to-cell infection of PK15 cells occurs via a neuron-cell interaction or by infectious free virions. The viral envelope protein gD is absolutely required for infection mediated by extracellular virions; however, PRV gD is not required for direct cell-cell spread of infection in vitro and in vivo (2, 46, 48, 52). We infected cell bodies in the S-compartment with either PRV Becker or PRV GS442, a gD null mutant that expresses green fluorescent protein (GFP). We produced infectious PRV GS442 by growing virus stocks on a gD-expressing cell line. Complemented viruses can infect once, but the resulting progeny do not contain gD and hence these gD null extracellular particles are noninfectious. If PRV GS442 can spread from neurons to PK15 cells, such spread cannot be mediated by standard infectious virions.
At 24 h postinfection, infected neurons and PK15 cells were harvested from the S- and N-compartments and titered. PRV Becker replicated in the cell bodies and infection spread to PK15 cells in the N-compartment. We detected no plaques in the N-compartment, but we did detect a few hundred PRV GS442 GFP-positive plaques in the S-compartment that most likely reflect residual extracellular complemented particles remaining from the input inoculum (Fig. 6A). The critical experiment was to look for evidence of axon-to-PK15 spread of infection. Indeed, by autofluorescence of GFP or by immunofluorescence, we could easily identify many clusters of GFP-expressing PK15 cells in the N-compartment (Fig. 6B). The clusters of infected PK15 cells ultimately form plaques and always lie adjacent to a DiI-labeled axon. At 14 h postinfection, all plaques were located near the central barriers where axons are found in the highest concentration. No infected PK15 cells were detected on the far edges of the Teflon ring in the N-compartment devoid of axons. Interestingly, we observed an increase of DiI staining in PK15 infected cells beyond the background signal. We conclude that neuron-to-cell transmission of infection to PK15 cells does not require gD, which, in turn, suggests that spread is not by typical gD-mediated extracellular infectious virions.
FIG. 6.
Neuron-to-cell transmission of infection does not require gD. A: Neurons in the S-compartment were infected at high MOI with either PRV Becker or GS442, a complemented gD null virus that expresses GFP. At 24 h postinfection, infected cells in the S- and N-compartments were harvested and titered in PK15 cells. Five chambers were used in each type of infection. The open circle beside each data set represents the average value for that particular set of data. The standard deviations are: N-compartment (Becker, ±1.0 × 107); S-compartment (Becker, ±1.6 × 105; GS442, ±5.9 × 101). B: Immunofluorescence experiment on PRV Becker- and GS442-infected neurons in the trichamber system. Culture samples in the S- (a to c), M- (d to f), and N-comparments (g to i) were labeled with antibodies against GFP (a, d, and g), DiI (b, e, and h), and the nuclear dye Hoechst shown in the merged image (c, f, and i). Scale bar: 20 μm.
PRV Bartha is defective in neuron-to-cell spread of infection.
PRV Bartha is an attenuated virus defective in anterograde spread from presynaptic neurons to postsynaptic neurons in a variety of animal models reviewed in (20). However, it retains the ability for retrograde spread from postsynaptic to presynaptic neurons and is widely used to trace neuronal circuits (19). This remarkable phenotype is due primarily to a small deletion that removes all or some of the coding sequences for gE, gI, and Us9 (33, 34, 40, 50). Using the trichamber system, we tested if PRV Bartha exhibited the same neuron-to-cell defect in vitro as observed in infection of neural circuits in animal models.
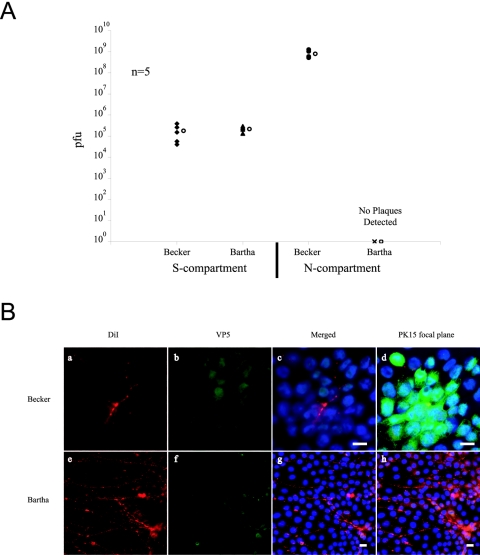
Superior cervical ganglia neurons were plated in the S-compartment, and 2 weeks later PK15 cells were plated in the presence of axons in the N-compartment. A day later, cell bodies in the S-compartment were infected at high MOI with PRV Becker or PRV Bartha. After 48 h, cells and medium from the S- and N-compartments were harvested and titered. PRV Bartha was completely defective in neuron-to-cell spread to the detector cells since no infectious particles were detected in the N-compartment. In comparison, more than 108 PFU/ml were produced in the N-compartment after PRV Becker infection (Fig. 7A). Even with the amplification potential of the detector cells and 48 h of incubation, no PRV Bartha infectious virions were ever detected in the N-compartment. In addition, by immunofluorescence, we found that despite the abundance of DiI-labeled axons, no infected PK15 cells were ever found (Fig. 7B). This massive neuron-to-cell spread defect indicates that the anterograde spread defect previously observed in neuronal circuits of animal models was recapitulated in this in vitro model.
FIG. 7.
PRV Bartha is defective in neuron-to-cell spread of infection. A: Neurons in the S-compartment were infected at high MOI with either PRV Becker or PRV Bartha. At 24 h postinfection, infected cells in the S- and N-compartments were harvested and titered in PK15 cells. Five chambers were used in each type of infection. The open circle beside each data set represents the average value for that particular set of data. The standard deviations are: N-compartment (Becker, ±3.4 × 108); S-compartment (Becker, ±1.5 × 105, Bartha, ±5.9 × 104). B: Immunofluorescence experiment on PRV Becker and PRV Bartha infected neurons in the trichamber system. PK15 cells and axons in the N-compartment of PRV Becker-infected cells (a to d) and PRV Bartha-infected cells (e to h) were labeled with antibodies against VP5 (b and f) and the lipid dye DiI (a and e). The cells were stained with the nuclear dye Hoechst and are shown in the merged image (c and g). Images at the focal plane of PK15 cells clearly show clusters of PRV Becker infected cells (d to h). Scale bar: 20 μm.
Individual gE, gI, and Us9 null mutants are partially defective in neuron-to-cell spread of infection.
In animal studies, deletion of any one of the three genes, gE, gI, or Us9, not expressed by PRV Bartha impaired the ability of the virus to spread from pre- to postsynaptic neurons (1, 5, 10, 11, 57, 62). Given the striking defect of PRV Bartha in the trichamber system, we examined the contribution of these three genes to neuron-to-cell spread.
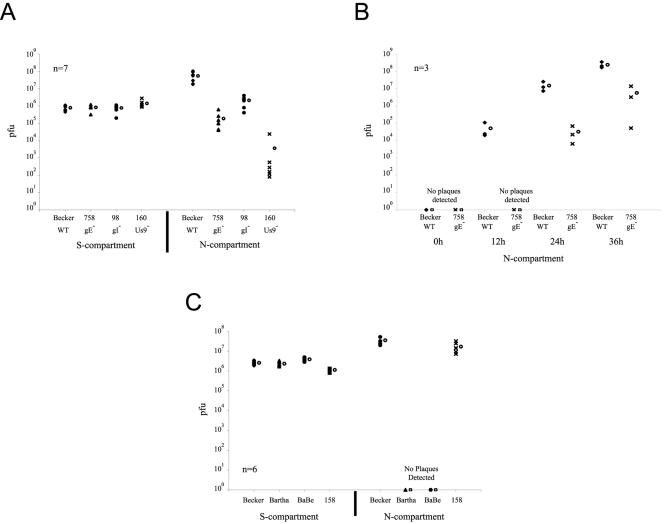
PK15 cells were plated on 2-week-old axons in the N-compartment. Neurons in the S-compartment then were infected at high MOI with PRV Becker, PRV 758 (gE null), PRV 98 (gI null), or PRV 160 (Us9 null). Twenty-four hours later, the contents of the S- and N-compartment were removed and titered (Fig. 8A). Surprisingly, unlike PRV Bartha, these mutants were partially defective in neuron-to-cell spread. Since the PK15 cells can amplify an infection, lower titers must reflect a kinetic defect in spread from neurons to PK15 cells given that these mutants have almost no replication defect in PK15 cells (6, 41). We also conducted a similar experiment using PRV 174, a Us2 null mutant, since Us2 is also partially deleted in PRV Bartha (33). PRV 174 had no spread defect and essentially spreads like PRV Becker (data not shown). These results indicate that the trichamber system is more sensitive than animal infections and can detect kinetic defects in neuron-to-cell spread.
FIG. 8.
gE, gI and Us9 are kinetically delayed during neuron-to-cell transmission of infection. A: Neurons in the S-compartment were infected at high MOI with PRV Becker, PRV 758, PRV 98 and PRV 160. At 24 h postinfection, infected cells in the S- and N-compartments were harvested and titered in PK15 cells. Seven chambers in two separate experiments were used in each type of infection. The standard deviations are: N-compartment (Becker, ±3.5 × 107, 758, ±2.1 × 105, 160, ±9.0 × 103, 98, ±1.2 × 106); S-compartment (Becker, ±2.3 × 107, 758, ±2.7 × 105, 160, ±1.2 × 104, 98, ±1.3 × 106). B: Neurons in the S-compartment were infected at high MOI with PRV Becker and PRV 758. At 0, 12, 24, and 36 h postinfection, infected cells in the N-compartment were harvested and titered in PK15 cells. Three chambers were used for each time point. The standard deviations are: N-compartment; Becker (12 h, ±5.0 × 104, 24 h, ±9.1 × 106, 36 h, ±9.3 × 107,); 758 (24 h, ±3.2 × 104, 36 h, ±7.1 × 106). C: Neurons in the S-compartment were infected at high MOI with PRV Becker, PRV Bartha, PRV BaBe, or PRV 158. At 24 h postinfection, infected cells in the S- and N-compartments were harvested and titered in PK15 cells. Six chambers were used in each type of infection. The standard deviations are: N-compartment (Becker, ±1.4 × 107; 158, ±9.5 × 106); S-compartment (Becker, ±5.6 × 105, Bartha, ±5.8 × 105; 158, ±2.4 × 105; BaBe, ±8.0 × 105). The open circles beside each data set represent the average value for that particular set of data.
To verify that there is a kinetic defect in spread from neurons to PK15 cells, we compared a time course infection of PRV Becker and PRV 758 (gE null). Neurons in the S-compartment were infected at high MOI and the spread of infection to PK15 cells in the N-compartment was assayed at 0, 12, 24, and 36 h after infection. At 12 h postinfection, PRV Becker has successfully spread and infected a substantial number of PK15 cells in the N-compartment. However, at this time, no infectious particles were detected in the PRV 758 infection. At 24 and 36 h postinfection, PRV 758 virions could be detected in the N-compartment at steadily increasing amounts (Fig. 8B). This experiment confirms that the gE null mutant spreads more slowly from neurons to PK15 cells, but once PK15 cells are infected, the infection is amplified at the same rate as a wild-type virus infection. Eventually, all the PK15 cells in the N-compartment will be infected and the amount of newly replicated gE null and PRV Becker virions will be identical.
It is likely that the gE, gI, and Us9 proteins have an additive, if not synergistic, effect on neuron-to-cell spread, given that PRV Bartha is completely defective. To test this hypothesis, we utilized two viral mutants in our collection: PRV BaBe is a PRV Becker mutant that carries the PRV Bartha Us deletion removing gE, gI, Us9, and part of the Us2 gene. Conversely, PRV 158 is a PRV Bartha mutant whose Us region has been restored with the gE, gI, Us9, and Us2 genes derived from PRV Becker. PRV Becker, PRV Bartha, PRV 158, and PRV BaBe were used to infect neurons in the S-compartment at a high MOI. As previously described, the titer of infectious virions in the S- and N-compartments was determined after 24 h postinfection. PRV BaBe is as defective in neuron-to-cell infection as is PRV Bartha (Fig. 8C). No infectious particles were recovered in the N-compartment. However, when the Us region is restored in PRV 158, neuron-to-cell spread is identical to PRV Becker. We conclude that the deletion of all three viral proteins gE, gI and Us9 is sufficient to recapitulate the neuron-to-cell spread defect by PRV Bartha.
Viral capsids in axons are enveloped and enclosed in a vesicle.
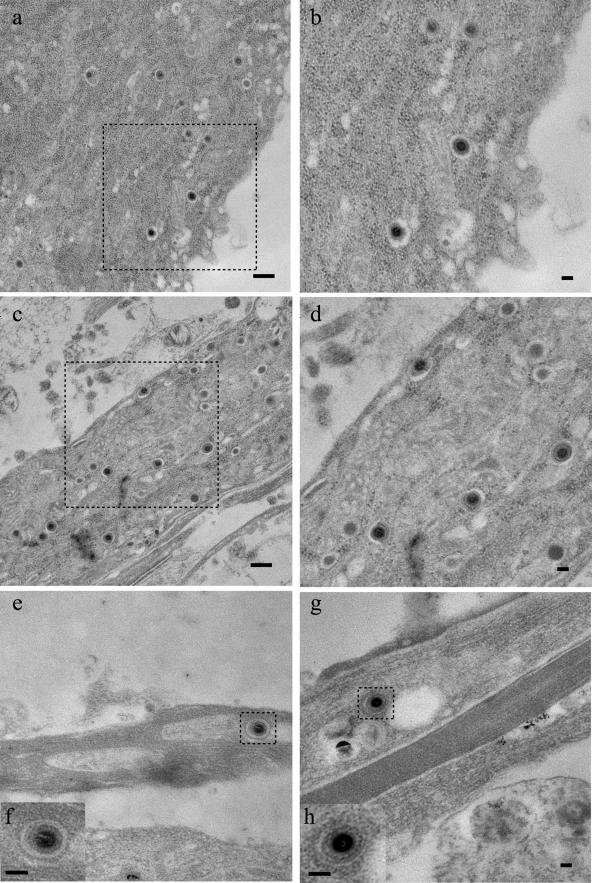
Recently, our laboratory reported that during egress, viral capsids localized within proximal axons were enclosed within vesicles (17). The trichamber system enables a further extension of these observations to distal axons. A technical difficulty in analysis of infected axons by electron microscopy is that the distribution of capsids in axons markedly decreases from the proximal to distal segments of the axons. By analyzing the distal axons in the M-compartment that were always higher in density, we increased the chances of finding viral capsids. In Fig. 9e and 9g, we illustrate a typical finding of capsids in axons enclosed within vesicles. Corresponding enlarged images of the capsids are shown within each respective inset (Fig. 9f and h). These particles, located mid-axon, were generally single capsids enclosed within an intact membrane, and were rarely found clustered in axonal swellings or enlargements. Electron micrographs of vesicle-enclosed capsids found in the neuron cell bodies (Fig. 9a and b) and in the proximal segment of the axon as it leave the cell body (Fig. 9c and d) are also shown in Fig. 9.
FIG.9.
Electron micrographs of distal axons show that viral capsids are enclosed in vesicles. Electron micrographs were obtained from infected cell bodies in the S-compartment (a, b, c, and d) and axons in the M-compartment (e, f, g, and h). The areas enclosed by the dotted boxes (a, c, e, and g) are enlarged and shown either to the right of the original micrograph (b and d) or as an inset (f and h). The micrographs taken from the S-compartment were either from the cell bodies (a and b) or from the proximal segment of the axons (c and d). Scale bars shown are either 500 nm (a and c) or 100 nm (b, d, e, f, g, and h).
Studying axon-mediated infection of cell bodies using the trichamber system.
We added 105 PFU to the N-compartment that was filled with axons, but without any PK15 cells. After a 1-hour incubation, the inoculum was removed and replaced with neuron medium and the infection was allowed to proceed for 24 h before the neurons and medium in the S-compartment were harvested and titered on PK15 cells. We expected that the virions would enter axons and travel to infect the neuron cell bodies. As expected, a productive infection with PRV Becker in the S-compartment generated 105 PFU. We found that a similar infection by PRV Bartha yielded titers in the S-compartment that were consistently lower compared to PRV Becker (Fig. 10A). This finding was replicated in five independent experiments. This result suggests that PRV Bartha has a modest defect either during entry, uncoating, axonal transport or subsequent events in the cell body compared to PRV Becker. This conclusion supports more qualitative observations that PRV Bartha spreads more slowly in vagus neurons of rats than does PRV Becker (11, 64). Preliminary evidence suggests that the rate of axonal transport of PRV Becker and Bartha capsids during entry is identical (Raldow, Ch'ng, and Enquist, unpublished data).
FIG. 10.
PRV Bartha but not gE, gI, or Us9 null single mutants have a slight defect in axon-mediated infection of neurons. A: High-titer viral inoculum from PRV Becker and PRV Bartha were incubated in the N-compartment for 1 hour before being replaced with regular medium. At 24 h postinfection, infected neuron cell bodies in the S-compartment were harvested, lysed, and titered on PK15 cells. A total of five chambers were used for each type of infection. The standard deviations are: S-compartment (Becker, ±1.2 × 105, Bartha, ±2.1 × 103). B: Immunofluorescence experiment on axon mediated infection of neurons by PRV Becker. Confocal microscopy images show the S- (a to c), M- (d to f), and N-compartments (g to i) being labeled with either antibodies against VP5 (a, d, and g), or DiI (b, e, and h). Merged images are shown in panel B (c, f, and i). Scale bar: 20 μm. C: High-titer viral inocula from PRV Becker, PRV 758, PRV 98, and PRV 160 were incubated in the N-compartment for 1 hour before being replaced with regular medium. At 24 h postinfection, infected neuron cell bodies in the S-compartment were harvested, lysed, and titered on PK15 cells. A total of five chambers were used for each type of infection. The standard deviations are: S-compartment (Becker, ±5.9 × 105; 758, ±9.1 × 105; 160, ±5.7 × 105; 98, ±6.7 × 105). The open circle beside each data set shown in the scatter plots represents the average value for that particular set of data.
To aid in visualizing the axons, we added DiI to the axon terminals in the N-compartment. At 14 h postinfection, neurons were fixed and labeled with antibodies against VP5. Confocal microscopy images reveal that not all cell bodies in the S-compartment were labeled with DiI. This observation reflects the fact that not all cell bodies extend axons into the N-compartment. We found that a subpopulation of cell bodies were colabeled with VP5 and DiI (Fig. 10B). In addition, we could also locate cells that were infected (VP5 positive) but had no DiI staining. These infected neurons most likely represent second-order infections resulting from spread from the initially infected neuron to other uninfected cells in the S-compartment that do not have axonal projections into the N-compartment.
We attempted to map the Bartha spread defect in axon-mediated cell body infection. Approximately 105 PFU of PRV Becker, PRV 758, PRV 98, and PRV 160 were incubated for 1 h in the N-compartment to allow entry of infectious particles into axons. At 24 h postinfection, the medium and neuron cell bodies in the S-compartment were harvested and titered on PK15 cells. All three viral mutants spread to the cell bodies and yielded comparable viral titers to PRV Becker (Fig. 10C). We conclude that the gE, gI and Us9 proteins have no obvious role in axon-mediated cell body infection.
DISCUSSION
The trichamber system provides a simple and tractable method for studying neuron-to-cell transmissions of infection in vitro. The presence of both the central Teflon barriers and the methocel diffusion barrier virtually eliminate virus leakage between compartments, providing a reliable method for virus infection studies. In addition, sensitive titer analyses to assess neuron-to-cell spread can be coupled with high resolution confocal microscopy imaging and electron microscopy of neuron cell bodies, axons and terminal processes.
In this report, we describe neuron-to-cell spread of infection from cultured superior cervical ganglia neurons to a variety of different cell types permissive to PRV. This robust spread requires intact axons, the presence of the gE, gI, and Us9 proteins, and is not mediated by gD. In addition, such spread requires viral mediated fusion as we could not detect any neuron-to-cell spread in PK15 cells when neurons were infected with complemented gB null virions (data not shown) (2, 3). By these criteria, we suggest that this system is a surrogate for transneuronal anterograde spread as defined by spread of infection from infected presynaptic neurons to postsynaptic cells in close contact. However, the trichamber system is not merely a substitute for animal infection studies, but rather provides a more sensitive assay free from the influence of the host immune system or other host cell interactions that may complicate observations.
Several new observations and insights have emerged from our studies. Despite a robust viral infection, axons do not retract and growth cones remain active early during infection. Our observations are consistent with those of Ziegler and Poros for herpes simplex virus type 1 infections of rat dorsal root ganglia (65). The lack of an effect of infection on axon growth is important since retraction after infection would result in the disruption of most cellular interactions critical for neuron-to-cell transmission of infection from neurons to target cells. Although axons start to degenerate and break apart approximately 48 h postinfection (data not shown), it is clear that the normal cellular interactions early after infection are maintained, allowing the infection to spread from the neuron to the detector cells.
PRV gD is an essential viral ligand required for the entry and fusion of extracellular virions to cells by binding various cellular receptors, including herpesvirus entry mediator (HVEM), nectin-1, and nectin-2 (23, 44, 61). In the rodent nervous system, gD is required for transneuronal spread of herpes simplex virus type 1 but not required for transneuronal spread of PRV (2, 18, 32, 46, 48). Using the trichamber system, we demonstrate that the gD receptor is not required for spread between neurons to detector cells. Our data imply that mature extracellular infectious particles may not be involved in this neuron-to-cell transmission of infection and that the spread of infection occurs through a yet uncharacterized neuron-cell interaction.
While most sympathetic neurons are noradrenergic and are not known to form synapses with epithelial cells, a small fraction of sympathetic neurons are cholinergic and have been shown, both in vivo and in vitro, to form functional cholinergic synapses with secretory epithelial cells such as sweat glands (22, 28, 53). In addition, various growth and differentiation factors secreted by different cell types, including neurotrophin-3, ciliary neurotrophic factor, leukemia inhibitory factor, cardiotrophin-1, oncostatin, and fibroblast growth factor have been shown to induce the switch of neurotransmitter release (7, 21). In short, it may be that functional synapses exist between the superior cervical ganglia neurons and the various permissive cells plated in the N-compartment.
While we suggest that neuron-to-cell infection occurs via a specific interaction between the neurons and detector cells, it is not yet clear how the infection actually is being transferred to the detector cells. It may be that mature virions released from the axon terminals infect adjacent PK15 cells in a gD-independent process. If so, viral entry can occur only within the confines of a neuron-cell junction or synaptic cleft where a different set of receptors could allow for the fusion and entry of the virus. It is also plausible that the neuron-cell interaction results in the fusion of membranes between the growth cones and PK15 cells, forming a type of syncytium. Formation of multinucleated syncytia due to membrane fusion of alphaherpesvirus-infected cultured cells has been well documented (16, 29, 39, 45, 60). Interestingly, we observed an increase of the fluorescent lipophilic dye DiI in infected PK15 cells that is above the background signal. The localization of DiI in PK15 cells correlates well with PRV-infected cells. DiI diffusion across membranes is thought to occur only if the membranes are continuous and fused or if cells have a unique interaction, such as cell junctions where the membranes are in close opposition (4).
The fact that gE, gI, and Us9 null mutants are delayed in neuron-to-cell spread complements our observations that gE null and Us9 null-infected neurons have dramatically reduced concentrations of selected viral structural proteins in the distal segments of the axon including varicosities and growth cones (12a, 58). Each mutant has a graded but not a complete defect in axonal localization. We suggest that the inefficient targeting and subsequent reduced concentration of viral proteins in axons is the primary reason for the partial defect in anterograde transmission of infection. When all three viral genes are deleted in PRV Bartha or PRV BaBe, the cumulative axonal targeting defects result in the absolute failure of anterograde spread of infection.
The trichamber system also can be used to study axon-mediated infection of neuron cell bodies. We discovered that PRV Bartha is not only is defective in anterograde transmission of infection, it is also moderately defective in axon-mediated infection of neuronal cell bodies. This defect could occur at various stages during infection, including, but not limited to, viral entry, uncoating, axonal transport, or upstream defects once the viral genome enters the cell body. Preliminary study of viral capsid movement in axons during entry indicates that PRV Becker and Bartha capsids have similar average velocities.
Analysis of infected axons by electron microscopy reveals viral capsids enclosed within cellular membranes in both proximal and distal axons. Two models have been proposed for the transport of viral capsids in axons during egress. The first model was proposed by Penfold et al. (49). The authors discovered that the rare herpes simplex virus type 1 capsids found in the axons of infected human fetal neurons were unenveloped and associated with tegument proteins, but not with viral membrane proteins (27). These authors also reported that capsids, but not viral glycoproteins, enter axons after brefeldin A treatment. Penfold et al. proposed that alphaherpesviruses were transported in axons as viral protein subassemblies rather than fully mature virions. Unenveloped capsids would be transported separately from glycoprotein-loaded vesicles, and these viral components would be reassembled into mature virions at specific sites of assembly along the distal segments of the axon. The second model posits that mature viral particles are packaged into transport vesicles in the cell body and subsequently are targeted to the axon and transported toward a distal site. These vesicle-enclosed and axonally targeted virions are not unlike the mature virions destined for release at the plasma membrane. Most of the evidence for the second model came from electron microscopy studies of infected neurons (9, 15, 25, 26, 30, 31, 35, 63).
We recently reported that axonal targeting of all tested PRV structural proteins, including viral capsids, is blocked by brefeldin A treatment (17). We hypothesized that for viral capsids to enter axons, they must be enclosed within cellular membranes. Electron micrographs of viral structures in axons were consistent with this idea (17). However, we were unable to distinguish whether the viral capsids were located in the proximal or distal axons. Furthermore, we could not completely rule out the possibility that the vesicle-enclosed viral capsids resulted from endocytosis of mature virions from the input inoculum.
Using the trichamber system, the input viral inoculum is physically isolated from the axons that were processed for electron microscopy. Thus, endocytosis of mature virions from the inoculum can be ruled out. Our electron micrographs indicate that capsids in cellular membranes can be detected within both proximal and distal segments of the axon during viral egress. The majority of membrane-bound capsids are located midaxon as single structures and not adjacent to any discernible axonal swelling or potential sites of assembly. Thus, we conclude that during viral egress, PRV capsids enter axons and are transported in cellular membranes. The nature of the viral particle as well as the cellular membrane remains to be determined (17). Our data suggest that these particles are most likely mature virions. However, it is possible that a subpopulation of capsids found in axons is enclosed within membranes derived from the trans-Golgi network, but do not represent mature infectious virions.
In summary, we have demonstrated the versatility of the trichamber system for studying the infection and subsequent neuron-to-cell spread of infection of an alphaherpesvirus from primary cultures of rat sympathetic neurons. This system is adaptable for culturing other types of neurons and target cells as well as for studying different infectious agents that invade the nervous system.
Acknowledgments
We thank A. Flood, L. Pomeranz, L. Olsen, B. Feierbach, and J. Yu for valuable advice during preparation of the manuscript and J. Goodhouse and M. Bisher for their technical help with confocal and electron microscopy, respectively.
This work was supported by the National Institute of Neurological Disorders and Stroke (NIH-NINDS; grant 1RO1 33506).
REFERENCES
- 1.Babic, N., B. Klupp, A. Brack, T. C. Mettenleiter, G. Ugolini, and A. Flamand. 1996. Deletion of glycoprotein gE reduces the propagation of pseudorabies virus in the nervous system of mice after intranasal inoculation. Virology 219:279-284. [DOI] [PubMed] [Google Scholar]
- 2.Babic, N., T. C. Mettenleiter, A. Flamand, and G. Ugolini. 1993. Role of essential glycoproteins gII and gp50 in transneuronal transfer of pseudorabies virus from the hypoglossal nerves of mice. J. Virol. 67:4421-4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babic, N., T. C. Mettenleiter, G. Ugolini, A. Flamand, and P. Coulon. 1994. Propagation of pseudorabies virus in the nervous system of the mouse after intranasal inoculation. Virology 204:616-625. [DOI] [PubMed] [Google Scholar]
- 4.Baker, G. E., and B. E. Reese. 1993. Using confocal laser scanning microscopy to investigate the organization and development of neuronal projections labeled with DiI. Methods Cell Biol. 38:325-344. [DOI] [PubMed] [Google Scholar]
- 5.Brideau, A. D., J. P. Card, and L. W. Enquist. 2000. Role of pseudorabies virus Us9, a type II membrane protein, in infection of tissue culture cells and the rat nervous system. J. Virol. 74:834-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brideau, A. D., M. G. Eldridge, and L. W. Enquist. 2000. Directional transneuronal infection by pseudorabies virus is dependent on an acidic internalization motif in the Us9 cytoplasmic tail. J. Virol. 74:4549-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodski, C., H. Schnurch, and G. Dechant. 2000. Neurotrophin-3 promotes the cholinergic differentiation of sympathetic neurons. Proc. Natl. Acad. Sci. USA 97:9683-9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campenot, R. B. 1977. Local control of neurite development by nerve growth factor. Proc. Natl. Acad. Sci. USA 74:4516-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Card, J. P., L. Rinaman, R. B. Lynn, B. H. Lee, R. P. Meade, R. R. Miselis, and L. W. Enquist. 1993. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport, and pathogenesis. J. Neurosci. 13:2515-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Card, J. P., M. E. Whealy, A. K. Robbins, and L. W. Enquist. 1992. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J. Virol. 66:3032-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Card, J. P., M. E. Whealy, A. K. Robbins, R. Y. Moore, and L. W. Enquist. 1991. Two alpha-herpesvirus strains are transported differentially in the rodent visual system. Neuron 6:957-969. [DOI] [PubMed] [Google Scholar]
- 12.Ch'ng, T. H., E. A. Flood, and L. W. Enquist. 2005. Culturing primary and transformed neuronal cells for studying pseudorabies virus infection. Methods Mol. Biol. 292:299-316. [DOI] [PubMed] [Google Scholar]
- 12a.Ch'ng, T. H., and L. W. Enquist. 2005. Efficient axonal localization of alphaherpesvirus structural proteins in cultured sympathetic neurons requires viral glycoprotein E. J. Virol. 79:8835-8846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowdhury, S. I., B. J. Lee, A. Ozkul, and M. L. Weiss. 2000. Bovine herpesvirus 5 glycoprotein E is important for neuroinvasiveness and neurovirulence in the olfactory pathway of the rabbit. J. Virol. 74:2094-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chowdhury, S. I., M. Onderci, P. S. Bhattacharjee, A. Al-Mubarak, M. L. Weiss, and Y. Zhou. 2002. Bovine herpesvirus 5 (BHV-5) Us9 is essential for BHV-5 neuropathogenesis. J. Virol. 76:3839-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook, M. L., and J. G. Stevens. 1973. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect. Immun. 7:272-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis-Poynter, N., S. Bell, T. Minson, and H. Browne. 1994. Analysis of the contributions of herpes simplex virus type 1 membrane proteins to the induction of cell-cell fusion. J. Virol. 68:7586-7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.del Rio, T., T. H. Ch'ng, E. A. Flood, S. P. Gross, and L. W. Enquist. 2005. Heterogeneity of a fluorescent tegument component in single pseudorabies virus virions and enveloped axonal assemblies. J. Virol. 79:3903-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dingwell, K. S., L. C. Doering, and D. C. Johnson. 1995. Glycoproteins E and I facilitate neuron-to-neuron spread of herpes simplex virus. J. Virol. 69:7087-7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enquist, L. W., and J. P. Card. 2003. Recent advances in the use of neurotropic viruses for circuit analysis. Curr. Opin. Neurobiol. 13:603-606. [DOI] [PubMed] [Google Scholar]
- 20.Enquist, L. W., P. J. Husak, B. W. Banfield, and G. A. Smith. 1998. Infection and spread of alphaherpesviruses in the nervous system. Adv. Virus Res. 51:237-347. [DOI] [PubMed] [Google Scholar]
- 21.Ernsberger, U., and H. Rohrer. 1999. Development of the cholinergic neurotransmitter phenotype in postganglionic sympathetic neurons. Cell Tissue Res. 297:339-361. [DOI] [PubMed] [Google Scholar]
- 22.Francis, N. J., and S. C. Landis. 1999. Cellular and molecular determinants of sympathetic neuron development. Annu. Rev. Neurosci. 22:541-566. [DOI] [PubMed] [Google Scholar]
- 23.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 24.Hampl, H., T. Ben-Porat, L. Ehrlicher, K. O. Habermehl, and A. S. Kaplan. 1984. Characterization of the envelope proteins of pseudorabies virus. J. Virol. 52:583-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill, T. J., and H. J. Field. 1973. The interaction of herpes simplex virus with cultures of peripheral nervous tissue: an electron microscopic study. J. Gen. Virol. 21:123-133. [DOI] [PubMed] [Google Scholar]
- 26.Hill, T. J., H. J. Field, and A. P. Roome. 1972. Intra-axonal location of herpes simplex virus particles. J. Gen. Virol. 15:233-235. [DOI] [PubMed] [Google Scholar]
- 27.Holland, D. J., M. Miranda-Saksena, R. A. Boadle, P. Armati, and A. L. Cunningham. 1999. Anterograde transport of herpes simplex virus proteins in axons of peripheral human fetal neurons: an immunoelectron microscopy study. J. Virol. 73:8503-8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iacovitti, L., T. H. Joh, D. H. Park, and R. P. Bunge. 1981. Dual expression of neurotransmitter synthesis in cultured autonomic neurons. J. Neurosci. 1:685-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klupp, B. G., R. Nixdorf, and T. C. Mettenleiter. 2000. Pseudorabies virus glycoprotein M inhibits membrane fusion. J. Virol. 74:6760-6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kristensson, K., B. Ghetti, and H. M. Wisniewski. 1974. Study on the propagation of Herpes simplex virus (type 2) into the brain after intraocular injection. Brain Res. 69:189-201. [DOI] [PubMed] [Google Scholar]
- 31.LaVail, J. H., K. S. Topp, P. A. Giblin, and J. A. Garner. 1997. Factors that contribute to the transneuronal spread of herpes simplex virus. J. Neurosci. Res. 49:485-496. [PubMed] [Google Scholar]
- 32.Ligas, M. W., and D. C. Johnson. 1988. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J. Virol. 62:1486-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lomniczi, B., M. L. Blankenship, and T. Ben-Porat. 1984. Deletions in the genomes of pseudorabies virus vaccine strains and existence of four isomers of the genomes. J. Virol. 49:970-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lomniczi, B., S. Watanabe, T. Ben-Porat, and A. S. Kaplan. 1984. Genetic basis of the neurovirulence of pseudorabies virus. J. Virol. 52:198-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lycke, E., B. Hamark, M. Johansson, A. Krotochwil, J. Lycke, and B. Svennerholm. 1988. Herpes simplex virus infection of the human sensory neuron. An electron microscopy study. Arch. Virol. 101:87-104. [DOI] [PubMed] [Google Scholar]
- 36.Lycke, E., K. Kristensson, B. Svennerholm, A. Vahlne, and R. Ziegler. 1984. Uptake and transport of herpes simplex virus in neurites of rat dorsal root ganglia cells in culture. J. Gen. Virol. 65:55-64. [DOI] [PubMed] [Google Scholar]
- 37.Lyman, M. G., G. L. Demmin, and B. W. Banfield. 2003. The attenuated pseudorabies virus strain Bartha fails to package the tegument proteins Us3 and VP22. J. Virol. 77:1403-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marchand, C. F., and M. E. Schwab. 1986. Binding, uptake and retrograde axonal transport of herpes virus suis in sympathetic neurons. Brain Res. 383:262-270. [DOI] [PubMed] [Google Scholar]
- 39.Maresova, L., T. J. Pasieka, and C. Grose. 2001. Varicella-zoster Virus gB and gE coexpression, but not gB or gE alone, leads to abundant fusion and syncytium formation equivalent to those from gH and gL coexpression. J. Virol. 75:9483-9492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mettenleiter, T. C., N. Lukacs, and H. J. Rziha. 1985. Pseudorabies virus avirulent strains fail to express a major glycoprotein. J. Virol. 56:307-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mettenleiter, T. C., C. Schreurs, F. Zuckermann, and T. Ben-Porat. 1987. Role of pseudorabies virus glycoprotein gI in virus release from infected cells. J. Virol. 61:2764-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mikloska, Z., and A. L. Cunningham. 2001. Alpha and gamma interferons inhibit herpes simplex virus type 1 infection and spread in epidermal cells after axonal transmission. J. Virol. 75:11821-11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mikloska, Z., P. P. Sanna, and A. L. Cunningham. 1999. Neutralizing antibodies inhibit axonal spread of herpes simplex virus type 1 to epidermal cells in vitro. J. Virol. 73:5934-5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Milne, R. S., S. A. Connolly, C. Krummenacher, R. J. Eisenberg, and G. H. Cohen. 2001. Porcine hvec, a member of the highly conserved hvec/nectin 1 family, is a functional alphaherpesvirus receptor. Virology 281:315-328. [DOI] [PubMed] [Google Scholar]
- 45.Muggeridge, M. I. 2000. Characterization of cell-cell fusion mediated by herpes simplex virus 2 glycoproteins gB, gD, gH and gL in transfected cells. J. Gen. Virol. 81:2017-2027. [DOI] [PubMed] [Google Scholar]
- 46.Mulder, W., J. Pol, T. Kimman, G. Kok, J. Priem, and B. Peeters. 1996. Glycoprotein D-negative pseudorabies virus can spread transneuronally via direct neuron-to-neuron transmission in its natural host, the pig, but not after additional inactivation of gE or gI. J. Virol. 70:2191-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peeters, B., N. de Wind, M. Hooisma, F. Wagenaar, A. Gielkens, and R. Moormann. 1992. Pseudorabies virus envelope glycoproteins gp50 and gII are essential for virus penetration, but only gII is involved in membrane fusion. J. Virol. 66:894-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peeters, B., J. Pol, A. Gielkens, and R. Moormann. 1993. Envelope glycoprotein gp50 of pseudorabies virus is essential for virus entry but is not required for viral spread in mice. J. Virol. 67:170-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Penfold, M. E., P. Armati, and A. L. Cunningham. 1994. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc. Natl. Acad. Sci. USA 91:6529-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petrovskis, E. A., J. G. Timmins, T. M. Gierman, and L. E. Post. 1986. Deletions in vaccine strains of pseudorabies virus and their effect on synthesis of glycoprotein gp63. J. Virol. 60:1166-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Platt, K. B., C. J. Mare, and P. N. Hinz. 1979. Differentiation of vaccine strains and field isolates of pseudorabies (Aujeszky's disease) virus: thermal sensitivity and rabbit virulence markers. Arch. Virol. 60:13-23. [DOI] [PubMed] [Google Scholar]
- 52.Rauh, I., and T. C. Mettenleiter. 1991. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration. J. Virol. 65:5348-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schotzinger, R. J., and S. C. Landis. 1988. Cholinergic phenotype developed by noradrenergic sympathetic neurons after innervation of a novel cholinergic target in vivo. Nature 335:637-639. [DOI] [PubMed] [Google Scholar]
- 54.Smith, G. A., and L. W. Enquist. 2002. Break ins and break outs: viral interactions with the cytoskeleton of Mammalian cells. Annu. Rev. Cell Dev. Biol. 18:135-161. [DOI] [PubMed] [Google Scholar]
- 55.Smith, G. A., S. P. Gross, and L. W. Enquist. 2001. Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc. Natl. Acad. Sci. USA 98:3466-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith, G. A., L. Pomeranz, S. P. Gross, and L. W. Enquist. 2004. Local modulation of plus-end transport targets herpesvirus entry and egress in sensory axons. Proc. Natl. Acad. Sci. USA 101:16034-16039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tirabassi, R. S., and L. W. Enquist. 1999. Mutation of the YXXL endocytosis motif in the cytoplasmic tail of pseudorabies virus gE. J. Virol. 73:2717-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tomishima, M. J., and L. W. Enquist. 2001. A conserved alpha-herpesvirus protein necessary for axonal localization of viral membrane proteins. J. Cell Biol. 154:741-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tomishima, M. J., G. S. Smith, and L. W. Enquist. 2001. Sorting and transport of alpha herpesvirus in axons. Traffic 2:429-436. [DOI] [PubMed] [Google Scholar]
- 60.Turner, A., B. Bruun, T. Minson, and H. Browne. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72:873-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Warner, M. S., R. J. Geraghty, W. M. Martinez, R. I. Montgomery, J. C. Whitbeck, R. Xu, R. J. Eisenberg, G. H. Cohen, and P. G. Spear. 1998. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology 246:179-189. [DOI] [PubMed] [Google Scholar]
- 62.Whealy, M. E., J. P. Card, A. K. Robbins, J. R. Dubin, H. J. Rziha, and L. W. Enquist. 1993. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. J. Virol. 67:3786-3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamamoto, T., S. Otani, and H. Shiraki. 1973. Ultrastructure of herpes simplex virus infection of the nervous system of mice. Acta Neuropathol. (Berlin). 26:285-299. [DOI] [PubMed] [Google Scholar]
- 64.Yang, M., J. P. Card, R. S. Tirabassi, R. R. Miselis, and L. W. Enquist. 1999. Retrograde, transneuronal spread of pseudorabies virus in defined neuronal circuitry of the rat brain is facilitated by gE mutations that reduce virulence. J. Virol. 73:4350-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ziegler, R. J., and R. E. Herman. 1980. Peripheral infection in culture of rat sensory neurons by herpes simplex virus. Infect. Immun. 28:620-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ziegler, R. J., and R. S. Pozos. 1981. Effects of lectins on peripheral infections by herpes simplex virus of rat sensory neurons in culture. Infect. Immun. 34:588-595. [DOI] [PMC free article] [PubMed] [Google Scholar]