Abstract
The majority of adenovirus serotypes utilize the coxsackievirus-adenovirus receptor (CAR) for virus-host cell attachment, but subgroup B and subgroup D (adenovirus type 37 [Ad37]) viruses recognize CD46. CD46 is a ubiquitously expressed receptor that serves as a cofactor for the inactivation of the complement components C3b and C4b, and it also serves as a receptor for diverse microbial pathogens. A reported consequence of CD46 engagement is a reduced capability of human immune cells to express interleukin-12 (IL-12), a cytokine involved in both the innate and adaptive immune responses. Studies were thus undertaken to determine whether CD46-utilizing Ads alter the expression of proinflammatory cytokines. Subgroup B (Ad16 and -35) and Ad37, but not Ad2 or -5, significantly reduced IL-12 production by human peripheral blood mononuclear cells stimulated with gamma interferon (IFN-γ) and lipopolysaccharide. IL-12 mRNA (p35 and p40 subunits) levels as well as other cytokine mRNA levels (IL-1α and -β, IL-1Ra, and IL-6) were decreased upon interaction with CD46-utilizing Ads. Analysis of transcription factor activity required for cytokine expression indicated that CD46-utilizing Ads preferentially inhibited IFN-γ-induced C/EBPβ protein expression, consequently reducing its ability to form DNA complexes. Interference with IFN-γ signaling events by CD46-utilizing Ads, but not CAR-utilizing Ads, reveals a potentially critical difference in the host immune response against distinct Ad vectors, a situation that has implications for gene delivery and vaccine development.
Accumulating knowledge has revealed that virus association with cell receptors may have important consequences beyond simple host cell recognition. For example, human adenovirus (Ad)-receptor interactions trigger multiple signaling pathways, some of which promote cell entry (32-34), while others may provoke acute inflammatory responses (8, 18, 36, 41, 57-59). Many of the 51 known Ad serotypes classified into subgroups A to F cause acute upper respiratory tract, gastrointestinal, and ocular diseases. Cell tropism is a major factor that influences the pathological consequences of Ad infection. Ad cell entry is initiated by binding of the virus fiber protein to a specific host receptor and is followed by the association of the penton base capsid protein with αv integrins leading to virus internalization (42). While most Ad serotypes use coxsackievirus-adenovirus receptor (CAR) as their primary attachment receptor (6, 60), subgroup B and at least one subgroup D virus use an alternative receptor, CD46 (13, 52, 55, 64).
CD46, also known as membrane cofactor protein, is a member of the family of complement regulatory proteins that bind the complement proteins C3b and C4b, serving as a cofactor for their inactivation by a serum protease (53). In addition to certain Ad serotypes, CD46 is a receptor for multiple microbial pathogens, including a gram-negative bacterium, Neisseria sp. (25), the Edmonston strain of measles virus (MV) (12, 40), and human herpesvirus 6 (HHV6) (49). The use of CD46 by diverse pathogens suggests that there may be a selective advantage to utilizing this receptor. Accumulating evidence suggests that engagement of CD46 may dampen the host immune response, thereby enabling the pathogen to gain a foothold in the host. Supporting evidence for this comes from studies that revealed that both MV and HHV6 suppress interleukin-12 (IL-12) production in infected human monocytes (26, 56). IL-12 is a critical cytokine produced by cells of the innate arm of the immune system that influences the development of the adaptive immune response. Specifically, IL-12 encourages the development of Th1 T-cell immune functions that are specialized to combat intracellular infection. While the mechanism of IL-12 reduction by MV is unresolved, the suppression of cytokine expression has been suggested to underlie the immunosuppressive state observed in MV-infected individuals, persisting months after initial exposure to the virus (2). Despite the knowledge of CD46 interactions with other viral and bacterial pathogens, the role of this receptor in Ad pathogenesis has not been determined. In addition, the ability of certain Ad types to influence expression of IL-12, as well as other inflammatory cytokines, has not been established. The host factors acting downstream of receptor ligation that are responsible for cytokine modulation by CD46-utilizing pathogens are also poorly characterized. In these studies, we examined the association of subgroup B and D Ads with human peripheral blood mononuclear cells (PBMCs) and analyzed the modulation of proinflammatory cytokines via specific transcription factors. These studies shed further light on the immunopathologic consequences of Ad infection and have important implications for the use of CD46-utilizing Ad vectors for gene delivery.
MATERIALS AND METHODS
Isolation of human blood cells.
Human PBMCs were isolated from heparin-treated blood by separation on Ficoll density gradients. The blood was first diluted with equal volumes of HEPES-buffed saline, pH 7.4 (HBS) and subsequently overlaid onto 12 ml room temperature Ficoll Histopaque 1077 (Sigma, St. Louis, MO) per 35 ml diluted blood. Gradients were spun at room temperature at 1,600 rpm for 30 min in a Sorvall RT6000 centrifuge. The mononuclear cell layer was removed and washed three times with four volumes of HBS prior to lysing residual red blood cells in buffer containing 150 mM NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA. Cells were washed a final time with four volumes of HBS and resuspended in culture medium (RPMI 1640 supplemented with 10 mM HEPES, pH 7.55, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 4 mM l-glutamine, 10% fetal calf serum) at 1 × 106 cells/ml and cultured in a 5% CO2 humidified incubator at 37°C. For monocyte enrichment, 7.5 × 106 PBMCs/ml were cultured in 96-well plates for 2 h to allow monocyte adhesion. Nonadherent cells were removed, and wells were washed twice with HEPES-buffered saline before the addition of fresh medium.
Quantitation of cytokine expression.
For IL-12 expression, PBMCs were primed overnight with 10 ng/ml gamma interferon (IFN-γ; Pierce Endogen, Rockford, IL) in the absence or presence of 104 adenoviral particles/cell (unless otherwise indicated) prior to the addition of 1 μg/ml Escherichia coli 0111:B4 lipopolysaccharide (LPS; Sigma). Culture supernatants were harvested 2 days after LPS addition and used to measure IL-12 concentrations by enzyme-linked immunosorbent assay (ELISA) using an IL-12 ELISA kit (BD Pharmingen, San Diego, CA) according to the manufacturer's instructions. Tumor necrosis factor alpha (TNF-α) expression was induced with 1 μg/ml LPS following a 2-h preexposure of PBMCs to 104 adenoviral particles/cell. Culture supernatants were assayed after 2 days by ELISA using a TNF-α ELISA kit (BD Pharmingen).
RPA.
PBMCs were cultured overnight at 1 × 106 cells/ml with 10 ng/ml IFN-γ and 104 adenoviral particles/cell. Cells were stimulated the following day with 1 μg/ml LPS for 3 h prior to harvesting total RNA using the Total RNA Isolation kit from BD Pharmingen according to the manufacturer's protocol. [32P]UTP-labeled (ICN, Irvine, CA) RNA probes were synthesized using the hCK-2b multicytokine probe template set (BD Pharmingen) with the in vitro transcription kit (BD Pharmingen) according to the manufacturer's suggestions. The hCK-2b probe set detects human IL-12 p35, IL-12 p40, IL-10, IL-1α, IL-1β, IL-1Ra, IL-6, IL-18, IFN-γ, and the control L32 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNAs. A 1.5-μg aliquot of total RNA was hybridized overnight with 6 ×105 Cherenkov CPM probe followed by RNase protein assay (RPA) analysis using BD Pharmingen's RPA kit. After processing the samples according to the manufacturer's instructions, the RNAs were separated on a 4.75% acrylamide gel (19:1 acrylamide-bis) for 2 h at 55 W. The gel was dried under vacuum and subsequently exposed to film (Kodak, Rochester, N.Y.) overnight at room temperature.
Adenovirus preparation.
Ad was propagated in A549 (WT-Ad37) or HEK-293 cells (WT-Ad2, Ad5.F5, Ad5.F37, Ad5.F16, and Ad5.F35) as previously described (64). Each of the replication-defective Ad vectors used in these studies was an Ad5-based virus with E1/E3 deleted that was genetically engineered to express Ad fibers derived from serotype 5 (Ad5.F5), 37 (Ad5.F37), 16 (Ad5.F16), or 35 (Ad5.F35). Briefly, cells were infected with virus at a multiplicity of infection of 200 particles/cell and cultured for 2 days before harvesting cells. Virus was purified from the cell lysates by separation on cesium chloride gradients and ultracentrifugation. Virus preparations were dialyzed in storage buffer (10% glycerol, 40 mM Tris-buffered saline, pH 8.1), snap-frozen in liquid nitrogen, and stored at −80°C. Ad5.F37 and wild-type Ad37 (WT-Ad37) were UV inactivated by exposure to 254-nm radiation for 20 min at a distance of 6 cm from the UV source, a Mineralight lamp, model UVG-11 (Ultra-Violet Products, San Gabriel, CA). The efficiency of UV inactivation was confirmed by comparing Ad-mediated green fluorescent protein expression in HeLa cells exposed to the control or UV-inactivated Ad5.F37 vector by flow cytometry.
FACS analysis of cell surface markers.
Human PBMCs were resuspended at 1 × 106 cells/ml in complete RPMI medium, infected overnight with 1 × 104 virus particles/cell, and then washed once with phosphate-buffered saline (PBS) and once with ice-cold fluorescence-activated cell sorter (FACS) buffer (PBS plus 0.2% fetal calf serum plus 0.2% sodium azide). To detect expression of various cell surface antigens, 6 × 105 virus-infected or uninfected PBMCs were mixed with 5 μl of a cell-specific antibody conjugated to phycoerythrin (PE) (see below) and incubated for 30 min on ice in a total volume of 100 μl of FACS buffer. Cells were washed twice with FACS buffer and analyzed on a FACScan flow cytometer using CellQuest software (BD Biosciences, San Jose, CA). Only viable cells (>96%), determined by forward and side scatter parameters, were used in the analyses. Antibodies including PE-conjugated monoclonal antibodies against human CD3, CD14, CD19, and CD56 and a control antibody, PE-immunoglobulin G, were purchased from eBioscience (San Diego, CA).
Protein analysis.
IκBα protein levels were assessed in PBMCs cultured with 104 Ad.F5, Ad.F37, or Ad.F16 particles/cell for 2 hours prior to addition of 1 μg/ml LPS. Cells were harvested 30 min after LPS stimulation and resuspended in lysis buffer (1% NP-40, 20 mM Tris, pH 7.4, 150 mM NaCl, and Complete protease inhibitor [Roche, Basel, Switzerland]). Lysates were prepared by gently rocking cells in lysis buffer for 1 h at 4°C, and cell debris was removed by centrifugation. Lysates, 50 μg/lane, were electrophoresed on an 8-to-16% Novex precast acrylamide gel (Invitrogen, Carlsbad, CA), proteins were transferred onto a polyvinylidene difluoride filter, and blocked by incubation in blocking buffer (5% milk, 50 mM Tris, pH 7.4, 150 mM NaCl, 0.1% Tween 20) for 1 hour at room temperature. The filter was incubated with anti-IκBα primary antibody (C-21; Santa Cruz) at a 1:2,000 dilution in blocking buffer for 1 hour at room temperature, washed with Tris-buffered saline-Tween, and then incubated with secondary anti-rabbit-horseradish peroxidase (Sigma) at a 1:2,000 dilution in blocking buffer for 30 min. After washing the filter in Tris-buffered saline-Tween for a final time, it was developed using the West Pico Super Signal reagent (Pierce, Rockford, IL) and exposed to film. The filter was subsequently stripped by incubating in stripping buffer (60 mM Tris, pH 6.8, 2% sodium dodecyl sulfate, and 0.1 M 2-mercaptoethanol) for 30 min at 65°C and reprobed with an anti-actin antibody at 1:1,000 (Sigma). For C/EBPβ analysis, PBMCs were exposed to 10 ng/ml IFN-γ with 104 Ad particles/cell for approximately 20 h and harvested before or after LPS stimulation for 1 h. Whole-cell lysates were prepared by boiling the cells pellets in 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis loading buffer for 5 min. Two million cell equivalents/lane were loaded onto an 8-to-16% acrylamide gel and processed as described above. C/EBPβ protein was detected using a rabbit polyclonal antibody (C-19; Santa Cruz) at a 1:100 dilution.
Relative levels of fiber expression on virus preparations were determined by Western blot analysis as described above using 2 μg total virus protein separated on a 4-to-20% Novex gel. Fiber protein was probed with the mouse monoclonal antibody 4D2 (NeoMarkers, Fremont, CA) at a 1:2,000 dilution, and the penton protein was probed with the rabbit polyclonal antibody 708 (63) at 1:500.
Measurement of AP-1 activity.
HeLa, a human cervical carcinoma cell line, and THP-1, a human monocytic cell line, were transiently transfected with a reporter plasmid containing multiple AP-1 binding sites upstream of a luciferase reporter gene for measurement of AP-1 activity. HeLa cells were plated in 12-well plates at a density of 1.5 × 105 cells/well. The following day, cells were transfected using the Effectine reagent (QIAGEN, Valencia, CA) according to the manufacturer's instructions. THP-1 cells were transfected by incubation of 3 × 106 cell in 3 ml of serum-free culture medium containing 5 μg AP-1 reporter DNA and 150 μl DEAE-dextran solution (1 M Tris, pH 7.4, 5 mg/ml DEAE-dextran) for 1 h at 37°C. The cells were pelleted and cultured at 1.5 × 105 cells/well in 12-well plates. The following day, the transfection mix was removed from HeLa cultures and replaced with fresh medium. Both HeLa and THP-1 cells were exposed to 104 Ad particles/cell and cultured for 24 h prior to stimulation with 10 ng/ml phorbol myristate acetate (PMA) and 1 μM ionomycin. Luciferase activity was measured 24 hours poststimulation using the Bright-Glo luciferase assay system (Promega, Madison, WI) and a MicroLumat Plus luminometer (EG&G Berthold, Oak Ridge, TN).
EMSAs.
C/EBP, NF-κB, and AP-1 protein-DNA complex formation were assessed by electromobility shift assay (EMSA) using nuclear extracts made from PBMCs stimulated overnight with IFN-γ in the absence or presence of 104 adenoviral particles/cell and followed by a 1-h stimulation with LPS. Cells were harvested and washed in PBS before resuspension in 1 ml cold wash buffer (10 mM HEPES, pH 7.9, 10 mM Tris, pH 7.8, 60 mM KCl, 1 mM EDTA, 1 mM dithiothreitol [DTT], and 1 mM phenylmethylsulfonyl fluoride)/pellet cell volume (PCV). Cytoplasmic proteins were removed by the addition of 2 μl of a 5% NP-40 solution with repeat pipetting using a Pasteur pipette until trypan blue incorporation of cell samples reached 90 to 100%, indicating efficient membrane disruption. Cells were spun for 1 min at 1,200 × g in a refrigerated Sorvall 6000B centrifuge, washed with 5 PCV of wash buffer, and centrifuged to pellet the nuclei. Nuclear extracts were obtained by suspension of nuclei in 200 μl nuclear extract buffer/PCV (250 mM Tris, pH 7.8, 60 mM KCl, 1 mM DTT, and 1 mM phenylmethylsulfonyl fluoride) with three cycles of freeze-thaw. Cellular debris was removed by centrifugation for 15 min at maximum speed in a cold microcentrifuge, and protein concentrations were determined using the Bio-Rad protein assay reagent (Hercules, CA). EMSAs were performed by incubating 10 μg nuclear protein in a 20-μl volume containing binding buffer (10 mM Tris, pH 7.5, 50 mM NaCl, 1 mM EDTA, 1 mM DTT, 5% glycerol, 0.5 μg dI-dC) and 2 μg antibody where indicated at room temperature for 15 min. 32P-end-labeled probe (20,000 cpm) was added to each sample followed by incubation for an additional 15 min. The probe sequences used contained consensus binding sites for the transcription factors of interest: C/EBP, 5′-TGCAGATTGCGCAATCTGCA-3′; NF-κB, 5′-AGTTGAGGGGACTTTCCCAGGC-3′; AP-1, 5′-CGCTTGATGACTCAGCCGGAA-3′.
Samples were loaded onto a 6.6% native acrylamide gel and run at 170 V for 3 h in 0.5× Tris-borate-EDTA buffer. Gels were dried under vacuum and exposed to film overnight at room temperature.
RESULTS
Subgroup D and B Ads suppress IL-12 production in IFN-γ- and LPS-stimulated human PBMCs.
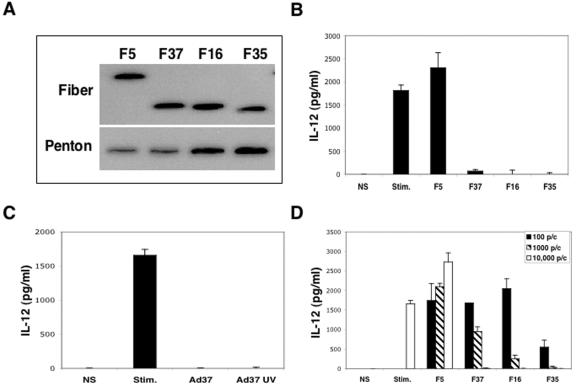
The induction of proinflammatory cytokines such as IL-12 is a prominent feature of infections with Ad type 2 or 5 (7). However, the capacity of CD46-utilizing Ad serotypes to influence cytokine expression has not been determined. Monocytes are the principal producers of IL-12 in human PBMCs and are known to express high levels of the cytokine when primed in vitro upon stimulation with IFN-γ in combination with bacterial LPS (11, 28). These culture conditions were therefore used to investigate whether replication-defective Ad5 vectors displaying fiber proteins derived from subgroup C (F5), subgroup D (F37), or subgroup B (F16 and F35) (Fig. 1A) were capable of altering IL-12 protein production. PBMCs were stimulated with IFN-γ and LPS in the presence or absence of Ad, and culture supernatants from these cells were analyzed after 48 h for IL-12 production. IFN-γ/LPS stimulation caused a substantial increase in IL-12 production compared to nonstimulated cells (Fig. 1B). Exposure of PBMCs to Ad particles displaying the subgroup C Ad5 fiber further enhanced IL-12 production, an effect that was observed in seven independent experiments with cells obtained from separate donors. In contrast, Ad5 particles displaying the subgroup D Ad37 fiber or the subgroup B Ad16 or Ad35 fiber significantly decreased cytokine production. Since the only difference among these replication-defective Ad particles is their fiber proteins, these findings strongly suggest that association of Ad37, Ad16, or Ad35 with CD46 preferentially downregulates cytokine production.
FIG. 1.
Inhibition of IL-12 expression by PBMCs upon infection with CD46-utilizing Ads. Fiber and penton base expression on the CAR-utilizing Ad5.F5 or CD46-utilizing Ad5-pseudotyped vectors Ad5.F37, Ad5.F16, and Ad5.F35 was analyzed by immunoblotting (A). An ELISA was used to measure IL-12 production in the culture supernates of PBMCs following stimulation with IFN-γ plus LPS in the absence or presence of 104 Ad/cell (B). The ability of wild-type Ad37 or UV-inactivated Ad37 to inhibit IL-12 expression was also measured in an ELISA (C). The dose of Ad vector (multiplicity of infection) required to alter IL-12 production in PBMCs was assessed 3 days postinfection in an ELISA (D).
Wild-type replication-competent Ad37 particles were also capable of suppressing IL-12 production (Fig. 1C), indicating that cytokine downregulation was not limited to fiber-pseudotyped Ad vectors. However, expression of E1 and/or E3 gene products encoded in wild-type viruses could also influence cytokine regulation (9, 21). Thus, we next examined whether UV-inactivated wild-type Ad particles were capable of suppressing cytokine production. UV-Ad particles retain the capacity to bind to cells but are defective in early and late viral genes expression. UV-inactivated Ad37 particles had a similar ability to suppress IL-12 as untreated wild-type Ad37 particles, indicating that expression of viral genes was not required for cytokine downregulation.
Further studies were performed to determine the number of Ad particles required to downregulate IL-12 production (Fig. 1D). As few as 100 particles/cell of Ad5.F35 nearly abolished IL-12 production, whereas 1,000 and 10,000 particles/cell of Ad5.F16 and Ad5.F37, respectively, were required to achieve this level of IL-12 inhibition.
Ad vectors displaying subgroup B and D fibers allow efficient infection of human peripheral blood mononuclear cells.
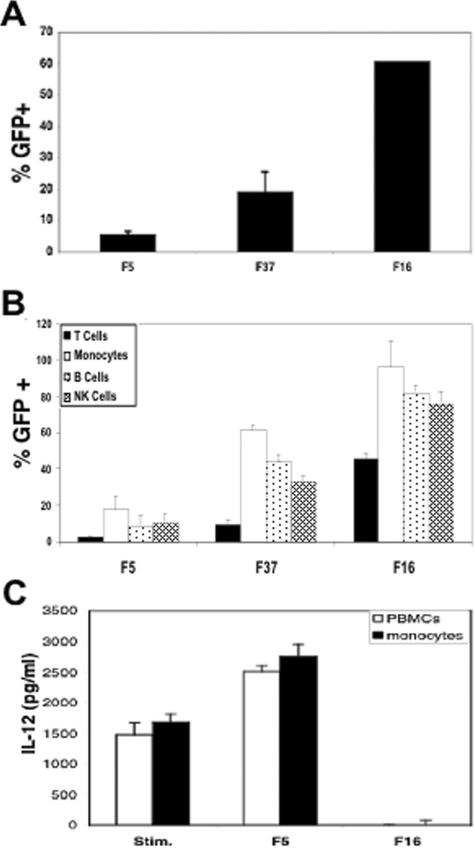
The ability of CD46-utilizing Ads to suppress IL-12 expression suggested that these virus types are capable of interacting with human PBMCs. To test this, we first examined gene delivery to unseparated PBMCs by fiber-pseudotyped Ad5 vectors. As seen in Fig. 2A, Ad5 vectors displaying the Ad5 fiber exhibited relatively poor gene delivery to PBMCs, as has been previously noted for CAR-utilizing serotypes (20, 24). In contrast, an equal number of Ad particles displaying the Ad37 or the Ad16 fiber allowed 19% or 61% delivery to these cells, respectively. We performed two-color analysis to identify the cell subpopulations transduced by Ad16 or Ad37 fiber-pseudotyped viruses. As shown in Fig. 2B, the Ad5.F5 vector allowed ∼3 to 17% transduction of the different PBMC cell populations (T, B, monocyte, and NK cells). In contrast, Ad5.F16 vectors facilitated much higher gene delivery to human T cells (45%), monocytes (96%), B cells (82%), and NK cells (77%). The Ad5.F37 vector also promoted more efficient gene delivery, although to somewhat lower levels than the Ad5.F16 vector, consistent with its somewhat lower capacity to suppress IL-12 production. To determine whether monocytes are the principle IL-12-producing cell type within the PBMC population, IL-12 expression was quantitated from monocyte-enriched cultures stimulated with IFN-γ/LPS and compared to PBMCs (Fig. 2C). The high level of IL-12 produced in the monocyte-enriched cultures was effectively inhibited by the addition of Ad5.F16, as is also seen with PBMCs. These studies confirmed that subgroup B- and D-based vectors have increased capacity to transduce human PBMCs, including the monocytes responsible for the majority of IL-12 expression.
FIG. 2.
Relative efficiency of hematopoietic cell transduction by CAR- and CD46-utilizing Ad vectors. Transduction of unseparated PBMC cultures by different fiber-pseudotyped Ad vectors was determined 48 h postinfection by flow cytometry (green fluorescent protein [GFP]) (A). Transduction of the major hematopoietic cell populations in PBMCs was determined by FACS staining with antibodies specific for T (CD3), B (CD19), monocyte/macrophage (CD14), and NK (CD56) cell surface markers (B). IL-12 production was measured in an ELISA with PBMCs and monocyte-enriched cultures stimulated with IFN-γ plus LPS and infected with either Ad5.F5 or Ad5.F16 (C).
Subgroup B and D Ads inhibit transcription of multiple inflammatory cytokines.
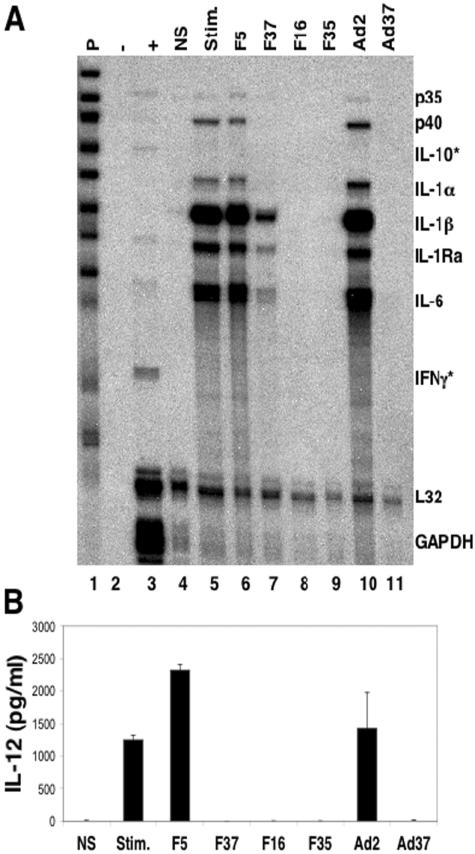
We next considered whether reduced IL-12 expression by the CD46-utilizing Ads was due to decreased cytokine mRNA expression, reduced protein synthesis, or diminished protein secretion. To examine the effects on cytokine mRNA expression, we used an RPA. PBMCs were primed overnight with IFN-γ in the presence or absence of Ad, and total RNA was prepared following a 3-h treatment with LPS. A multi-cytokine mRNA probe set was utilized to detect the mRNAs that encode both the p35 and p40 subunits that make up the bioactive IL-12 cytokine, as well as other proinflammatory cytokine mRNAs.
Stimulation of cells with IFN-γ/LPS caused a significant increase in the expression of IL-12 p35 and p40 subunits, IL-1α, IL-1β, IL-1Ra, and IL-6 (Fig. 3A, lane 5), and the addition of Ad5.F5 did not reduce the expression of these mRNAs (lane 6). In contrast, incubation of PBMCs with Ad5.F37 caused a dramatic decrease in the expression of multiple cytokine mRNAs (lane 7). Ad particles displaying the Ad16 and Ad35 fibers had the greatest effect on cytokine mRNA levels, as these fiber-pseudotyped Ads prevented the cells from producing detectable levels of any of the cytokines analyzed (lanes 8 and 9). The ability to inhibit steady-state mRNA levels was not restricted to the fiber-pseudotyped viral vectors with E1/E3 deleted, since similar results were observed with wild-type Ad37 particles (lane 11). In contrast, wild-type Ad2 virus, which uses CAR as its primary attachment receptor, slightly increased cytokine mRNA levels (lane 10). It is important to note that IL-10 expression was not detected in any of the samples. As IL-10 has been shown to inhibit IL-12 production, our findings indicate that upregulation of IL-10 is unlikely to account for the observed IL-12 suppression by CD46-utilizing Ads. Finally, the reduction in IL-12 mRNAs was also apparent at the protein level (Fig. 3B), indicating that the reduction in IL-12 p35 and p40 mRNA was not due to a decrease in the kinetics of mRNA expression, since no IL-12 protein was detected from stimulated cell cultures 3 days postinfection with fiber-pseudotyped Ad37, Ad16, or Ad35 vectors or wild-type Ad37. The IL-12 protein data correlate with the level of inhibition seen for p35 and p40 mRNA. These data indicate that Ad5.F37, Ad5.F16, and Ad5.F35 association with CD46 leads to a substantial inhibition of cytokine mRNA expression that likely explains the observed reduction in IL-12 protein expression.
FIG. 3.
CD46-utilizing Ads suppress expression of proinflammatory cytokine mRNA. (A) Detection of cytokine mRNAs was performed by RPA using RNA harvested from cells stimulated with LPS for 3 h following an overnight incubation with IFN-γ in the presence of the indicated Ad vectors or wild-type virions. A multiprobe template set (P) was used to detect mRNAs for IL-12 p35, IL-12 p40, IL-10, IL-1α, IL-1β, IL-1Ra, IL-6, and IFN-γ as well as the control L32 and GAPDH mRNAs. *, mRNAs that were not detected. (B) IL-12 production in culture supernates from the same PBMC prep used for the RPA was measured on day 3 postinfection in an ELISA. The results are representative of two independent experiments from two separate donors.
Effect of Ads on transcription factor-mediated cytokine expression.
The promoters of many proinflammatory cytokine genes involve association with and regulation by a host cell transcription factor(s). Thus, we considered the possibility that cytokine suppression by subgroup B and D Ads could be linked to impairment of specific transcription factor expression and/or activity. Interestingly, the IL-12 p35, IL-12 p40, IL-1α, IL-1β, IL-1Ra, and IL-6 genes involve not just one but three transcription factors in common to all promoters: NF-κB, AP-1, and C/EBP (3-5, 15, 16, 30, 37). We therefore investigated whether the activity of these factors was altered in cells exposed to CD46-utilizing Ads.
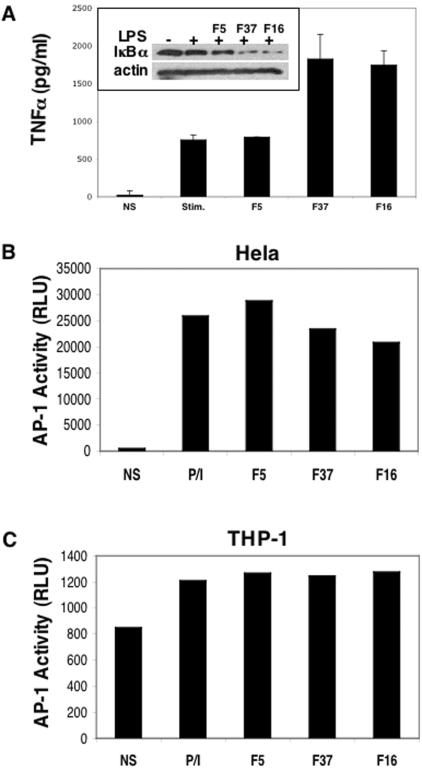
Activation of the NF-κB transcription factor and subsequent induction of the TNF-α gene are known to occur in response to LPS stimulation alone (14, 54); therefore, we assessed whether these two processes were altered upon infection with different fiber-pseudotyped Ad vectors. PBMCs were infected with Ad5.F5, Ad5.F37, or Ad5.F16 vectors prior to LPS stimulation and analyzed for NF-κB activation by monitoring the changes in IκBα protein levels by Western blotting. IκBα is an inhibitory protein that binds and retains NF-κB in the cytoplasm. Degradation of IκBα upon stimulation releases NF-κB, allowing its translocation to the nucleus, where it activates gene transcription (65). Therefore, a decrease in IκBα protein levels is generally indicative of NF-κB activation. As can be seen in Fig. 4A, LPS alone or LPS plus Ad5.F5 both caused a slight decrease in IκBα levels. Interestingly, Ad5.F37 and Ad5.F16 actually decreased IκBα levels beyond that of LPS alone, presumably leading to greater levels of NF-κB activity. To substantiate these findings, we measured downstream events indicative of NF-κB activation, including the expression of TNF-α, and TNF-α protein levels from stimulated cultures correlated with the observed increased NF-κB activity (Fig. 4A). LPS alone or LPS in conjunction with Ad5.F5 induced higher levels of expression of TNF-α. Induction of this cytokine by LPS with either Ad5.F37 or Ad5.F16 was even more pronounced than that elicited by Ad5.F5. Together, these findings suggest that CD46-utilizing Ads do not suppress cytokine expression via downregulation of NF-κB activation induced by LPS.
FIG. 4.
Effect of Ads on NF-κB and AP-1 transcription factor activity. (A) Degradation of IκBα (inset) and the induction of TNF-α production in response to LPS stimulation of PBMCs in the presence of different Ad vectors were analyzed as described in Materials and Methods. (B and C) Cells were harvested 30 min after LPS addition for lκBα analysis by Western blotting, while replicate culture supernatants were collected and assayed for TNF-α. AP-1-driven luciferase reporter gene activity, expressed as relative light units (RLU), was measured in transfected HeLa (B) and THP-1 (C) cells following stimulation with PMA and ionomycin.
We next examined whether AP-1 activity is altered upon Ad infection as measured by transient expression of an AP-1-driven reporter gene (luciferase). PBMCs are relatively resistant to transfection; thus, we utilized the human HeLa and THP-1 cell lines to test AP-1 activity by transfection of an AP-1-inducible reporter gene construct. HeLa and THP-1 cells are also permissive to Ad infection. Although LPS is known to induce AP-1 activity via the activation of the JNK mitogen-activated protein kinase cascade (17), this stimulation was not sufficient to generate AP-1-regulated luciferase activity in our experiments (data not shown). Therefore, we used the phorbol ester PMA and the calcium ionophore ionomycin to induce AP-1 activity (Fig. 4B and C). Under these conditions, we did not observe a significant inhibition of AP-1 activity by subgroup B and D Ads and, therefore, we conclude that this transcription factor is not a likely target of Ad-mediated cytokine suppression.
Subgroup B and D Ads restrict C/EBPβ DNA binding activity.
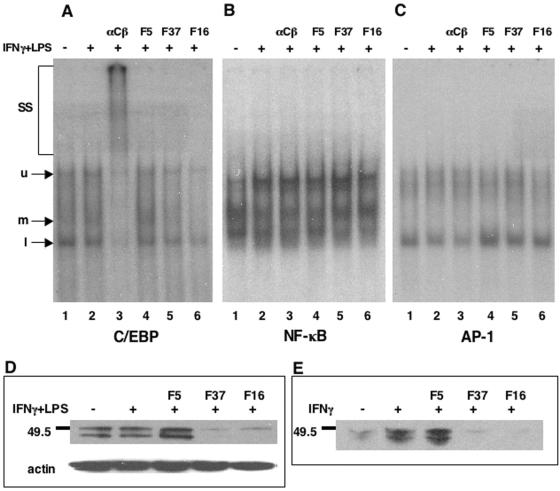
The third common transcription factor known to participate in the expression of the IL-12 p35, IL-12 p40, IL-1α, IL-1β, IL-1Ra, and IL-6 genes is C/EBP. C/EBP has been shown, upon either IFN-γ or LPS stimulation, to translocate from the cytosol to the nucleus, where it is capable of binding a CCAAT DNA consensus sequence found in a variety of gene promoters. While the C/EBP family of transcription factors consists of six members, the C/EBPβ protein is preferentially expressed in monocytes and macrophages and is involved in the expression of myeloid genes, such as IL-1, IL-6, IL-8, and macrophage inflammatory protein (46, 47). To determine whether Ad infection alters C/EBP function in PBMCs, we analyzed DNA-binding activity of this transcription factor by EMSA using nuclear extracts derived from IFN-γ/LPS-treated cells that had also been infected with Ad. The C/EBP DNA probe bound three distinct complexes in the presence of unstimulated cell extracts (Fig. 5A, lane 1), and a slight induction of the middle complex was observed upon stimulation (lane 2). This middle complex is completely supershifted upon addition of antibody to C/EBPβ, while the upper and lower bands are only partially shifted (lane 3). This result indicated that C/EBPβ is present in all complexes formed on the probe but that other proteins, most likely other C/EBP family members (i.e., α, δ, ɛ, etc.), comprise the lower and upper complexes. Interestingly, Ad5.F5 infection increased the formation of the middle complex above that of stimulated cells alone while leaving the others unaffected (lane 4). In contrast to Ad5.F5, the Ad5.F37 (lane 5) and Ad5.F16 (lane 6) vectors reduced all of the protein-DNA complexes on the C/EBP probe, with Ad5.F16 causing the complete disappearance of the middle complex. The binding activity observed with extracts derived from Ad5.F16-infected cells (lane 6) is comparable to the complexes unaffected by the C/EBPβ-specific antibody in lane 3.
FIG. 5.
CD46-utilizing Ads suppress C/EBP DNA binding activity and transcription factor expression. Transcription factor binding to DNA probes following infection with different AD vectors was assessed by EMSA for C/EBPβ (A), NF-κB (B), and AP-1 (C). Nuclear extracts generated from IFN-γ/LPS-stimulated and Ad-infected PBMC cultures were used for these studies. To detect the presence of C/EBPβ in the DNA-protein complexes, samples in lanes 3 (αCβ) were incubated with antibody to C/EBPβ. SS, super shifted species; u, upper band; m, middle band; l, lower band. C/EBPβ protein levels were assessed by Western blot analysis from cells stimulated with IFN-γ/LPS (D) or IFN-γ only (E) in the presence of the various Ad vectors.
We also analyzed the DNA-binding capabilities of NF-κB and AP-1 by EMSA. As seen in Fig. 5B and C, there was no significant decrease in either NF-κB or AP-1 complex formation in the PBMC extracts from Ad5.F37- or Ad5.F16-infected cells compared to IFN-γ/LPS treatment alone. These data are consistent with the functional studies described above and indicate that CD46-utilizing Ads preferentially inhibit C/EBPβ activation.
To better understand the reduction in C/EBPβ DNA binding activity, we looked at C/EBPβ protein expression following stimulation and Ad infection. A substantial reduction in total C/EBPβ protein levels was observed in Ad5.F37- and Ad5.F16-infected but not in Ad5.F5-infected cells (Fig. 5D). This finding is thus consistent with the reduced DNA complex formation seen with this transcription factor. As IFN-γ stimulation is known to induce C/EBPβ gene expression independent of LPS (23), we studied the effects of the CD46-utilizing vectors on C/EBPβ expression in the absence of LPS stimulation and found an equivalent decrease in protein levels compared with double stimulation (Fig. 5E). These results indicate that CD46 usage by specific Ad types results in a block in IFN-γ signaling that ultimately restricts C/EBPβ expression.
DISCUSSION
The 51 serotypes that comprise the family of human Ads vary in their abilities to infect different cell types. The Ad fiber is the major capsid protein that dictates the tropism of individual members of the Ad family. While the fiber proteins of most Ad serotypes use CAR as their host receptor, subgroup B Ads and the subgroup D Ad37 have recently been shown to recognize CD46 rather than CAR (13, 52, 55, 64). The normal role of CD46 is the regulation of complement activation, but this receptor has also been implicated in modification of the host immune response upon engagement with ligand (1, 27, 29, 39) or by diverse pathogens that use CD46, including the Edmonston strain of MV and HHV-6. CD46 ligation by the Edmonston strain of MV and HHV-6 leads to the downregulation of IL-12 (26, 56), a cytokine predominantly expressed by activated monocytes/macrophages which provides an important link between the innate and adaptive immune response (62). The studies presented here demonstrated that CD46-utilizing Ad37, -16, and -35 also reduce IL-12 production due to a suppression of cytokine mRNA levels, extending the findings from the MV and HHV-6 systems. Cytokine downregulation is independent of viral replication and early viral gene expression, since replication-defective and UV-inactivated Ads retain their potent ability to inhibit IL-12 expression. Moreover, subgroup B and D Ad suppression of IL-12 was not limited to this proinflammatory molecule, since several other cytokines, including IL-1 and IL-6, were also dramatically decreased upon infection.
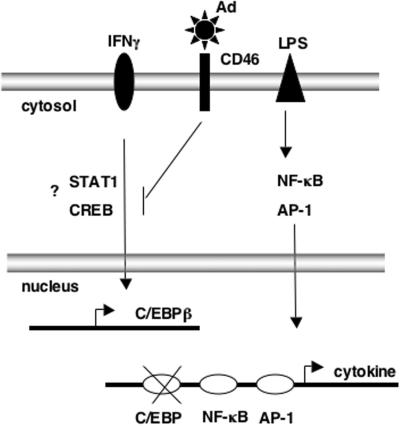
In vivo, cytokine expression is a transient event that is rapidly induced by microbial antigens. Stimulation of cells leads to the orchestrated transcription of multiple target genes that are induced by the coordinated activation of a few critical transcription factors. Specifically, many proinflammatory cytokines produced by monocytes, such as IL-1, IL-12, and IL-6, are induced by the NF-κB, AP-1, and C/EBP transcription factors. Therefore, in an effort to understand the mechanism by which Ads inhibit cytokine expression, we investigated whether virus infection altered the activities of these transcription factors. While there was no functional or biochemical evidence for the inhibition of the LPS-responsive factors NF-κB or AP-1 in PBMCs exposed to Ads, C/EBPβ-DNA complex formation was specifically decreased by the CD46-utilizing vectors. The decrease in DNA binding is a consequence of reduced C/EBPβ protein expression normally induced by IFN-γ. Therefore, we hypothesize that cytokine inhibition by the subgroup B and D (Ad37) serotypes is due to a block in IFN-γ signaling that ultimately restricts C/EBPβ expression of proinflammatory cytokines (Fig. 6). This model represents the first description of a signaling pathway that is inhibited, rather than activated, by Ad.
FIG. 6.
Model summarizing the events leading to inhibition of IFN-γ signaling and decreased C/EBPβ-mediated cytokine induction by adenoviruses utilizing CD46. Current analyses suggest that LPS signaling to NF-κB and AP-1 are not significantly impacted.
It is also tempting to speculate that these processes provide a distinct advantage for these virus types in vivo in terms of their abilities to evade the host immune response. A dampened immune response against Ad would provide an ideal environment for viral replication. This possibility is supported by studies in mice, where a decrease in IL-12 production improved adenoviral transgene expression (45, 67). Destruction of the IL-12-producing dendritic cells (DCs) and macrophages in mice prior to Ad administration also significantly blocks the immune response elicited by Ad5-based vectors (67). Moreover, studies in nonhuman primates and mice show that despite a dramatic increase in monocyte and DC transduction by CD46-utilizing Ads, humoral and/or cellular immune responses were reduced compared to that elicited by Ad5 (22, 44, 50). These findings were initially unexpected, since DCs are major antigen-presenting cells, and an increase in the expression of a transgene in this cell type is speculated to cause a concomitant increase in the immune response against it. In light of the present in vitro findings, suppression of monokines might explain these earlier in vivo observations.
In addition to the in vivo implications of immune suppression, the current studies may provide clues to the signaling pathways involved in cytokine regulation. IFN-γ stimulation of monocytes is critical for efficient cytokine production in response to pathogens. For example, in the absence of IFN-γ stimulation prior to Toll-like receptor 4 engagement (known as “IFN-γ priming”) by gram-negative bacteria or LPS, little to no IL-12 is produced (38). The presumption is that IFN-γ stimulation induces the expression of host factors that are needed at optimal concentrations for cytokine induction at the time of Toll-like receptor engagement. The best understood mechanism of gene activation by IFN-γ is through the JAK-STAT signaling pathway. Upon binding to its receptor, IFN-γ causes the activation of the tyrosine kinases JAK1 and JAK2, leading to the phosphorylation of STAT1 and STAT3 transcription factors, allowing for their dimerization and nuclear translocation. While little is known about the transcription factors regulating the C/EBPβ gene in hematopoietic cells, IFN-γ has been proven to be a potent inducer of its expression (10, 48). A STAT binding sequence has been identified in the C/EBPβ promoter and may explain its expression in monocytes/macrophages in response to IFN-γ. Alternatively, activation of the CREB transcription factor by IFN-γ may also induce C/EBPβ expression, as this factor has been implicated in its regulation (35, 43). Future studies will determine whether these factors or novel molecules are targets of inhibition by CD46-utilizing Ads. Regardless, the current data suggest that CD46-utilizing Ads interfere with IFN-γ priming, since C/EBPβ protein levels are significantly reduced in response to infection. In addition to cytokine induction, IFN-γ induces the expression of many genes and can act on a variety of cell types, influencing many cell processes such as antiviral and antimicrobial immune responses, apoptosis, and cell proliferation (51). Therefore, a disruption in IFN-γ signaling by Ad may have pleiotropic effects.
A lingering question that remains is whether fiber-CD46 interactions alone restrict cytokine expression or whether subsequent steps in the infectious process are required. Ad5.F5 vectors can infect monocytes, albeit at a lower rate than the CD46-utilizing serotypes. Nonetheless, Ad5.F5 consistently enhances IL-12 expression above that observed with IFN-γ plus LPS stimulation alone (Fig. 1 and 2C), suggesting that specific CD46 engagement, and not infectivity per se, is involved in downregulation of cytokine expression. It is presently unclear how CD46 engagement could interfere with IFN-γ signaling events. CD46 possesses one of two possible intracellular domains that are products of alternative splicing. The CYT1 cytoplasmic tail contains 16 amino acids, while the CYT2 tail is 23 amino acids long and can be tyrosine phosphorylated by src family kinases upon receptor engagement (31, 61). Differential activation of these CD46 isoforms can impact macrophage and T-cell responses (19). In addition, various signaling molecules have been implicated in CD46 signaling events, such as SHP-1, Vav, p120Cbl, LAT, Rac, and mitogen-activated protein kinase (1, 29, 66). Despite this knowledge, there is still a lack of understanding of the downstream effects of these interactions. More detailed analysis of C/EPBβ downregulation by Ads may provide additional clues into CD46 biology and cytokine signaling pathways.
Finally, our studies may have important implications for the design and use of adenoviral vectors for clinical applications. On the one hand, the use of CD46-utilizing Ad vectors may improve the duration of transgene expression by virtue of their abilities to dampen the host immune response. On the other hand, the use of CD46-tropic Ad vectors for vaccine administration may have undesirable consequences for these same reasons. Further studies will be necessary to define the precise signaling responses by CD46-tropic viruses and to determine the result of their balancing act with the host immune response.
Acknowledgments
This work was supported by NIH grants EY11431 and EY14174 R24 to G.R.N. and NINDS grant 5T32NS41219-05 to M.I.-M.
This is manuscript 17032 from The Scripps Research Institute.
We thank Joan Gausepohl for her help in the preparation of the manuscript and Jason G. Smith for critical review of the manuscript.
REFERENCES
- 1.Astier, A., M. C. Trescol-Biemont, O. Azocar, B. Lamouille, and C. Rabourdin-Combe. 2000. Cutting edge: CD46, a new costimulatory molecule for T cells, that induces p120CBL and LAT phosphorylation. J. Immunol. 164:6091-6095. [DOI] [PubMed] [Google Scholar]
- 2.Atabani, S. F., A. A. Byrnes, A. Jaye, I. M. Kidd, A. F. Magnusen, H. Whittle, and C. L. Karp. 2001. Natural measles causes prolonged suppression of interleukin-12 production. J. Infect. Dis. 184:1-9. [DOI] [PubMed] [Google Scholar]
- 3.Awad, S., H. Yokozeki, Y. Miyazaki, K. Igawa, K. Minatohara, T. Satoh, and K. Nishioka. 2002. Glucocorticoids induced the production and gene expression of IL-1α through AP-1 and partially NF-κB activation in murine epidermal cells. J. Med. Dent. Sci. 49:27-35. [PubMed] [Google Scholar]
- 4.Baccam, M., S. Y. Woo, C. Vinson, and G. A. Bishop. 2003. CD40-mediated transcriptional regulation of the IL-6 gene in B lymphocytes: involvement of NF-κB, AP-1, and C/EBP. J. Immunol. 170:3099-3108. [DOI] [PubMed] [Google Scholar]
- 5.Becker, C., S. Wirtz, X. Ma, M. Blessing, P. R. Galle, and M. F. Neurath. 2001. Regulation of IL-12 p40 promoter activity in primary human monocytes: roles of NF-κB, CCAAT/enhancer-binding protein β, and PU.1 and identification of a novel repressor element (GA-12) that responds to IL-4 and prostaglandin E2. J. Immunol. 167:2608-2618. [DOI] [PubMed] [Google Scholar]
- 6.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 7.Bessis, N., F. J. GarciaCozar, and M. C. Boissier. 2004. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther. 11:S10-S17. [DOI] [PubMed] [Google Scholar]
- 8.Bowen, G. P., S. L. Borgland, M. Lam, T. A. Libermann, N. C. Wong, and D. A. Muruve. 2002. Adenovirus vector-induced inflammation: capsid-dependent induction of the C-C chemokine RANTES requires NF-κB. Hum. Gene Ther. 13:367-379. [DOI] [PubMed] [Google Scholar]
- 9.Burgert, H. G., Z. Ruzsics, S. Obermeier, A. Hilgendorf, M. Windheim, and A. Elsing. 2002. Subversion of host defense mechanisms by adenoviruses. Curr. Top. Microbiol. Immunol. 269:273-318. [DOI] [PubMed] [Google Scholar]
- 10.Cantwell, C. A., E. Sterneck, and P. F. Johnson. 1998. Interleukin-6-specific activation of the C/EBPδ gene in hepatocytes is mediated by Stat3 and Sp1. Mol. Cell. Biol. 18:2108-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassatella, M. A., L. Meda, S. Gasperini, A. D'Andrea, X. Ma, and G. Trinchieri. 1995. Interleukin-12 production by human polymorphonuclear leukocytes. Eur. J. Immunol. 25:1-5. [DOI] [PubMed] [Google Scholar]
- 12.Dorig, R. E., A. Marcil, A. Chopra, and C. D. Richardson. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75:295-305. [DOI] [PubMed] [Google Scholar]
- 13.Gaggar, A., D. M. Shayakhvetov, and A. Lieber. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 9:1408-1412. [DOI] [PubMed] [Google Scholar]
- 14.Goldfeld, A. E., C. Doyle, and T. Maniatis. 1990. Human tumor necrosis factor α gene regulation by virus and lipopolysaccharide. Proc. Natl. Acad. Sci. USA 87:9769-9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goriely, S., D. Demonte, S. Nizet, D. De Wit, F. Willems, M. Goldman, and C. Van Lint. 2003. Human IL-12(p35) gene activation involves selective remodeling of a single nucleosome within a region of the promoter containing critical Sp1-binding sites. Blood 101:4894-4902. [DOI] [PubMed] [Google Scholar]
- 16.Goto, M., K. I. Katayama, F. Shirakawa, and I. Tanaka. 1999. Involvement of NF-κB p50/p65 heterodimer in activation of the human pro-interleukin-1β gene at two subregions of the upstream enhancer. Cytokine 11:16-28. [DOI] [PubMed] [Google Scholar]
- 17.Hambleton, J., S. L. Weinstein, L. Lem, and A. L. DeFranco. 1996. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc. Natl. Acad. Sci. USA 93:2774-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higginbotham, J. N., P. Seth, R. M. Blaese, and W. J. Ramsey. 2002. The release of inflammatory cytokines from human peripheral blood mononuclear cells in vitro following exposure to adenovirus variants and capsid. Hum. Gene Ther. 13:129-141. [DOI] [PubMed] [Google Scholar]
- 19.Hirano, A., Z. Yang, Y. Katayama, J. Korte-Sarfaty, and T. C. Wong. 1999. Human CD46 enhances nitric oxide production in mouse macrophages in response to measles virus infection in the presence of gamma interferon: dependence on the CD46 cytoplasmic domains. J. Virol. 73:4776-4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horvath, J., and J. M. Weber. 1988. Nonpermissivity of human peripheral blood lymphocytes to adenovirus type 2 infection. J. Virol. 62:341-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horwitz, M. S. 2004. Function of adenovirus E3 proteins and their interactions with immunoregulatory cell proteins. J. Gene Med. Suppl. 1:S172-S183. [DOI] [PubMed] [Google Scholar]
- 22.Hsu, C., M. Boysen, L. D. Gritton, P. D. Frosst, G. R. Nemerow, and D. J. Von Seggern. 2005. In vitro dendritic cell infection by pseudotyped adenoviral vectors does not correlate with their in vivo immunogenicity. Virology 332:1-7. [DOI] [PubMed] [Google Scholar]
- 23.Hu, J., S. K. Roy, P. S. Shapiro, S. R. Rodig, S. P. Reddy, L. C. Platanias, R. D. Schreiber, and D. V. Kalvakolanu. 2001. ERK1 and ERK2 activate CCAAT/enhancer-binding protein-beta-dependent gene transcription in response to interferon-γ. J. Biol. Chem. 276:287-297. [DOI] [PubMed] [Google Scholar]
- 24.Huang, S., R. I. Endo, and G. R. Nemerow. 1995. Upregulation of integrins αvβ3 and αvβ5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J. Virol. 69:2257-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kallstrom, H., M. K. Liszewski, J. P. Atkinson, and A. B. Jonsson. 1997. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol. Microbiol. 25:639-647. [DOI] [PubMed] [Google Scholar]
- 26.Karp, C. L., M. Wysocka, L. M. Wahl, J. M. Ahearn, P. J. Cuomo, B. Sherry, G. Trinchieri, and D. E. Griffin. 1996. Mechanism of suppression of cell-mediated immunity by measles virus. Science 273:228-231. [DOI] [PubMed] [Google Scholar]
- 27.Kemper, C., A. C. Chan, J. M. Green, K. A. Brett, K. M. Murphy, and J. P. Atkinson. 2003. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature 421:388-392. [DOI] [PubMed] [Google Scholar]
- 28.Kubin, M., J. M. Chow, and G. Trinchieri. 1994. Differential regulation of interleukin-12 (IL-12), tumor necrosis factor α, and IL-1β production in human myeloid leukemia cell lines and peripheral blood mononuclear cells. Blood 83:1847-1855. [PubMed] [Google Scholar]
- 29.Kurita-Taniguchi, M., A. Fukui, K. Hazeki, A. Hirano, S. Tsuji, M. Matsumoto, M. Watanabe, S. Ueda, and T. Seya. 2000. Functional modulation of human macrophages through CD46 (measles virus receptor): production of IL-12 p40 and nitric oxide in association with recruitment of protein-tyrosine phosphatase SHP-1 to CD46. J. Immunol. 165:5143-5152. [DOI] [PubMed] [Google Scholar]
- 30.La, E., J. E. Rundhaug, A. Pavone, and S. M. Fischer. 2002. Regulation of transcription of the intracellular interleukin-1 receptor antagonist gene by AP-1 in mouse carcinoma cells. Mol. Carcinog. 33:237-243. [DOI] [PubMed] [Google Scholar]
- 31.Lee, S. W., R. A. Bonnah, D. L. Higashi, J. P. Atkinson, S. L. Milgram, and M. So. 2002. CD46 is phosphorylated at tyrosine 354 upon infection of epithelial cells by Neisseria gonorrhoeae. J. Cell Biol. 156:951-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, E., D. Stupack, G. Bokoch, and G. R. Nemerow. 1998. Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by Rho family GTPases. J. Virol. 72:8806-8812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, E., D. Stupack, D. Cheresh, R. Klemke, and G. Nemerow. 1998. Adenovirus endocytosis via αv integrins requires phosphoinositide-3-OH-kinase. J. Virol. 72:2055-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li, E., D. G. Stupack, S. L. Brown, R. Klemke, D. D. Schlaepfer, and G. R. Nemerow. 2000. Association of p130cas with phosphatidylinositol-3-OH kinase mediates adenovirus cell entry. J. Biol. Chem. 275:14729-14735. [DOI] [PubMed] [Google Scholar]
- 35.Liu, L., Y. Wang, Y. Fan, C. L. Li, and Z. L. Chang. 2004. IFN-gamma activates cAMP/PKA/CREB signaling pathway in murine peritoneal macrophages. J. Interferon Cytokine Res. 24:334-342. [DOI] [PubMed] [Google Scholar]
- 36.Liu, Q., A. K. Zaiss, P. Colarusso, K. Patel, G. Haljan, T. J. Wickham, and D. A. Muruve. 2003. The role of capsid-endothelial interactions in the innate immune response to adenovirus vectors. Hum. Gene Ther. 14:627-643. [DOI] [PubMed] [Google Scholar]
- 37.Ma, W., K. Gee, W. Lim, K. Chambers, J. B. Angel, M. Kozlowski, and A. Kumar. 2004. Dexamethasone inhibits IL-12p40 production in lipopolysaccharide-stimulated human monocytic cells by down-regulating the activity of c-Jun N-terminal kinase, the activation protein-1, and NF-κB transcription factors. J. Immunol. 172:318-330. [DOI] [PubMed] [Google Scholar]
- 38.Ma, X., J. M. Chow, G. Gri, G. Carra, F. Gerosa, S. F. Wolf, and R. Dzialo. 1996. The interleukin 12 p40 gene promoter is primed by interferon γ in monocytic cells. J. Exp. Med. 183:147-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marie, J. C., A. L. Astier, P. Rivailler, T. Rabourdin-Combe, T. F. Wild, and B. Horvat. 2002. Linking innate and acquired immunity: divergent role of CD46 cytoplasmic domains in T cell-induced inflammation. Nat. Immunol. 3:659-666. [DOI] [PubMed] [Google Scholar]
- 40.Naniche, D., G. Varior-Krishnan, F. Cervoni, T. F. Wild, B. Rossi, C. Rabourdin-Combe, and D. Gerlier. 1993. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67:6025-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Natarajan, K., M. S. Rajala, and J. Chodosh. 2003. Corneal IL-8 expression following adenovirus infection is mediated by c-Src activation in human corneal fibroblasts. J. Immunol. 170:6234-6243. [DOI] [PubMed] [Google Scholar]
- 42.Nemerow, G. R. 2000. Cell receptors involved in adenovirus cell entry. Virology 274:1-4. [DOI] [PubMed] [Google Scholar]
- 43.Niehof, M., M. P. Manns, and C. Trautwein. 1997. CREB controls LAP/C/EBP β transcription. Mol. Cell. Biol. 17:3600-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ophorst, O. J. A. E., S. Kostense, J. Goudsmit, R. L. De Swart, S. Verhaagh, A. Zakhartchouk, M. Van Meijer, M. Spangers, G. Van Amerongen, S. Yüksel, A. D. M. E. Osterhaus, and M. J. Havenga. 2004. An adenoviral type 5 vector carrying a type 35 fiber as a vaccine vehicle: DC targeting, cross neutralization, and immunogenicity. Vaccine 22:3035-3044. [DOI] [PubMed] [Google Scholar]
- 45.Peng, Y., E. Falck-Pedersen, and K. B. Elkon. 2001. Variation in adenovirus transgene expression between BALB/c and C57BL/6 mice is associated with differences in interleukin-12 and gamma interferon production and NK cell activation. J. Virol. 75:4540-4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poli, V. 1998. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J. Biol. Chem. 273:29279-29282. [DOI] [PubMed] [Google Scholar]
- 47.Ramji, D. P., and P. Foka. 2002. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem. J. 365:561-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roy, S. K., S. J. Wachira, X. Weihua, J. Hu, and D. V. Kalvakolanu. 2000. CCAAT/enhancer-binding protein-beta regulates interferon-induced transcription through a novel element. J. Biol. Chem. 275:12626-12632. [DOI] [PubMed] [Google Scholar]
- 49.Santoro, F., P. E. Kennedy, G. Locatelli, M. S. Malnati, E. A. Berger, and P. Lusso. 1999. CD46 is a cellular receptor for human herpesvirus 6. Cell 99:817-827. [DOI] [PubMed] [Google Scholar]
- 50.Schnell, M. A., Y. Zhang, J. Tazelaar, G. P. Gao, Q. C. Yu, R. Qian, S. J. Chen, A. N. Varnavski, C. LeClair, S. E. Raper, and J. M. Wilson. 2001. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol. Ther. 3:708-722. [DOI] [PubMed] [Google Scholar]
- 51.Schroder, K., P. J. Hertzog, T. Ravasi, and D. A. Hume. 2004. Interferon-gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75:163-189. [DOI] [PubMed] [Google Scholar]
- 52.Segerman, A., J. P. Atkinson, M. Marttila, V. Dennerquist, G. Wadell, and N. Arnberg. 2003. Adenovirus type 11 uses CD46 as a cellular receptor. J. Virol. 77:9183-9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seya, T., T. Hara, M. Matsumoto, Y. Sugita, and H. Akedo. 1990. Complement-mediated tumor cell damage induced by antibodies against membrane cofactor protein (MCP, CD46). J. Exp. Med. 172:1673-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shakhov, A. N., M. A. Collart, P. Vassalli, S. A. Nedospasov, and C. V. Jongeneel. 2004. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor α gene in primary macrophages. J. Exp. Med. 171:35-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sirena, D., B. Lilienfeld, M. Eisenhut, S. Kälin, K. Boucke, R. R. Beerli, L. Vogt, C. Ruedl, M. F. Bachmann, U. F. Greber, and S. Hemmi. 2004. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J. Virol. 78:4454-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith, A., F. Santoro, G. DiLullo, L. Dagna, A. Verani, and P. Lusso. 2003. Selective suppression of IL-12 production by human herpesvirus 6. Blood 102:2877-2884. [DOI] [PubMed] [Google Scholar]
- 57.Stilwell, J. L., and R. J. Samulski. 2004. Role of viral vectors and virion shells in cellular gene expression. Mol. Ther. 9:337-346. [DOI] [PubMed] [Google Scholar]
- 58.Tamanini, A., R. Rolfini, E. Nicolis, P. Melotti, and G. Cabrini. 2003. MAP kinases and NF-κB collaborate to induce ICAM-1 gene expression in the early phase of adenovirus infection. Virology 307:228-242. [DOI] [PubMed] [Google Scholar]
- 59.Tibbles, L. A., J. C. L. Spurrell, G. P. Bowen, Q. Liu, M. Lam, and A. K. Zaiss. 2002. Activation of p38 and ERK signaling during adenovirus vector cell entry lead to expression of the C-X-C chemokine IP-10. J. Virol. 76:1559-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tomko, R. P., R. Xu, and L. Philipson. 1997. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 94:3352-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang, G., M. K. Liszewski, A. C. Chan, and J. P. Atkinson. 2000. Membrane cofactor protein (MCP; CD46): isoform-specific tyrosine phosphorylation. J. Immunol. 164:1839-1846. [DOI] [PubMed] [Google Scholar]
- 62.Watford, W. T., M. Moriguchi, A. Morinobu, and J. J. O'Shea. 2003. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 14:361-368. [DOI] [PubMed] [Google Scholar]
- 63.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]
- 64.Wu, E., S. A. Trauger, L. Pache, T.-M. Mullen, D. J. Von Seggern, G. Siuzdak, and G. R. Nemerow. 2004. Membrane cofactor protein is a receptor for adenoviruses associated with epidemic keratoconjunctivitis. J. Virol. 78:3897-3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamamoto, Y., and R. B. Gaynor. 2004. IκB kinases: key regulators of the NF-κB pathway. Trends Biochem. Sci. 29:72-79. [DOI] [PubMed] [Google Scholar]
- 66.Zaffran, Y., O. Destaing, A. Roux, S. Ory, T. Nheu, P. Jurdic, C. Rabourdin-Combe, and A. L. Astier. 2001. CD46/CD3 costimulation induces morphological changes of human T cells and activation of Vav, Rac, and extracellular signal-regulated kinase mitogen-activated protein kinase. J. Immunol. 167:6780-6785. [DOI] [PubMed] [Google Scholar]
- 67.Zhang, Y., N. Chirmule, G.-P. Gao, R. Qian, M. Croyle, B. Joshi, J. Tazelaar, and J. M. Wilson. 2001. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol. Ther. 3:697-707. [DOI] [PubMed] [Google Scholar]