Abstract
The relative contribution of measles virus hemagglutinin (H)- or fusion protein (F)-specific antibodies to virus neutralization (VN) has not been demonstrated. We have depleted these specific antibodies from sera collected from young adults, who had been vaccinated during childhood, by prolonged incubation with viable transfected human melanoma cells expressing H or F. Simultaneous depletion of antibodies of both specificities completely abrogated VN activity. Depletion of F-specific antibodies only had a minimal effect, whereas removal of H-specific antibodies resulted in almost complete reduction of VN activity. These results demonstrate that measles virus neutralizing antibodies are mainly directed to the H protein.
Measles virus (MV) is a member of the family Paramyxoviridae, genus Morbillivirus. It is transmitted via the respiratory route and has an incubation phase of 9 to 19 days (7). Whereas the cellular immune response is thought to be crucial for clearance of infection, virus-neutralizing (VN) antibodies are an important correlate of protection (4). VN antibody levels of 0.1 to 0.2 international units (IU) per ml, equivalent to titers of approximately 1:8 to 1:16, have been shown to protect from disease (2, 10) and interfere with the efficacy of live-attenuated measles vaccines (6, 14). VN antibodies are exclusively directed to the hemagglutinin (H) or fusion protein (F) and mostly recognize conformational epitopes (1). It has been suggested that H-specific antibodies are the major contributors to VN activity (1), but this has never formally been demonstrated.
We have recently developed a fluorescence-activated cell scanner (FACS)-measured immunofluorescence assay for the detection of MV H- and F-specific antibodies, using the stably transfected human melanoma cells Mel-JuSo/MV-H or Mel-JuSo/MV-F as target cells (3), and used this assay for the detection of specific antibodies in humans and experimentally infected animals (5, 8, 12). Here, we have used the same cells to deplete H- or F-specific antibodies from polyclonal sera. Interactions between antibody and antigen result in an equilibrium between bound and unbound antibody. For depletion of unbound antibodies the antigen/antibody complexes should be removed from the system. We achieved this by culturing H- or F-expressing cells in medium supplemented with 10% of the test serum.
From our serum bank we selected 160 samples collected in 2002 or 2003 from healthy subjects born in the period 1980 to 1985. Measles vaccination was introduced in The Netherlands in 1976 and was replaced by vaccination with measles-mumps-rubella in 1987 (15). The subjects included in this study therefore most likely received a monovalent measles vaccination at the age of 14 months and a measles-mumps-rubella vaccination at the age of 9 years. Measles has become a rare disease in The Netherlands, with sporadic outbreaks in communities with low vaccination coverage. Therefore, the majority of these subjects was probably never exposed to wild-type MV. Since samples with low MV-specific antibody levels were considered of limited use for this study, the sera were first tested in an in-house MV-specific immunoglobulin G (IgG) enzyme-linked immunosorbent assay. The 64 samples (40%) with the lowest signals in this test were excluded from further analysis. The remaining 96 samples were heat inactivated (30 min, 56°C) and tested anonymously.
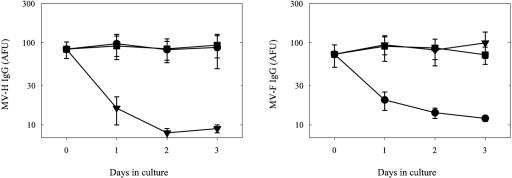
Mel-JuSo cells expressing the MV H or F protein or the untransfected parental cell line (Mel-JuSo/wt) were seeded in 96-well flat-bottom plates in RPMI 1640 medium supplemented with antibiotics and 10% fetal bovine serum (FBS). When monolayers were nearly confluent, supernatants were replaced with medium containing 10% of the different test sera (150 μl/well). Supernatants were collected after 0, 1, 2, or 3 days of culture for 10 different samples and were tested for the presence of H- or F-specific IgG as previously described (3) after 1:10 dilution in phosphate-buffered saline (PBS) supplemented with 3% FBS. After 3 days of culture on H-expressing cells, H-specific IgG antibodies were efficiently depleted while F-specific antibody levels remained unaffected (Fig. 1). The inverse pattern was observed for sera incubated on F-expressing cells, while incubation on the untransfected cell line left both H- and F-specific antibody levels virtually unchanged (Fig. 1).
FIG. 1.
Depletion of MV glycoprotein-specific antibodies from polyclonal serum by prolonged incubation with viable transfected human melanoma cells expressing the MV H or F protein. Serum samples collected from healthy young adults (n = 10) vaccinated during childhood were heat inactivated and diluted 1:10 in culture medium. The resulting media were used to grow the human melanoma cell line Mel-JuSo (square symbols) or stably transfected Mel-JuSo cells expressing the MV-Edmonston H (triangles) or F (circles) protein. Supernatants were collected on days 0, 1, 2, and 3 and tested for the presence of H- or F-specific IgG antibodies by a FACS-measured immunofluorescence assay (left and right panels, respectively). Results are shown as means ± standard errors and are expressed in arbitrary fluorescence units (AFU).
Next, H- and/or F-specific antibodies were removed sequentially and VN activity of the resulting samples was determined. Mel-JuSo cells were grown in medium supplemented with 10% of the test serum for a period of 3 days (round 1). Supernatants were then transferred to freshly seeded Mel-JuSo cells and cultured for another three days (round 2). To make certain that all samples had a comparable culture history, samples from which no or only a single specific antibody was depleted were also cultured on untransfected Mel-JuSo cells. Four conditions were used: condition 1 (no depletion), Mel-JuSo/wt (round 1)→Mel-JuSo/wt (round 2); condition 2 (H depletion), Mel-JuSo/MV-H (round 1)→Mel-JuSo/wt (round 2); condition 3 (F depletion), Mel-JuSo/MV-F (round 1)→Mel-JuSo/wt (round 2); and condition 4 (H and F depletion), Mel-JuSo/MV-H (round 1)→Mel-JuSo/MV-F (round 2). All assays were also performed on the original sera (referred to as condition 0).
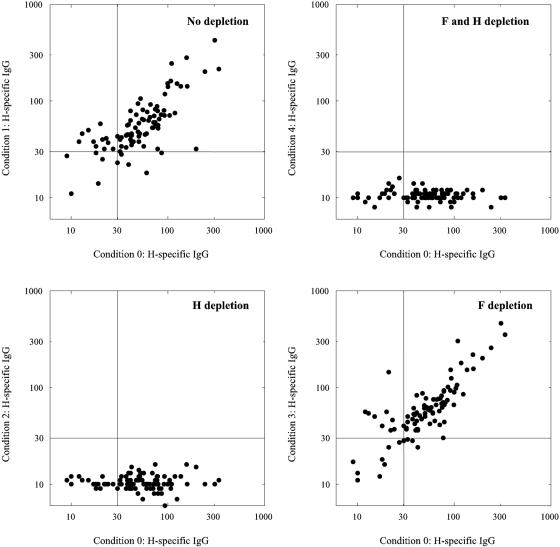
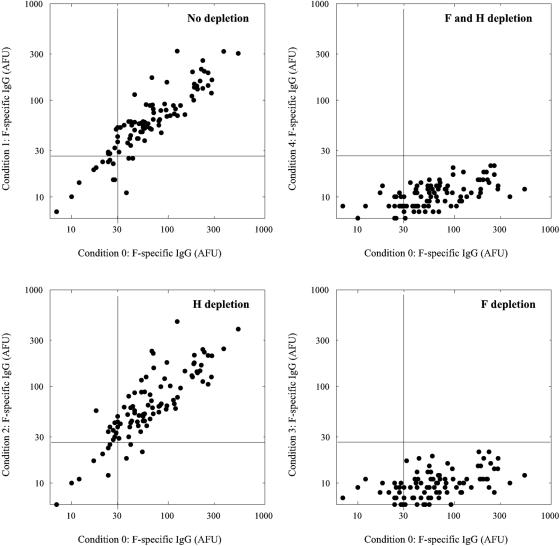
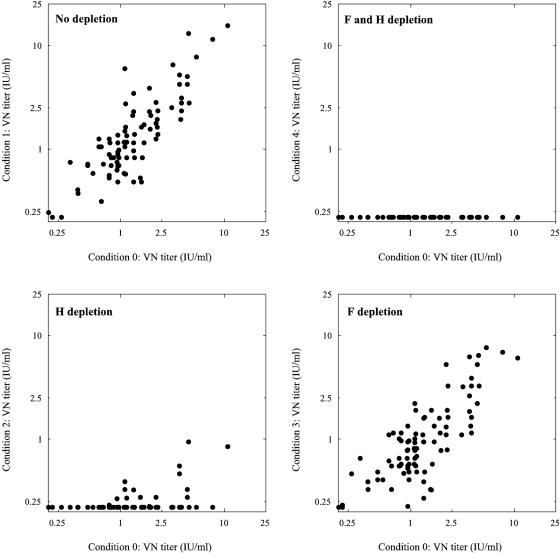
As shown in Fig. 2 and 3, this protocol was successful in depleting H- and/or F-specific IgG from the polyclonal test sera. Virus neutralization was measured by endpoint titration as previously described (12) and expressed in international units (IU) per milliliter, using the international reference serum for measles (serum 66/202; 5 IU per ml; World Health Organization International Laboratory for Biological Standards, National Institute for Biological Standards and Control, Hertfordshire, United Kingdom). Effective depletion was confirmed by showing that depletion of both H- and F-specific antibodies resulted in complete abrogation of the capacity to neutralize MV strain Edmonston (Fig. 4). Depletion of F-specific antibodies only had a minimal effect on the VN titers of the serum, whereas depletion of H-specific antibodies resulted in an almost complete reduction of VN activity (Fig. 4).
FIG. 2.
H-speciifc IgG antibody levels (expressed in AFU) after two consecutive 3-day culture periods on Mel-JuSo cells either expressing or not expressing MV glycoproteins, as compared to the levels in untreated sera. Serum samples collected from healthy young adults (n = 96) vaccinated during childhood were heat inactivated and diluted 1:10 in culture medium, referred to as condition 0. Subsequently, Mel-JuSo cells were cultured in these media for two consecutive periods of 3 days each. In condition 1 (no depletion) the samples were incubated twice on Mel-JuSo/wild-type cells, in condition 2 (H depletion) the samples were first incubated on Mel-JuSo/MV-H and next on Mel-JuSo/wild-type cells, in condition 3 (F depletion) the samples were first incubated on Mel-JuSo/MV-F and next on Mel-JuSo/wild-type cells, while in condition 4 (H and F depletion) the samples were first incubated on Mel-JuSo/MV-H and next on Mel-JuSo/MV-F cells.
FIG. 3.
F-specific IgG antibody levels (expressed in AFU) after two consecutive 3-day culture periods on Mel-JuSo cells, as described in the legend to Fig. 2.
FIG. 4.
MV-specific VN antibody levels after two consecutive 3-day culture periods on Mel-JuSo cells, as described in the legend to Fig. 2. VN antibody levels were determined by endpoint neutralization of a 50% cell culture infectious dose of 60 MV-Edmonston (12) and are expressed in international units (IU) per ml.
We hypothesize that the depletion of specific antibodies in this protocol is the result of internalization and degradation of bound antibodies by the H- or F-expressing cells. Since new recombinant protein is continuously produced, expressed on the cell surface, and available for binding antibody, this eventually depletes unbound antibodies from the sample without affecting antibodies with other specificities. Protein-specific antibodies in sera with VN antibody levels up to 10 IU/ml were effectively depleted (Fig. 4), demonstrating that the depletion assay had a dynamic range with a relatively high upper limit.
The effectiveness of this approach would be expected to depend on the avidity of the serum. This was confirmed by preliminary depletion studies using a limited number of early-convalescent-phase sera collected from patients infected with wild-type MV from which H- or F-specific antibodies could not effectively be depleted (results not shown). However, in the present study all sera were collected several years after vaccination and thus may be expected to have contained only high-avidity antibodies. Another prerequisite is the absence of toxicity: a few (3 out of 96) serum samples in our study did not accommodate growth of the Mel-JuSo cells, resulting in incomplete depletion (these samples were excluded from the final analysis).
Genetic variability of the virus under investigation may also play an important role. Although MV is a monotypic virus, some genetic variability exists, with the highest level in the H gene (16). In the present study we have used a homologous system: both the vaccine and the genes used for transfecting the cells belonged to clade A. It has been suggested that genetic variability of wild-type MV strains could in part be due to positive selection of viruses less susceptible to neutralization by vaccination-induced antibodies (9, 11). Therefore, depletion of H- and F-specific antibodies induced by infection might not completely remove the capacity to neutralize the wild-type virus. To test this hypothesis we are currently collecting late-convalescent-phase serum samples from subjects infected with recent wild-type strains.
Finally, wild-type MV strains use the signaling lymphocyte activation molecule (SLAM; CD150) as a cellular receptor, while MV-Edmonston and MV vaccine strains can use both SLAM and CD46 (13). The neutralization assay used in the present study is based on MV Edmonston infection of Vero cells, which is completely dependent on CD46 as a receptor. It would therefore be interesting to study the effect of depletion of H- and F-specific antibodies induced by infection on neutralization of wild-type MV strains in SLAM-expressing cells.
In conclusion, we have depleted H- and/or F-specific antibodies from polyclonal sera collected from young adults more than 10 years after measles vaccination. Successful depletion was demonstrated by the loss of protein-specific IgG fluorescence signals and by the complete abrogation of VN activity in samples from which both H- and F-specific antibodies were depleted. The results described indicate that H-specific antibodies are the main correlate of vaccination-induced MV neutralization. Studies on monitoring vaccination efficacy in relation to newly emerging MV genotypes should therefore largely focus on the H protein, which could ultimately have consequences for the design of new generations of measles vaccines. Finally, the depletion assay used may also be of value for determining the role of protein-specific antibody responses in other virus infections.
Acknowledgments
We thank Ann Vossen and Hans Kruining for their contributions to this study.
REFERENCES
- 1.Bouche, F. B., O. T. Ertl, and C. P. Muller. 2002. Neutralizing B cell response in measles. Viral Immunol. 15:451-471. [DOI] [PubMed] [Google Scholar]
- 2.Chen, R. T., L. E. Markowitz, P. Albrecht, J. A. Stewart, L. M. Mofenson, S. R. Preblud, and W. A. Orenstein. 1990. Measles antibody: reevaluation of protective titers. J. Infect. Dis. 162:1036-1042. [DOI] [PubMed] [Google Scholar]
- 3.De Swart, R. L., H. W. Vos, F. G. C. M. UytdeHaag, A. D. M. E. Osterhaus, and R. S. Van Binnendijk. 1998. Measles virus fusion protein- and hemagglutinin-transfected cell lines are a sensitive tool for the detection of specific antibodies by a FACS-measured immunofluorescence assay. J. Virol. Methods 71:35-44. [DOI] [PubMed] [Google Scholar]
- 4.Duke, T., and C. S. Mgone. 2003. Measles: not just another viral exanthem. Lancet 361:763-773. [DOI] [PubMed] [Google Scholar]
- 5.El Mubarak, H. S., S. A. Ibrahim, H. W. Vos, M. M. Mukhtar, O. A. Mustafa, T. F. Wild, A. D. M. E. Osterhaus, and R. L. De Swart. 2004. Measles virus protein-specific IgM, IgA and IgG subclass responses during the acute and convalescent phase of infection. J. Med. Virol. 72:290-298. [DOI] [PubMed] [Google Scholar]
- 6.Garly, M.-L., and P. Aaby. 2003. The challenge of improving the efficacy of measles vaccine. Acta Trop. 85:1-17. [DOI] [PubMed] [Google Scholar]
- 7.Griffin, D. E. 2001. Measles virus, p. 1401-1441. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 8.Hartter, H. K., R. L. De Swart, F. Hanses, H. W. Vos, F. B. Bouche, A. D. M. E. Osterhaus, F. Schneider, and C. P. Muller. 2000. Evaluation of different measles IgG assays based on recombinant proteins using a panel of low-titre sera. J. Virol. Methods 84:191-200. [DOI] [PubMed] [Google Scholar]
- 9.Klingele, M., H. K. Hartter, F. Adu, W. Ammerlaan, W. Ikusika, and C. P. Muller. 2000. Resistance of recent measles virus wild-type isolates to antibody-mediated neutralization by vaccinees with antibody. J. Med. Virol. 62:91-98. [DOI] [PubMed] [Google Scholar]
- 10.Samb, B., P. Aaby, H. C. Whittle, A. M. Coll Seck, S. Rahman, J. Bennett, L. Markowitz, and F. Simondon. 1995. Serologic status and measles attack rates among vaccinated and unvaccinated children in rural Senegal. Pediatr. Infect. Dis. J. 14:203-209. [DOI] [PubMed] [Google Scholar]
- 11.Santibanez, S., S. Niewiesk, A. Heider, J. Schneider-Schaulies, G. A. M. Berbers, A. Zimmermann, A. Halenius, A. Wolbert, I. Deitemeier, A. Tischer, and H. Hengel. 2005. Probing neutralizing-antibody responses against emerging measles viruses (MVs): immune selection of MV by H protein-specific antibodies? J. Gen. Virol. 86:365-374. [DOI] [PubMed] [Google Scholar]
- 12.Stittelaar, K. J., L. S. Wyatt, R. L. De Swart, H. W. Vos, J. Groen, G. Van Amerongen, R. S. Van Binnendijk, S. Rozenblatt, B. Moss, and A. D. M. E. Osterhaus. 2000. Protective immunity in macaques vaccinated with a modified vaccinia virus Ankara-based measles vaccine in the presence of passively acquired antibodies. J. Virol. 74:4236-4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tatsuo, H., N. Ono, K. Tanaka, and Y. Yanagi. 2000. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406:893-897. [DOI] [PubMed] [Google Scholar]
- 14.Van Binnendijk, R. S., M. C. M. Poelen, G. Van Amerongen, P. De Vries, and A. D. M. E. Osterhaus. 1997. Protective immunity in macaques vaccinated with live attenuated, recombinant and subunit measles vaccines in the presence of passively acquired antibodies. J. Infect. Dis. 175:524-534. [DOI] [PubMed] [Google Scholar]
- 15.Van den Hof, S., G. A. M. Berbers, H. E. De Melker, and M. A. E. Conyn-van Spaendonck. 1999. Sero-epidemiology of measles antibodies in the Netherlands, a cross-sectional study in a national sample and in communities with low vaccination coverage. Vaccine 18:931-940. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. 2003. Update of the nomenclature for describing the genetic characteristics of wild-type measles viruses: new genotypes and reference strains. Wkly. Epidemiol. Rec. 78:229-232. [PubMed] [Google Scholar]