Abstract
The flavivirus nonstructural protein NS1 is expressed as three discrete species in infected mammalian cells: an intracellular, membrane-associated form essential for viral replication, a cell surface-associated form that may be involved in signal transduction, and a secreted form (sNS1), the biological properties of which remain elusive. To determine the distribution of the dengue virus (DEN) sNS1 protein in vivo, we have analyzed by immunohistological means the tissue tropism of purified DEN sNS1 injected intravenously into adult mice. The sNS1 protein was found predominantly associated with the liver, where hepatocytes appeared to represent a major target cell. We further showed that sNS1 could be efficiently endocytosed by human Huh7 and HepG2 hepatocytes in vitro. After its internalization, the protein was detected intracellularly for at least 48 h without being substantially degraded. Colocalization studies of sNS1 with markers of the endolysosomal compartments revealed that the protein was specifically targeted to lysobisphosphatidic acid-rich structures reminiscent of late endosomes, as confirmed by electron microscopy. Intracellular accumulation of sNS1 in Huh7 cells enhanced the fluid phase uptake of rhodamine-labeled dextran. Furthermore, preincubation of Huh7 cells with sNS1 increased dengue virus production after infection with the homologous strain of DEN-1 virus. Our results demonstrate that the accumulation of DEN sNS1 in the late endosomal compartment of hepatocytes potentializes subsequent dengue virus infection in vitro, raising the possibility that sNS1 may contribute to viral propagation in vivo.
Dengue viruses (DEN) are among the most prevalent arboviruses in tropical and subtropical areas. Each year, over 100 million dengue infections occur, leading to 500,000 hospitalizations and an estimated 50,000 deaths (18). Dengue viruses are transmitted to humans by Aedes aegypti mosquitoes and cause a wide range of symptoms from an unapparent or mild disease (dengue fever) to a severe hemorrhagic form (dengue hemorrhagic fever). Dengue hemorrhagic fever is characterized by abnormalities of hemostasis and vascular permeability and by an elevated risk of hypovolemic shock (dengue shock syndrome) that may be fatal (53). To date, the physiopathology of dengue hemorrhagic fever/dengue shock syndrome remains poorly understood (19, 31, 55).
Dengue viruses (serotypes 1 to 4) belong to the Flavivirus genus of the Flaviviridae family, which comprises other major human pathogens such as yellow fever, Japanese encephalitis, tick-borne encephalitis, and West Nile viruses. Flaviviruses are enveloped, single-stranded, positive-sense RNA viruses. The genome is approximately 11 kilobases long and contains a single open reading frame encoding a polyprotein precursor of about 3,400 amino acids. Co- and posttranslational processing of the polyprotein by cellular and viral proteases gives rise to three structural proteins, designated C (core), M (membrane), and E (envelope), and seven nonstructural proteins, NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5 (4, 38). The two cytosolic proteins NS3 and NS5 have been identified as the viral protease/helicase and polymerase, respectively (27, 61).
The nonstructural NS1 glycoprotein is essential for virus viability but has no established biological activity. During infection in vitro, NS1 is translocated into the endoplasmic reticulum through a hydrophobic signal sequence localized at the carboxyl terminus of the E protein (13). In the endoplasmic reticulum, NS1 becomes associated as a homodimer which interacts with membranous components (63, 64). A fraction of NS1 remains associated with intracellular organelles, where it appears to be involved in the early steps of viral replication (39, 40, 46). Alternatively, the NS1 protein is exported along the secretory pathway to the plasma membrane, where it remains anchored, possibly via a glycosylphosphatidylinositol group (23), or is released as a soluble hexamer (sNS1) from infected mammalian cells (8, 14).
In vivo, the sNS1 protein is found circulating in sera from dengue virus-infected patients throughout the clinical phase of the disease (1, 65). Concentrations of sNS1 in sera may exceed 1 to 10 μg/ml depending on the virus serotype, the time course of infection, and the individual host and appear to be higher in patients who developed dengue hemorrhagic fever rather than dengue fever (32). Antibodies elicited by NS1 during infection may play a role in pathogenesis by cross-reacting with cell surface antigens of endothelial cells or platelets, inducing their activation, or causing their death by apoptosis or complement-mediated lysis (11, 33-35).
In order to get some insights into the role of DEN sNS1 during its circulation in blood, we have investigated the fate of the protein in vivo, in a mouse model of intravenous injection. We found that sNS1 predominantly associates with the liver, where hepatocytes represent a major target cell. This prompted us to analyze the intracellular trafficking of endocytosed sNS1 in human hepatocytes in vitro and the consequences of its accumulation in late endosomes.
MATERIALS AND METHODS
Cells and virus.
The virus was purified and titrated as focus-forming units on AP61 cells. The human hepatoma cell lines Huh7 and HepG2 were cultured at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (Invitrogen, Gibco, France) supplemented with 10% heat-inactivated fetal calf serum, 4 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Vero cells were grown in Iscove medium (Invitrogen) supplemented with 2% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. The FGA/89 strain of DEN-1 virus (French Guyana, 1989) was isolated from a patient with dengue fever and propagated a limited number of times on C6/36 and AP61 mosquito cells (9).
Antibodies.
Monoclonal antibodies directed against DEN NS1 were prepared by fusing SP2/0/AG14 mouse myeloma cells with splenocytes isolated from BALB/c mice immunized with purified sNS1. Hybridomas secreting NS1-specific antibodies were selected by a cell-based enzyme-linked immunosorbent assay using dengue virus-infected Vero cell lysates, cloned, and further characterized by immunoblotting. Five monoclonal antibodies (G18, 9C5, 14F12, 4F7, and 15C6) were selected for their high reactivity against nonreduced dimeric or monomeric DEN NS1. NS1-specific rabbit polyclonal antibodies were raised against DEN-1 sNS1, immunopurified on immobilized sNS1, and concentrated by ultrafiltration (Sartorius, Vivascience, United Kingdom).
Antibodies used for subcellular localization studies were specific for the transferrin receptor (57), the lysosome-associated membrane protein (LAMP)-1 (Developmental Studies Hybridoma Bank, Iowa), and the lysobisphosphatidic acid (LBPA) lipid (clone 6C4) (25).
Expression and purification of DEN sNS1.
Vero cells grown on monolayers were infected with the DEN-1 FGA/89 virus at a multiplicity of infection (MOI) of 1. Supernatants were harvested 5 days postinfection, passed through a 0.2-μm filter, and concentrated by ultrafiltration (Sartorius, United Kingdom). Virus particles were precipitated overnight at 4°C with 7% polyethylene glycol (Sigma-Aldrich) and pelleted for 30 min at 10,000 × g. The supernatant was passed through an immunoaffinity column and the eluted sNS1 protein was further purified by size exclusion chromatography as described previously (12, 14). The purity of DEN-1 sNS1 was assessed by staining acrylamide gels with Coomassie brilliant blue R250 (Bio-Rad, France) and protein concentrations were determined using the MicroBCA protein assay (Perbio Science, Pierce, France). The endotoxin content was quantified by the PyroGene Recombinant Factor C assay (Cambrex, Belgium) and found to be lower than 0.05 EU/ml.
Mouse model.
Two independent sets of experiments were carried out using 6-week-old B6 × SJL female mice (Janvier Laboratories, France). In each set of experiments, four groups of three mice and one control were injected intravenously with 25 μg of purified DEN-1 sNS1 or an equivalent volume (100 μl) of phosphate-buffered saline (PBS), respectively. At different times postinjection, a group of mice were sacrificed and different organs (spleen, liver, kidney, and brain) were recovered. In one set of experiments, mice were anesthetized with Imalgene (Merial, France) and perfused with PBS followed by 4% neutral-buffered paraformaldehyde before the organs were recovered. Organs were fixed for 2 h at room temperature in 4% paraformaldehyde and dehydrated overnight at 4°C in 60% ethanol. Tissues were embedded in paraffin and processed for histology and immunohistochemistry.
Immunohistochemistry.
Tissue sections (5 μm) were deparaffinized in xylene (VWR-Prolabo, France), dehydrated in ethanol, washed in PBS, and placed for 8 min in a solution containing 49.5% acetone, 49.5% hydrogen peroxide, and 1% ammonium hydroxide. Samples were treated for 45 min at 75°C in a specific target retrieval solution (DakoCytomation, France), and incubated for 30 min at room temperature with 2% goat serum, for 10 min in 10 mM NH4Cl, and for 1 h with purified anti-NS1 rabbit polyclonal antibodies in PBS with 1% bovine serum albumin. Tissue sections were washed three times in PBS and overlaid for 1 h with a 1:50 dilution of fluorescein isothiocyanate-conjugated anti-rabbit antibodies (The Jackson Laboratory, Maine). For counterstaining of cell nuclei, cells were permeabilized with 0.1% TritonX-100, treated for 35 min at 37°C with RNase and DNase (10 μg/ml each) (Roche, Paris, France) and stained for 20 min with 1 μg/ml propidium iodide in 50 mM citrate buffer, pH 6.0.
Indirect immunofluorescence.
Subconfluent Huh7 and HepG2 cells were cultured for 6, 24, or 48 h in the presence of purified sNS1 (10 μg/ml). Cells were washed with PBS, fixed with 3% paraformaldehyde in PBS-4% sucrose, treated for 10 min with 10 mM NH4Cl, and incubated with primary antibodies specific for NS1, transferrin receptor, LAMP-1, or LBPA in PBS-1 mg/ml bovine serum albumin-0.05% saponin for 1 h at room temperature. Slides were washed three times in PBS-1 mg/ml bovine serum albumin-0.05% saponin, and incubated with secondary fluorescein isothiocyanate- or cyanin-3-conjugated antibodies (GE Healthcare, Amersham Biosciences, France) as previously described. Single-labeled samples were examined using a Leica microscope at 63x magnification and images were acquired using a Coolpix digital camera (Nikon, France). Dual-labeled samples were examined by confocal microscopy (LSM 510 META microscope) and images were analyzed using the LSM Image Browser 3.2 software (Zeiss, Germany).
Western blotting.
Subconfluent Huh7 cells were cultured for 6, 24, or 48 h in the presence of purified sNS1 (10 μg/ml). After a chase of 24 or 48 h, cells were collected in 50 mM borate buffer, pH 9, centrifuged for 10 min at 1,500 × g, resuspended in borate buffer, and lysed for 20 min at 4°C in PBS, 1% Triton X-100. Cell lysates were centrifuged for 10 min at 6,000 × g and the supernatants were collected and stored at −20°C until processed. Proteins were separated on sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and transferred onto a nitrocellulose membrane (Whatman, Schleicher & Schuell, Germany). After saturation with 3% milk in 0.2 M Tris-HCl, pH 8.5, 0.5 M NaCl, the membrane was incubated at room temperature with a mixture of five anti-NS1 monoclonal antibodies, each at a final concentration of 5 μg/ml in Tris-NaCl. The membrane was washed three times in Tris-NaCl, incubated for 1 h with alkaline phosphatase-conjugated anti-mouse immunoglobulin G (Sigma-Aldrich), further washed three times, and stained with 0.35 mM 5-bromo-4-chloro-3-indolylphosphate, 0.35 mM nitroblue tetrazolium in 100 mM Tris-HCl, pH 9.5, 100 mM NaCl, and 50 mM MgCl2.
Electron microscopy.
Purified DEN NS1 was covalently labeled with horseradish peroxidase (HRP) according to the manufacturer's instructions (Invitrogen, Molecular Probes, France), except that HRP was reduced with dithiothreitol rather than succinimidyl 3-(2-pyridyldithio)propionate. Thiolated HRP was deprotected by the reducing agent tris-(2-carboxyethyl)phosphine and succinimidyl trans-4-(maleimidylmethyl)cyclohexane-1-carboxylate was used to add thiol-reactive maleimide groups to the sNS1 protein. Activated HRP was incubated for 2 h at room temperature with the maleimide derivative of sNS1 at a molar ratio of 1:1, which allowed the formation of a stable thioether bond. N-Ethylmaleimide was added to the reaction mixture for quenching free thiol groups. Unbound HRP was removed by size exclusion chromatography as described for the purification of unlabeled sNS1.
Subconfluent Huh7 cells were cultured for 6, 24, or 48 h with NS1-HRP (10 μg/ml), washed three times in PBS, and fixed for 15 min at 4°C in 2.5% glutaraldehyde, 0.1 M sodium cacodylate buffer, pH 7.4. Cells were washed three times in PBS, incubated for 10 min at room temperature with a mixture of 3,3′-diaminobenzidine (0.7 mg/ml, Sigma-Aldrich) and hydrogen peroxide (0.17 mg/ml) in 60 mM Tris buffer, washed three times with PBS, postfixed with 1% osmium tetroxide for 30 min at room temperature, dehydrated in graded ethanol series and propylene oxide, and embedded in Epoxy resin. Ultrathin sections were observed without counterstaining with a Jeol 1010 electron microscope (Jeol, France).
Quantification of the endocytic activity of hepatocytes.
Huh7 cells (104 cells/ml) were cultured for 48 h in the presence of purified sNS1 (10 μg/ml), followed by 6-h-fluid-phase uptake of fluorescein- and tetramethylrhodamine-labeled dextran (2 mg/ml) (10,000 molecular weight, Invitrogen, Molecular Probes, France). Cells were then washed thoroughly with cold PBS and fixed for 20 min with cold PBS, 3% paraformaldehyde, 4% sucrose. Cells were examined by confocal microscopy (LSM 510 META microscope). Images were recorded as nonoverlapping sections on the z axis and fused prior to treatment with the LSM Image Browser 3.2 software. Fluorescent signals were quantified per cell or per surface unit for subconfluent and confluent cultures, respectively. Average values corresponded to three different image analyses from two independent experiments.
Dengue virus production by hepatocytes.
Subconfluent Huh7 cells (104 cells/ml) were cultured for 24 h in the presence of purified sNS1 (10 μg/ml) or an equivalent volume of PBS. Cells were washed twice with medium and infected for 2 h at room temperature with the DEN-1 FGA/89 isolate at a multiplicity of infection of 2, 10, or 50. Cells were washed four times with medium and cultured for another 24 or 48 h. Supernatants were recovered and virus titers were determined by a focus-forming assay on AP61 cells, as described previously (9).
RESULTS
Hepatocytes are target cells for the DEN sNS1 protein in vivo.
In order to analyze the tissue tropism of the DEN sNS1 protein, adult B6 × SJL mice (four groups of three animals) were injected intravenously with 25 μg of purified hexameric DEN-1 sNS1. Initial sNS1 protein concentration represented around 10 μg/ml of blood. Mice inoculated with an equivalent volume of PBS were used as negative controls. At different times postinjection (1, 3, 6, or 24 h), the spleen, liver, kidney, and brain were recovered from mice that received sNS1 and from the control animals, and tissues were embedded in paraffin for histology and immunohistochemistry studies. No obvious histopathological alterations or inflammatory infiltrates could be observed by standard microscopy.
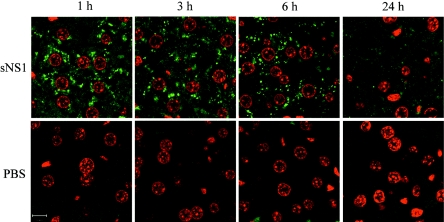
Detection of the NS1 protein was carried out by immunofluorescence on 5-μm tissue sections using purified anti-NS1 rabbit polyclonal antibodies as primary antibodies. Sections from spleen, kidney, and brain were negative in all animals (data not shown). In contrast, liver sections from mice injected with sNS1 but not from control animals were found massively positive in sNS1 antigen (Fig. 1). Analysis by confocal microscopy revealed that the NS1-specific signal was prominent from 1 h to 6 h postinjection and could still be detected, although to a lesser extent, after 24 h. The sNS1 protein localized in numerous discrete punctate structures within the cytoplasm of cells that display a distinctive circular nucleus characteristic of hepatocytes (Fig. 1). These cells could also be unambiguously identified as positive for sNS1 by histochemical staining (Fig. S1 in the supplemental material). Thus, hepatocytes appeared to be a major target cell of the DEN sNS1 protein in mice.
FIG. 1.
Detection of the DEN sNS1 protein in mouse liver. Mice were injected intravenously with purified DEN-1 sNS1 (25 μg) or PBS and sacrificed at 1, 3, 6, or 24 h postinjection. Histological sections of paraffin-embedded livers were immunostained with purified NS1-specific polyclonal rabbit antibodies and a fluorescein isothiocyanate-labeled secondary antibody. Cell nuclei were counterstained with propidium iodide and samples were analyzed by confocal microscopy (63× magnification). sNS1 localized in punctate cytoplasmic structures (green). Bar, 10 μm.
Internalization and stability of sNS1 in human hepatocytes in vitro.
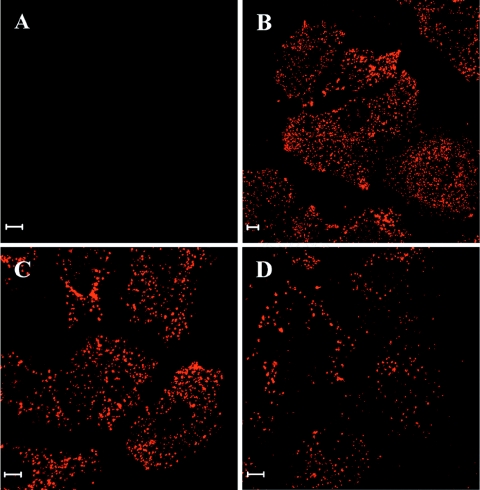
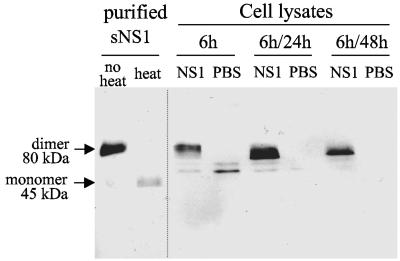
We then investigated the interactions of the DEN-1 sNS1 protein with human Huh7 or HepG2 hepatocytes. sNS1 was efficiently internalized by both cell types at concentrations ranging from 1 to 10 μg/ml of medium and persisted in cultured cells for 3 to 4 days without inducing any cytopathic effect (data not shown). Strikingly, the sNS1 protein was found in discrete punctate structures in the cytoplasm of Huh7 and HepG2 cells (Fig. 2 and data not shown), which resembled those observed in mouse liver sections. The sNS1-positive signals remained intense after a 48-h chase, indicating that the protein might be particularly resistant to degradation. This was confirmed by immunoblot analysis of sNS1 internalized in Huh7 cells for 6 h and chased for 24 h or 48 h. Cell lysates were separated by SDS-PAGE, transferred onto a nitrocellulose membrane, and probed with a mix of NS1-specific monoclonal antibodies (Fig. 3). Purified NS1, included as a positive control, resolved as a dimer with an apparent size of 80 kDa when unheated prior to electrophoresis and was converted to the 45-kDa monomer by boiling. The sNS1 protein internalized by hepatocytes was still detected as a dimer after a 48-h chase, and its amount showed only a slight decrease between 24 h and 48 h of chase (Fig. 3). These results demonstrated the efficient internalization and marked stability of the DEN sNS1 protein in human hepatocytes.
FIG. 2.
DEN sNS1 protein is efficiently internalized in human hepatocytes. Subconfluent Huh7 cells were incubated for 6 h with DEN-1 sNS1 (10 μg/ml) (B to D) or PBS (A). Cells were fixed directly (B) or after 24 h or 48 h of chase (C, D). sNS1 was detected by immunofluorescence using specific rabbit polyclonal antibodies and a cyanin-3-labeled secondary antibody. Bars, 10 μm.
FIG. 3.
Stability of the internalized sNS1 protein in human hepatocytes. Huh7 cells were incubated for 6 h with DEN-1 sNS1 (10 μg/ml) or PBS, and lysed directly (6 h) or after 24 h or 48 h of chase (6 h/24 h and 6 h/48 h, respectively). Proteins were separated by SDS-PAGE and analyzed by immunoblotting. The internalized sNS1 protein was probed using a mix of specific monoclonal antibodies and an alkaline phosphatase-conjugated secondary antibody. Samples were unheated, except for a control in which purified sNS1 was heated for 3 min at 95°C to convert the dimeric subunit into monomers.
Accumulation of sNS1 in LBPA-rich structures.
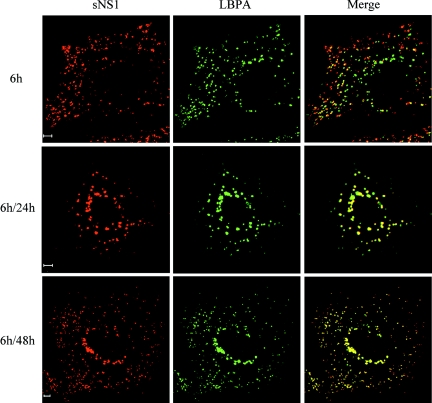
We next examined the intracellular distribution of DEN sNS1 during the internalization process in human hepatocytes with markers of the endolysosomal system. Colocalization studies of sNS1 were carried out in Huh7 cells by immunofluorescence and confocal microscopy using antibodies specific for the transferrin receptor, a marker of early endosomes (16); LAMP-1, which cycles between late endosomes and lysosomes (5); and LBPA, a specific constituent of late endosomes (26). After a 24-h or 48-h chase, the stains for sNS1 and the transferrin receptor were distinct, indicating that the protein did not accumulate in early endosomes (data not shown). In contrast, the sNS1 and LBPA signals partially colocalized after a 6-h pulse and became indistinguishable at 24 h or 48 h of chase (Fig. 4). The NS1-positive structures could also be labeled with an anti-LAMP-1 antibody (Fig. S2 in the supplemental material). Taken together, these results demonstrated that the DEN sNS1 protein is specifically targeted to late endosomes in human hepatocytes.
FIG. 4.
sNS1 protein accumulates in LBPA-rich structures. Huh7 cells were incubated for 6 h with DEN-1 sNS1 (10 μg/ml) or PBS, followed by 24 h or 48 h of chase (6 h/24 h and 6 h/48 h, respectively). Intracellular localization of sNS1 was analyzed by immunofluorescence and confocal microscopy. The sNS1 protein was detected using NS1-specific rabbit polyclonal antibodies and a cyanin-3-conjugated secondary antibody (red). Late endosomes were labeled using a lysobisphosphatidic acid (LBPA)-specific mouse monoclonal antibody and a fluorescein isothiocyanate-conjugated secondary antibody (green). Colocalization of sNS1 and LBPA is revealed on the merged images (yellow). Bars, 5 μm.
Ultrastructural analysis of sNS1-treated human hepatocytes.
To determine whether the accumulation of the DEN sNS1 protein in late endosomes could induce any cytopathic effect or change in cell morphology, Huh7 cells were incubated with DEN-1 sNS1 (10 μg/ml) or PBS for 48 h and analyzed by standard electron microscopy. No obvious cytopathic effect could be identified in sNS1-treated hepatocytes except for the formation of voluminous vesicles with a diameter greater than l μm that were not observed in control cells (data not shown).
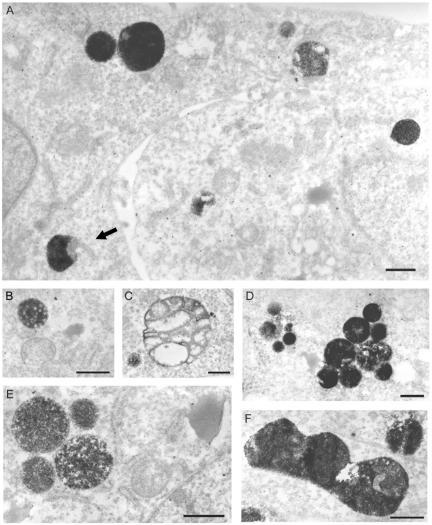
In order to better characterize the sites of sNS1 accumulation at the ultrastructural level, Huh7 cells were incubated with HRP-conjugated sNS1, fixed, stained with diaminobenzidine (DAB), and observed by electron microscopy (Fig. 5). After a 6-h incubation followed by a 24-h chase, a considerable amount of HRP-conjugated sNS1 protein was detected inside vacuoles 0.5 to 1 μm in diameter (Fig. 5A), occasionally connected to unlabeled tubules (arrow). When Huh7 cells were incubated for a longer period of time (48 h) with HRP-conjugated sNS1, the protein accumulated in similar structures (Fig. 5B to D), some of which clearly contained internal membranes characteristic of late endosomes (Fig. 5B). In addition, some large NS1-positive organelles were observed that resembled either swelling multivesicular bodies (Fig. 5C) or autophagic vacuoles (Fig. 5F). Overall, most of the NS1-positive structures were reminiscent of late endosomes, in accordance with the results obtained by confocal microscopy.
FIG. 5.
Ultrastructural morphology of sNS1-positive structures in human hepatocytes. Huh7 cells were incubated with HRP-labeled DEN-1 sNS1 (10 μg/ml) for 6 h followed by a 24-h chase (A) or for 48 h without chase (B to F). sNS1-positive structures stained with DAB are revealed by electron microscopy as dense cytoplasmic vacuoles 0.5 to 1 μm in diameter, some of which contained distinct internal vesicular bodies. Connection of an NS1-positive vacuole to a nonlabeled tubule was occasionally observed (arrow). Bars, 500 nm.
DEN sNS1-treated Huh7 cells exhibit enhanced endocytic activity.
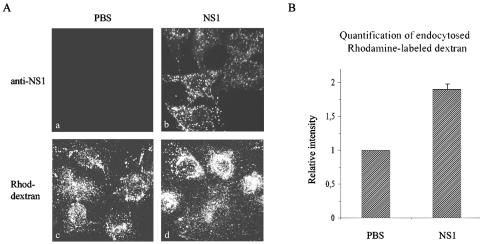
Since the DEN sNS1 protein did not perturb cell survival and was not associated with major cytopathic effects, we next investigated a possible effect of the intracellular accumulation of sNS1 on the endocytic activity of hepatocytes. For this purpose, Huh7 cells were pretreated or not with sNS1 for 48 h and incubated with rhodamine-labeled dextran for 6 h. Fluorescent signals were examined by confocal microscopy and quantified by image analysis. Rhodamine-labeled dextran displayed a stronger perinuclear distribution (Fig. 6A) and was internalized twice as much in cells pretreated with sNS1 compared to PBS-treated cells (Fig. 6B). These effects were observed in both confluent and subconfluent Huh7 cells at the time rhodamine-labeled dextran was added to the medium. Thus, the DEN sNS1 protein increases the endocytic activity of hepatocytes and modulates the intracellular trafficking of endocytosed dextran molecules.
FIG. 6.
sNS1 protein increases the endocytic activity of human hepatocytes. (A) Huh7 cells were incubated for 48 h with PBS (a and c) or DEN-1 sNS1 (10 μg/ml) (b and d) and further incubated for 6 h with PBS (a and b) or tetramethylrhodamine-labeled dextran (Rhod-dextran, 2 mg/ml) (c and d). Cells were fixed and stained with anti-NS1 rabbit polyclonal antibodies (a and b) or untreated (c and d) and observed by confocal microscopy. (B) Rhodamine-labeled dextran signals were quantified, and control values from PBS-treated cell samples were arbitrarily set to 1. Means and standard deviation from two independent experiments are shown.
sNS1 protein increases productive dengue virus infection in hepatocytes.
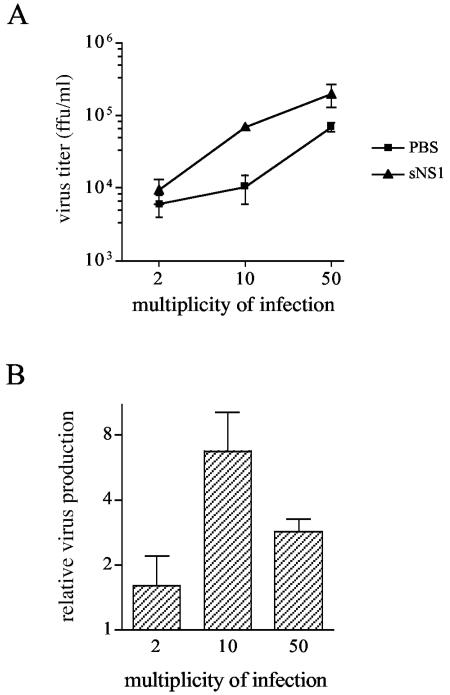
We hypothesized that the accumulation of the DEN sNS1 protein in late endosomes could influence the subsequent dengue virus infection of hepatocytes. To test this hypothesis, Huh7 cells were incubated with sNS1 or PBS for 24 h and infected for 2 h with the homologous DEN-1 FGA/89 isolate at an MOI of 2, 10, or 50. Dengue virus production was quantified 24 h postinfection in the culture supernatants using a focus-forming assay on AP61 cells.
Dengue virus titers ranged from 104 to 2.105 FFU/ml in NS1-pretreated cells and from 6.103 to 7.104 FFU/ml in PBS-treated cells (Fig. 7A), corresponding to a 1.6- to 6.7-fold increase in cell cultures that have preinternalized sNS1 (Fig. 7B). The ratio of virus particle release in sNS1- versus PBS-treated Huh7 cells was higher when cells were infected at an intermediate MOI of 10 (6.7-fold) compared to MOIs of 2 and 50 (1.6- and 2.9-fold, respectively) (Fig. 7B). Similar results were obtained at 48 h postinfection, with a 2- and 4.3-fold increase of dengue virus production in sNS1- versus PBS-treated cells infected at MOIs of 2 and 10, respectively. A marked cytopathic effect was associated with viral replication at an MOI of 50 (data not shown). Altogether, these results indicate that the sNS1 protein increases dengue virus production in hepatocytes, a phenomenon dependent on the virus load and the kinetics of viral multiplication.
FIG. 7.
sNS1 protein enhances dengue virus production by human hepatocytes. Huh7 cells were incubated for 24 h with DEN-1 sNS1 (10 μg/ml) or PBS, and infected for 2 h with the homologous DEN-1 FGA/89 virus at a multiplicity of infection of 2, 10, or 50. (A) Cell supernatants were harvested at 24 h postinfection and DEN-1 virus titers were determined by focus-forming assay in AP61 cells. (B) Ratios of DEN-1 viral titers from infected Huh7 cells treated with sNS1 versus PBS. Means and standard deviations of duplicate experiments are shown.
DISCUSSION
Secretion of the nonstructural protein NS1 is a hallmark of dengue virus infection in humans (1, 32, 65), but its biological significance remains unknown. In this study, we have identified the liver as a major site of DEN sNS1 accumulation in vivo, in a mouse model of intravenous injection. We further showed that the sNS1 protein is internalized in human hepatocytes in vitro, where it accumulates in late endosomes. Remarkably, the intracellular accumulation of sNS1 leads to increased endocytic activity of hepatocytes and enhances dengue virus production after subsequent infection, pointing to a possible role of the sNS1 protein in virus multiplication.
In mice, the sNS1 protein was detected in the liver of all animals from 1 h to 6 h postinjection, but not in the spleen, kidney, or brain. Liver sections highly positive for sNS1 antigen did not show obvious sign of inflammatory infiltrate or physiological disorder. A majority of hepatocytes contained punctate cytoplasmic structures intensely labeled with anti-NS1 antibodies, indicating that they represented an important target cell type for the protein. However, this does not rule out the possibility that sNS1 may interact with other cell types, such as sinusoidal endothelial cells and Kupffer cells present in the liver, or cells circulating in the blood.
Interestingly, sNS1 appears to be endocytosed with varying efficiency by a wide range of cell types in vitro, including primary human endothelial cells (M. Flamand and S. Kayal, unpublished observations). These observations suggest a common route of entry of sNS1 into cells, possibly through interaction with an as yet unknown ubiquitous receptor. Given the broad spectrum of target cells in vitro, it is unclear why sNS1 exhibits a restricted tissue tropism in vivo. This may be due to the poor accessibility of certain cell types to circulating sNS1, combined with its rapid and efficient uptake by the liver, in particular hepatocytes. Alternatively, the internalization of sNS1 by potential target cells might be modulated by the tissue-specific expression of cellular factors that remain to be identified. From several points of view, the biodistribution and clearance of reporter proteins or protein complexes circulating in the blood are highly variable and depend on the nature of the target cells, the mode of entry, and resistance to degradation rather than the actual size of the proteins and do not necessarily involve hepatocytes (6, 22, 28, 54, 60).
A striking finding of the in vitro studies with human hepatocytes was that the internalized sNS1 protein did not reach the lysosomal compartment but instead accumulated in late endosomes, as demonstrated by its strict colocalization with LBPA after 24 h or 48 h of chase in Huh7 cells. The LBPA phospholipid, in association with cellular proteins such as Alix, drives the formation of intralumenal membranous structures constitutive of late endosomes (26, 43), thereby representing a specific marker of the late endosomal compartment in contrast to proteins such as LAMP-1, which recycle between late endosomes and lysosomes (5). Moreover, the sNS1 protein internalized by human Huh7 and HepG2 hepatocytes persisted as an undegraded form over 48 h in both cell types. By comparison, the DEN E protein and the ovalbumin are degraded in these cells within 4 h (21) and 2 h (data not shown), respectively. Although it was not possible to assess the native state of the protein once internalized, as the hexameric form of sNS1 is sensitive to detergents (14, 24), the sNS1 dimeric subunits could be detected in cell extracts after 48 h of chase. Furthermore, the protein dimers have been found to be particularly resistant to denaturation by acidic pH (8) and cleavage by various proteases such as trypsin and chymotrypsin (3) (data not shown). Our observations suggest that the sNS1 protein has the ability to resist denaturation at a pH of about 5.5 inside late endosomes and degradation by cellular proteases (50, 58). It is noteworthy that the intracellular fate of the sNS1 protein differs notably from that of well-characterized bacterial toxins that circumvent the endolysosomal degradation pathway by being directly transported from early endosomes to the Golgi apparatus, such as Shiga toxin (41), or making use of a caveola-mediated endocytic pathway for their direct targeting from the plasma membrane to the Golgi, such as cholera toxin (48, 49). Additional experiments are required to characterize the interactions of the DEN sNS1 protein with the constituents of late endosomes and to better understand the mechanism of resistance to proteolysis.
Surprisingly, the sNS1 protein did not impair the viability of Huh7 or HepG2 hepatocytes or induce any obvious cytopathic effect. However, at the ultrastructural level, numerous enlarged vacuoles (0.5 to 1 μm) were observed in Huh7 cells treated with sNS1, some of which contained substantial amounts of the viral protein. It is known that interference with the endolysosomal system at the level of protein or lipid transport (17, 51) and signaling (59) can lead to severe human pathologies. One illustration is the antiphospholipid syndrome, characterized by the production of antibodies reactive against phospholipids such as LBPA that accumulate in late endosomes and lead to a redistribution of cholesterol as well as the insulin-like growth factor 2 or mannose-6-phosphate receptor (10, 25). We observed that the distribution of cholesterol and of the fluid phase marker rhodamine-labeled dextran was modified in sNS1-treated Huh7 cells, in which both displayed a stronger perinuclear localization than in untreated cells (Fig. 6 and data not shown). Thus, sNS1 accumulation affects the intracellular trafficking of endogenous and exogenous molecules, suggesting that the sNS1 protein may alter cell physiology.
In humans, liver failure consistently occurs during clinical dengue virus infections, resulting in hepatomegaly and/or elevated transaminase concentrations in patient sera (29, 44). Furthermore, components of the acute-phase response such as cytokines, coagulation, and complement factors that may be synthesized by the liver are particularly elevated in individuals who develop dengue hemorrhagic fever (30, 47, 52). The actual cause of liver damage is still unknown and may in part be ascribed to dengue virus replication. Viral antigen has been detected around foci of liver necrosis in human biopsies (7) and in vitro studies in hepatocytes have demonstrated that replication of dengue virus induces nitric oxide and RANTES synthesis (36) and cell death by apoptosis (37, 42). Moreover, severe combined immunodeficient mice transplanted with HepG2 cells and infected intraperitoneally with dengue virus produced infectious virus particles in the liver and developed clinical signs with some similarities to those observed in humans (2), emphasizing the role of hepatocytes in dengue pathogenesis.
Our study raises the possibility that sNS1 could also contribute to liver dysfunction. We have shown that sNS1 induces a substantial increase, up to sevenfold, of DEN-1 virus production in treated Huh7 cells, suggesting that, in addition to its effect on cell physiology, the sNS1 protein may contribute to viral multiplication. Accordingly, concentrations of both sNS1 and dengue virus particles appear to be higher in dengue hemorrhagic fever compared to dengue fever (32). The enhanced productive dengue virus infection in sNS1-exposed hepatocytes may correlate with the greater endocytic activity of these cells that would lead to an increased uptake of virus particles. Alternatively, the sNS1 protein may trigger specific signaling pathways that could upregulate cell surface expression of the virus receptor(s), alter the cellular proteolytic machinery thereby preventing extensive degradation of virus particles as demonstrated for human immunodeficiency virus (15), facilitate the fusion process of dengue virus particles with endosomal membranes (20, 45), or provide an optimized environment for the initial stages of viral replication (38, 56, 62).
The present study demonstrates the preferential uptake of circulating DEN sNS1 by the liver in vivo, in particular by hepatocytes. In vitro, its accumulation in the late endosomal compartment of human hepatocytes correlates with a modification of the trafficking in the endocytic pathway and a marked enhancement of dengue virus production. Deciphering the role of sNS1 during dengue virus infection will require better characterization of its interaction with hepatocytes and its potential effects on other target cell types, such as endothelial cells or cells of the immune system. A contribution of the DEN sNS1 protein to the physiopathology of dengue fever and dengue hemorrhagic fever could substantiate sNS1 as a potent candidate for the development of novel antiviral therapeutic approaches.
Supplementary Material
Acknowledgments
We thank Fasséli Coulibaly for the study of NS1 stability to proteases, Emmanuelle Perret and Spencer Shorte for confocal microscopy analyses, Agnès Pouzet for the peroxidase labeling of NS1, Farida Nato, Sylvie Dartevelle, Françoise Mégret, and BIOTEM for the preparation of DEN NS1-specific monoclonal antibodies, Philippe Camparo, Nathalie Cordonnier, and Drew Syder for their contribution to the immunohistological analyses, and Agathe Subtil and Jean-Claude Antoine for helpful discussions. We are particularly grateful to Jérôme Salmon and Myriam Ermonval for critical reading of the manuscript.
Sophie Alcon-LePoder was the recipient of Ph.D. funding from the Ecole Nationale Vétérinaire d'Alfort, France.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org.
REFERENCES
- 1.Alcon, S., A. Talarmin, M. Debruyne, A. Falconar, V. Deubel, and M. Flamand. 2002. Enzyme-linked immunosorbent assay specific to dengue virus type 1 nonstructural protein reveals circulation of the antigen in blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 40:376-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An, J., J. Kimura-Kuroda, Y. Hirabayashi, and K. Yasui. 1999. Development of a novel mouse model for dengue virus infection. Virology 263:70-77. [DOI] [PubMed] [Google Scholar]
- 3.Cauchi, M. R., E. A. Henchal, and P. J. Wright. 1991. The sensitivity of cell-associated dengue virus proteins to trypsin and the detection of trypsin-resistant fragments of the nonstructural glycoprotein NS1. Virology 180:659-667. [DOI] [PubMed] [Google Scholar]
- 4.Chambers, T. J., C. S. Hahn, R. Galler, and C. M. Rice. 1990. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 44:649-688. [DOI] [PubMed] [Google Scholar]
- 5.Chen, J. W., W. Pan, M. P. D'Souza, and J. T. August. 1985. Lysosome-associated membrane proteins: characterization of LAMP-1 of macrophage P388 and mouse embryo 3T3 cultured cells. Arch. Biochem. Biophys. 239:574-586. [DOI] [PubMed] [Google Scholar]
- 6.Coffey, G., J. Fox, S. Pippig, S. Palmieri, B. Reitz, M. Gonzales, A. Bakshi, J. Padilla-Eagar, and P. Fielder. 2005. Tissue distribution and receptor-mediated clearance of anti-cd11a antibody in mice. Drug. Metab. Dispos. 33:623-629. [DOI] [PubMed] [Google Scholar]
- 7.Couvelard, A., P. Marianneau, C. Bedel, M. T. Drouet, F. Vachon, D. Henin, and V. Deubel. 1999. Report of a fatal case of dengue infection with hepatitis: demonstration of dengue antigens in hepatocytes and liver apoptosis. Hum. Pathol. 30:1106-1110. [DOI] [PubMed] [Google Scholar]
- 8.Crooks, A. J., J. M. Lee, L. M. Easterbrook, A. V. Timofeev, and J. R. Stephenson. 1994. The NS1 protein of tick-borne encephalitis virus forms multimeric species upon secretion from the host cell. J. Gen. Virol. 75:3453-3460. [DOI] [PubMed] [Google Scholar]
- 9.Despres, P., M. P. Frenkiel, and V. Deubel. 1993. Differences between cell membrane fusion activities of two dengue type-1 isolates reflect modifications of viral structure. Virology 196:209-219. [DOI] [PubMed] [Google Scholar]
- 10.Dunoyer-Geindre, S., E. K. O. Kruithof, B. Galve-de Rochemonteix, C. Rosnoblet, J. Gruenberg, G. Reber, and P. de Moerloose. 2001. Localization of β2-glycoprotein 1 in late endosomes of human endothelial cells. Thromb. Haemost. 85:903-907. [PubMed] [Google Scholar]
- 11.Falconar, A. K. I. 1997. The dengue virus nonstructural-1 protein (NS1) generates antibodies to common epitopes on human blood clotting, integrin/adhesin proteins and binds to human endothelial cells: potential implications in haemorrhagic fever pathogenesis. Arch. Virol. 142:897-916. [DOI] [PubMed] [Google Scholar]
- 12.Falconar, A. K. I., and P. R. Young. 1990. Immunoaffinity purification of native dimer forms of the flavivirus nonstructural glycoprotein NS1. J. Virol. Methods 30:323-332. [DOI] [PubMed] [Google Scholar]
- 13.Falgout, B., and L. Markoff. 1995. Evidence that flavivirus NS1-NS2a cleavage is mediated by a membrane-bound host protease in the endoplasmic reticulum. J. Virol. 69:7232-7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flamand, M., F. Megret, M. Mathieu, J. Lepault, F. A. Rey, and V. Deubel. 1999. Dengue-1 non structural glycoprotein NS1 is secreted from mammalian cells as a soluble hexamer in a glycosylation-dependent fashion. J. Virol. 73:6104-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fredericksen, B. L., B. L. Wei, J. Yao, T. Luo, and J. V. Garcia. 2002. Inhibition of endosomal/lysosomal degradation increases the infectivity of human immunodeficiency virus. J. Virol. 76:11440-11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldenthal, K. L., K. Hedman, J. W. Chen, J. T. August, P. Vihko, I. Pastan, and M. C. Willingham. 1988. Pre-lysosomal divergence of alpha 2-macroglobulin and transferrin: a kinetic study using a monoclonal antibody against a lysosomal membrane glycoprotein (LAMP-1). J. Histochem. Cytochem. 36:391-400. [DOI] [PubMed] [Google Scholar]
- 17.Gruenberg, J. 2001. The endocytic pathway: a mosaic of domains. Nat. Rev. Mol. Cell. Biol. 2:721-730. [DOI] [PubMed] [Google Scholar]
- 18.Gubler, D. 2002. The global emergence/resurgence of arboviral disease as public health problems. Arch. Med. Res. 33:330-342. [DOI] [PubMed] [Google Scholar]
- 19.Halstead, S. B. 1970. Observations related to pathogenesis of dengue hemorrhagic fever. VI. Hypothsesis and discussion. Yale J. Biol. Med. 42:350-362. [PMC free article] [PubMed] [Google Scholar]
- 20.Heinz, F. X., K. Stiasny, and S. L. Allison. 2004. The entry machinery of flaviviruses. Arch. Virol. Suppl. 18:133-137. [DOI] [PubMed] [Google Scholar]
- 21.Hilgard, P., and R. Stockert. 2000. Heparan sulfate proteoglycans initiate dengue virus infection of hepatocytes. Hepatology 32:1069-1077. [DOI] [PubMed] [Google Scholar]
- 22.Jackle, S., E. A. Runquist, S. Miranda-Brady, and R. J. Havel. 1991. Trafficking of the epidermal growth factor receptor and transferrin in three hepatocytic endosomal fractions. J. Biol. Chem. 266:1396-1402. [PubMed] [Google Scholar]
- 23.Jacobs, M. G., P. J. Robinson, C. Bletchly, J. M. Mackenzie, and P. R. Young. 2000. Dengue virus nonstructural protein 1 is expressed in a glycosyl-phosphatidyl-linked form that is capable of signal transduction. FASEB J. 14:1603-1610. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs, S. C., J. R. Stephenson, and G. W. Wilkinson. 1992. High-level expression of the tick-borne encephalitis virus NS1 protein by using an adenovirus-based vector: protection elicited in a murine model. J. Virol. 66:2086-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi, T., E. Stang, K. S. Fang, P. Moerloose, R. G. Parton, and J. Gruenberg. 1998. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature 392:193-197. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi, T., K. Startchev, A. Whitney, and J. Gruenberg. 2001. Localization of lysobiphosphatidic acid-rich membrane domains in late endosomes. Biol. Chem. 382:483-485. [DOI] [PubMed] [Google Scholar]
- 27.Koonin, E. V. 1993. Computer-assisted identification of a putative methyltransferase domain in NS5 protein of flaviviruses and lambda 2 protein of reovirus. J. Gen. Virol. 74:733-740. [DOI] [PubMed] [Google Scholar]
- 28.Kosugi, I., H. Muro, H. Shirasawa, and I. Ito. 1992. Endocytosis of soluble IgG immune complex and its transport to lysosomes in hepatic sinusoidal endothelial cells. J. Hepatol. 16:106-114. [DOI] [PubMed] [Google Scholar]
- 29.Kuo, C. H., D. I. Taï, C. S. Chang-Chien, C. K. Lan, S. S. Chiou, and Y. F. Liaw. 1992. Liver biochemical tests and dengue fever. Am. J. Trop. Med. Hyg. 47:265-270. [DOI] [PubMed] [Google Scholar]
- 30.Kurane, I., B. L. Innis, S. Nimmannitya, A. Nisalak, A. Meager, and F. A. Ennis. 1991. Activation of T-lymphocytes in dengue virus infection. High levels of soluble interleukin 2 receptor, soluble CD4, soluble CD8, interleukin 2, and interferon-gamma in sera of children with dengue. J. Clin. Investig. 88:1473-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lei, H. Y., T. M. Yeh, H. S. Liu, Y. S. Lin, S. H. Chen, and C. C. Liu. 2001. Immunopathogenesis of dengue virus infection. J. Biomed. Sci. 8:377-388. [DOI] [PubMed] [Google Scholar]
- 32.Libraty, D. H., P. R. Young, D. Pickering, T. P. Endy, S. Kalayanarooj, S. Green, D. W. Vaughn, A. Nisalak, F. A. Ennis, and A. L. Rothman. 2002. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J. Infect. Dis. 186:1165-1168. [DOI] [PubMed] [Google Scholar]
- 33.Lin, C., S. Chiu, Y. Hsiao, S. Wan, H. Lei, A. Shiau, H. Liu, T. Yeh, S. Chen, C. Liu, and Y. Lin. 2005. Expression of cytokine, chemokine, and adhesion molecules during endothelial cell activation induced by antibodies against dengue virus nonstructural protein 1. J. Immunol. 174:395-403. [DOI] [PubMed] [Google Scholar]
- 34.Lin, C. F., H. Y. Lei, C. C. Liu, H. S. Liu, T. M. Yeh, S. H. Chen, and Y. S. Lin. 2003. Antibodies from dengue patient cross-react with endothelial cells and induce damage. J. Med. Virol. 69:82-90. [DOI] [PubMed] [Google Scholar]
- 35.Lin, C. F., H. Y. Lei, A. L. Shiau, H. S. Liu, T. M. Yeh, S. H. Chen, C. C. Liu, S. C. Chiu, and Y. S. Lin. 2002. Endothelial cell apoptosis induced by antibodies against dengue virus nonstructural protein 1 via production of nitric oxyde. J. Immunol. 169:657-664. [DOI] [PubMed] [Google Scholar]
- 36.Lin, Y. L., C. C. Liu, J. I. Chuang, H. Y. Lei, T. M. Yeh, Y. S. Lin, Y. H. Huang, and H. S. Liu. 2000. Involvement of oxidative stress, NF-Il-6, and RANTES expression in dengue-2 virus infected human liver cells. Virology 276:114-126. [DOI] [PubMed] [Google Scholar]
- 37.Lin, Y. L., C. C. Liu, H. Y. Lei, T. M. Yeh, Y. S. Lin, R. M. Y. Chen, and H. S. Liu. 2000. Infection of five human liver cell lines by dengue-2 virus. J. Med. Virol. 60:425-431. [DOI] [PubMed] [Google Scholar]
- 38.Lindenbach, B. D., and C. M. Rice. 2003. Molecular biology of flaviviruses. Adv. Virus Res. 59:23-61. [DOI] [PubMed] [Google Scholar]
- 39.Lindenbach, B. D., and C. M. Rice. 1997. trans-Complementation of yellow fever virus NS1 reveals a role in early RNA replication. J. Virol. 71:9608-9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mackenzie, J. M., M. K. Jones, and P. R. Young. 1996. Immunolocalization of the dengue virus nonstructural glycoprotein NS1 suggests a role in viral RNA replication. Virology 220:232-240. [DOI] [PubMed] [Google Scholar]
- 41.Mallard, F., C. Antony, D. Tenza, J. Salamero, B. Goud, and L. Johannes. 1998. Direct pathway from early/recycling endosome to the Golgi apparatus revealed through the study of Shiga toxin B-fragment transport. J. Cell Biol. 143:973-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marianneau, P., A. Cardona, L. Edelman, V. Deubel, and P. Desprès. 1997. Dengue virus replication in human hepatoma cells activates NF-kappa B which in turn induces apoptotic cell death. J. Virol. 71:3244-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsuo, H., J. Chevallier, N. Mayran, I. Le Blanc, C. Ferguson, J. Faure, N. S. Blanc, S. Matile, J. Dubochet, R. Sadoul, R. G. Parton, F. Vilbois, and J. Gruenberg. 2004. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science 303:531-534. [DOI] [PubMed] [Google Scholar]
- 44.Mohan, B., A. K. Patwari, and V. K. Anand. 2000. Hepatic dysfunction in childhood dengue infection. J. Trop. Pediatr. 46:40-43. [DOI] [PubMed] [Google Scholar]
- 45.Mukhopadhyay, S., R. J. Kuhn, and M. G. Rossmann. 2005. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 3:13-22. [DOI] [PubMed] [Google Scholar]
- 46.Muylaert, I. R., R. Galler, and C. M. Rice. 1997. Genetic analysis of yellow fever virus NS1 protein: identification of a temperature-sensitive mutation which blocks RNA accumulation. J. Virol. 71:291-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nimmannitya, S. 1993. Clinical manifestations of dengue/dengue haemorrhagic, p. 48-54. In Monograph on dengue/dengue haemorrhagic fever. W.H.O. Regional Office for South-East Asia, New Delhi. World Health Organization, Geneva, Switzerland.
- 48.Pelkmans, L., J. Kartenbeck, and A. Helenius. 2002. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3:473-483. [DOI] [PubMed] [Google Scholar]
- 49.Phuong, U., and I. R. Nabi. 2003. Distinct caveolae-mediated endocytic pathways target the Golgi apparatus and the endoplasmic reticulum. J. Cell. Sci. 116:1059-1071. [DOI] [PubMed] [Google Scholar]
- 50.Pillay, C. S., E. Elliott, and C. Dennison. 2002. Endolysosomal proteolysis and its regulation. Biochem. J. 363:417-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piper, R. C., and J. P. Luzio. 2001. Late endosomes: sorting and partitioning in multivesicular bodies. Traffic 2:612-621. [DOI] [PubMed] [Google Scholar]
- 52.Ramadori, G., and T. Armbrust. 2001. Cytokines in the liver. Eur. J. Gastroenterol. Hepatol. 13:777-784. [DOI] [PubMed] [Google Scholar]
- 53.Rigau-Pérez, J., G. Clark, D. Gubler, P. Reiter, E. Sanders, and A. Vorndam. 1998. Dengue and dengue haemorrhagic fever. Lancet 352:971-977. [DOI] [PubMed] [Google Scholar]
- 54.Rijken, D., M. Otter, J. Kuiper, and T. van Berkel. 1990. Receptor-mediated endocytosis of tissue-type plasminogen activator (t-PA) by liver cells. Thromb. Res. Suppl. 10:63-71. [DOI] [PubMed] [Google Scholar]
- 55.Rothman, A. L. 2004. Dengue: defining protective versus pathologic immunity. J. Clin. Investig. 113:946-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salonen, A., T. Ahola, and L. Kaariainen. 2005. Viral RNA replication in association with cellular membranes. Curr. Top. Microbiol. Immunol. 285:139-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sutherland, R., D. Delia, C. Schneider, R. Newman, J. Kemshead, and M. Greaves. 1981. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc. Natl. Acad. Sci. USA 78:4515-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tjelle, T. E., A. Brech, L. K. Juvet, G. Griffiths, and T. Berg. 1996. Isolation and characterization of early endosomes, late endosomes and terminal endosomes: their role in protein degradation. J. Cell. Sci. 109:2905-2914. [DOI] [PubMed] [Google Scholar]
- 59.Ulloa, F., and F. X. Real. 2003. Benzyl-N-acetyl-alpha-D-galactosaminide induces a storage disease-like phenotype by perturbing the endocytic pathway. J. Biol. Chem. 278:12374-12383. [DOI] [PubMed] [Google Scholar]
- 60.Weigel, P. H., B. L. Clarke, and J. A. Oka. 1986. The hepatic galactosyl receptor system: two different ligand dissociation pathways are mediated by distinct receptor populations. Biochem. Biophys. Res. Commun. 140:43-50. [DOI] [PubMed] [Google Scholar]
- 61.Wengler, G., G. Czaya, P. M. Färber, and J. H. Hegemann. 1991. In vitro synthesis of West Nile virus proteins indicates that the amino terminal fragment of the NS3 protein contain the active centre multiple basic amino acids. J. Gen. Virol. 72:851-858. [DOI] [PubMed] [Google Scholar]
- 62.Westaway, E. G., J. M. Mackenzie, and A. A. Khromykh. 2002. Replication and gene function in Kunjin virus. Curr. Top. Microbiol. Immunol. 267:323-351. [DOI] [PubMed] [Google Scholar]
- 63.Winkler, G., S. E. Maxwell, C. Ruemmler, and V. Stollar. 1989. Newly synthesized dengue-2 virus nonstructural protein NS1 is a soluble protein but becomes partially hydrophobic and membrane-associated after dimerization. Virology 171:302-305. [DOI] [PubMed] [Google Scholar]
- 64.Winkler, G., V. B. Randolph, G. R. Cleaves, T. E. Ryan, and V. Stollar. 1988. Evidence that the mature form of the flavivirus nonstructural protein NS1 is a dimer. Virology 162:187-196. [DOI] [PubMed] [Google Scholar]
- 65.Young, P., A. Paige, C. Bletchly, and W. Halloran. 2000. An antigen capture enzyme-linked immunosorbent assay reveals high levels of dengue virus protein NS1 in the sera of infected patients. J. Clin. Microbiol. 38:1053-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.