Abstract
In an in vitro assay employing reconstituted nuclei, importin 7 (IPO7) has been implicated in nuclear translocation of human immunodeficiency virus type 1 (HIV-1) cDNA. Using RNA interference technology, we inhibited expression of IPO7 by 80 to 95% in primary macrophages and in HeLa cells and monitored their ability to support HIV-1 and simian immunodeficiency virus (SIV) cDNA synthesis, nuclear translocation, and infection efficiency. Marked IPO7 deficiency did not alter the rate or extent of HIV-1 or SIV cDNA synthesis or nuclear translocation. The infection efficiency of HIV-1 was similarly unaltered. Therefore, in natural, nondividing targets of HIV-1, IPO7 may be dispensable for infection.
Following infection of the cell by lentiviruses and retroviruses, viral cDNA is synthesized within a reverse transcription complex (RT complex). The RT complex containing nascent viral cDNA is subsequently translocated to the host cell nucleus, where viral cDNA integrates into cellular DNA. A striking feature of primary lentiviruses, such as human immunodeficiency virus type 1 (HIV-1), is their ability to infect nondividing cells (3, 9, 12). This feature requires that the viral RT complex traverse the intact nuclear envelope prior to contacting cellular DNA. While several virion proteins with karyophilic properties are present in the RT complex, there is no consensus as to which of these proteins directs nuclear translocation (2, 6, 7). Presumably, virion proteins contained within the RT complex interact with proteins of the cellular nuclear import apparatus, but the nature of the cellular import receptors that regulate nuclear import of HIV-1 cDNA are not known.
Importin 7 (IPO7) is a nuclear import receptor which can form a heterodimer with importin β and has been shown to participate in import of histone H1 (1, 8). A recent investigation of the mechanism of the HIV-1 RT complex nuclear import has identified IPO7 as a potential mediator in an in vitro cell permeabilization nuclear import assay (5). IPO7 together with a Ran mix were sufficient to induce accumulation of RT complexes at the nuclear envelope, while importins 4, 5, 9, and 13 displayed little to no such activity.
The potential impact of this discovery led us to investigate the role of IPO7 in HIV-1 and simian immunodeficiency virus (SIV) nuclear import using a more biologically relevant experimental system. The advent of RNA inhibition (RNAi) technology has allowed the unique capability to examine the impact of specific protein targets on HIV-1 infection in living primary macrophages during the course of a natural infection process (4, 11). Utilizing this method, we could find no conditions under which loss of IPO7 in macrophages or HeLa cells affected HIV-1 or SIV nuclear import or infection.
We initially studied the effect of RNAi-mediated knockdown of IPO7 on HIV-1 infection of HeLa cells. Cells in a 24-well plate were transfected once a day for 2 days (Fig. 1A) with 60 pmol of one of two IPO7-specific small interfering RNA (siRNA) sequences (DNA target sequences 5′-GGCAGGTGTTATCTATCTG-3′ [Ambion 17721] or 5′-GATGGAGCCCTGCATATGA-3′ [IPO7-1395]; QIAGEN [5]) or a nonspecific control (NS; 5′-TTCTCCGAACGTGTCACGT-3′; QIAGEN or Ambion) siRNA in OptiMEM (Invitrogen) using Oligofectamine reagent (Invitrogen). Equivalent IPO7 knockdown and other data obtained were the same using either IPO7 siRNA; therefore, results were combined. The extent of IPO7 mRNA knockdown was determined on the day following transfection using RNA isolated with TRIzol reagent (Invitrogen). Real-time quantitative RT-PCR (Quantitect Sybr Green kit; QIAGEN) was performed using IPO7-specific primers (IPO7-941F, TGGCAATGTTTCCAAGGAGTA; IPO7-1143R, TATGGGGCTTCAGATTCTTCC), and mRNA equivalence was determined using DNA ligase 3 primers (LIG3-up, GGCTGGGAAGAGCTGGAAGATAAT; LIG3-dn, TGATCTGGGTCTTCGTGTTGTAGC).
FIG. 1.
HIV-1 nuclear import in HeLa cells. Nuclear import of VSV-G-pseudotyped HIV was analyzed in HeLa cells following siRNA-mediated IPO7 knockdown. (A) Experimental timeline showing that cells were transfected twice with siRNA and infected, and DNA or cells were taken for PCR or flow cytometric analysis, respectively. Western blotting shows IPO7 knockdown; numbers below lanes indicate the relative fraction of IPO7 protein remaining. Each lane was loaded with 20 μg total protein. FACS, flow-activated cell sorting; M, size marker. (B) Total DNA collected from cultures 20 h postinfection was analyzed for 2LTR circles/cell and late cDNAs/cell. No effect from IPO7 knockdown was observed. White bars, 2LTR circles; black bars, late cDNAs. (C) Magi cells were siRNA transfected before infection with serial dilutions of wild-type enveloped HIV. At high virus concentrations, cell death was considerable and accounts for lower Vmax values. Kinetic analysis of product formation was measured to determine Vmax. (D) NS control and IPO7-depleted HeLa cells infected with VSV-G-pseudotyped HIV/GFP and analyzed by flow cytometry 4 days postinfection. The proportion of GFP+ cells was greater in IPO7-deficient cells than in NS control cells.
Using this transfection scheme, IPO7 mRNA levels on the day following transfection were 13% of NS control transfected cells (data not shown). To assess protein levels, Western blotting was performed on protein extracts using a rabbit anti-IPO7 polyclonal antibody (5). Densitometry revealed that IPO7 protein knockdown was substantial, with 10% remaining on day 2, decreasing to <3% by day 4 (Fig. 1A).
Upon nuclear import of the RT complex, cellular activities cause circularization of a proportion of the viral DNA to form two-long-terminal-repeat (2LTR) circles. The quantity of 2LTR circles can be measured using real-time PCR as an indicator of nuclear import. Serum-starved HeLa cells were infected following IPO7 siRNA transfection (Fig. 1A) with vesicular stomatitis virus G protein (VSV-G)-pseudotyped HIV-1LAI, and total DNA was recovered 20 h postinfection using DNAzole. DNA was treated with DpnI to eliminate possible plasmid DNA carryover and subjected to real-time PCR in order to quantify 2LTR circles (forward primer, TAGACCAGATCTGAGCCTGGGA; reverse primer, GTAGTTCTGCCAATCAGGGAAG), late viral cDNA products (late cDNAs; forward primer, GGGAGCTCTCTGGCTAACT; reverse primer, GGATTAACTGCGAATCGTTC), and CCR5 gene copies (for cell equivalents) (10). The number of 2LTR circles/cell in IPO7 knockdown cells was 0.117/cell, compared to 0.103/cell in NS control cells, indicating loss of IPO7 did not influence the ability of the RT complex to be imported into the nucleus (Fig. 1B). Quantitation of late cDNAs showed that the amount of viral cDNA formed was similar between IPO7 knockdown (1.27/cell) and NS control (0.691/cell) cells and, therefore, a potential difference in LTR circles was not masked by a difference in infection.
A defect in RT complex nuclear import should also manifest as a decrease in infection efficiency. We measured the infection efficiency of wild-type enveloped and VSV-G-pseudotyped HIV-1 in Magi cells and HeLa cells, respectively. In Magi cells, IPO7 was knocked down before infection with HIV-1LAI. After 2 days, β-galactosidase (β-Gal) expression was measured by kinetic analysis of substrate formation and Vmax was determined (Fig. 1C). The Vmax of IPO7-deficient cells was consistently greater than that of NS control cells over a greater than 2,000-fold range of multiplicities of infection (MOI), indicating IPO7 deficiency was not detrimental to Magi cell infection. To test infection by VSV-G-pseudotyped virus, we infected HeLa cells with HIV-1NL4-3/GFP carrying a green fluorescent protein (GFP) gene in place of nef as a marker for infected cells. IPO7 knockdown HeLa cells did not display an infection defect compared to NS control cells, with 30% versus 13% GFP+, respectively (Fig. 1D). These data indicate that loss of IPO7 did not impair infection by wild-type enveloped or VSV-G-pseudotyped HIV-1 in HeLa cells.
Macrophages are an important target of HIV-1 infection, in which active nuclear transport of the RT complex is essential (3, 12). IPO7 was knocked down in monocyte-derived macrophages seeded into 24-well plates with 180 pmol siRNA transfected once a day for 2 days (Fig. 1A) using Lipofectamine 2000 (Invitrogen). IPO7 mRNA levels the day after transfection as measured by real-time RT-PCR were 23% of NS control (data not shown). IPO7 protein level was 17% of NS control on day 2 and remained depleted through day 5 (Fig. 2A).
FIG. 2.
IPO7 RNAi in macrophages. Macrophages were transfected with NS control or IPO7 siRNA according to the scheme in Fig. 1A. (A) IPO7 Western blot assay showing knockdown of IPO7 for up to 4 days posttransfection. Numbers below lanes indicate relative fractions of IPO7 remaining. Each lane was loaded with 20 μg total protein. M, size marker. (B) Macrophages were transfected with NS control or IPO7 siRNA once a day for 2 days before infection with VSV-G-pseudotyped or wild-type enveloped HIV. Real-time PCR quantitation of viral DNA products was performed 20 h postinfection. Neither VSV-G-pseudotyped nor wild-type enveloped virus displayed altered 2LTR circle or late cDNA formation. (C) Kinetic analysis of 2LTR and late cDNA product formation in macrophages was performed starting at 24 h postinfection. Both 2LTR circles and late cDNA products were similar between NS control and IPO7 knockdown infections.
IPO7 RNAi was performed, macrophages were infected with either VSV-G-pseudotyped HIV-1LAI or wild-type enveloped HIV-1ADA, and total DNA was isolated 20 h after infection (Fig. 1A). In independent experiments, analysis of 2LTR circles found no significant difference in IPO7-depleted versus NS control macrophages (P > 0.4; n = 3) (Fig. 2B). Synthesis of late cDNAs in IPO7-depleted macrophages was also similar to NS control cells (P > 0.3; n = 3) (Fig. 2B).
IPO7 knockdown could have altered the kinetics of RT complex nuclear import, such that analysis of a single time point may not have shown an apparent phenotype. To address this possibility, we quantified 2LTR circles and late cDNAs at 24, 36, and 60 h following infection of IPO7-depleted or NS control macrophages with VSV-G-pseudotyped HIV-1LAI (Fig. 2C). The quantity of 2LTR circles and late cDNAs was approximately equivalent between NS control and IPO7-depleted macrophages at all time points tested, demonstrating that lack of a 2LTR circle phenotype in IPO7-depleted macrophages was not due to choice of an improper time point for analysis.
Similarly, when NS control and IPO7-depleted macrophages were infected with VSV-G-pseudotyped HIV-1NL4-3/GFP, no defect in infection as measured by flow cytometry of GFP was detected (Fig. 3). As observed in HeLa cells, IPO7-depleted macrophages tended toward a 2.5-fold-higher level of infection compared to NS control cells. Virus production in infected IPO7-depleted cultures was also slightly increased (data not shown). No defect in the context of a spreading infection of HIV-1ADA was observed in IPO7-depleted cultures carried for 7 days postinfection (data not shown).
FIG. 3.
Infection of macrophages. NS control and IPO7-depleted macrophages were infected with HIV expressing GFP and analyzed by flow cytometry 4 days postinfection. In each of three experiments, the proportion of GFP+ cells was greater in IPO7-deficient cells than in NS control cells.
These observations were extended to include SIV. IPO7 was knocked down in macrophages using a 2-day siRNA-transfection scheme (Fig. 1A). Macrophages were then infected with VSV-G-pseudotyped SIVPBj. Total DNA was isolated after 20 h, and a Taqman PCR was used to quantify late viral cDNAs, 2LTR circles, and CCR5 (genome equivalents). No differences in 2LTR circles/cell or late cDNAs were observed between IPO7 knockdown and NS control macrophages (Fig. 4). In NS control cells, we found 0.393 2LTR circles/cell, while IPO7-depleted cultures contained 0.552 2LTR circles/cell. Late cDNAs/cell were similar between NS control cells and IPO7-depleted cells, with 6.02/cell versus 6.68/cell, respectively. Analogous to our observations with HIV, these data show that the RT complex of SIV is not imported into the nucleus through an IPO7-dependent pathway.
FIG. 4.
SIV nuclear import in macrophages. Macrophages were infected with SIV following IPO7 knockdown and analyzed for RT complex nuclear import and formation of late cDNAs by real-time PCR. IPO7 knockdown did not affect 2LTR or late cDNA formation. White bars, 2LTR circles/cell; black bars, late cDNAs/cell.
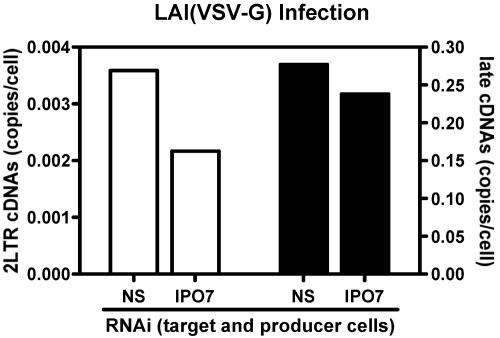
To rule out the possibility that RT complex nuclear import was mediated by IPO7 incorporated into virions during production, we transfected 293T cells with NS control or IPO7 siRNA and produced VSV-G-pseudotyped HIV-1LAI from cells exhibiting an IPO7 knockdown. A single siRNA transfection of 293T cells led to knockdown of IPO7 mRNA levels by 77% when measured at the time of virus collection. Virus derived from NS control and IPO7 knockdown producer cells was normalized by RT activity and used to infect macrophages that were transfected with NS or IPO7 siRNA (Fig. 2A).
Formation of 2LTR circles/cell and late viral cDNAs/cell measured 24 h postinfection showed similar levels between NS control and IPO7 knockdown producer-target cell combinations (Fig. 5). The quantity of 2LTR circles/cell in the IPO7-depleted system was 2.17 × 10−3, similar to the NS control system result of 3.59 × 10−3 2LTR circles/cell. Late cDNA formation was 1.30 × 10−2/cell, compared to 0.912 × 10−2/cell in NS control versus IPO7 knockdown producer-target cell combinations. Thus, potential incorporation of IPO7 protein into virions does not substitute for IPO7 deficiency in target cells.
FIG. 5.
Infection with virus from IPO7-deficient producer cells. NS control or IPO7-deficient macrophages were infected with HIV-1LAI produced from IPO7-deficient 293T cells. 2LTR circles and late viral cDNAs were measured 24 h postinfection by real-time PCR. Knockdown of IPO7 in producer cells in combination with target cells did not result in a nuclear import defect compared to NS control siRNA-transfected cells. White bars, 2LTR circles/cell; black bars, late cDNAs/cell.
Using the transfection scheme in Fig. 1A, a small amount of IPO7 protein was detectable for up to 4 days posttransfection (Fig. 2A). HIV nuclear import of the RT complex via IPO7 could still be accomplished by a small amount of remaining protein, essentially a threshold effect, rather than exhibiting a shallow dose response. To achieve a greater knockdown of IPO7 protein, we transfected macrophages every other day a total of three times (Fig. 6A). Levels of IPO7 protein were progressively reduced to maximum on day 5. Western blotting showed IPO7 protein levels to be 4.6% and 9.3% of NS control macrophages on days 5 and 6, respectively (Fig. 6A).
FIG. 6.
Extended RNAi in macrophages. Multiple IPO7 siRNA transfections over an extended period were used to achieve greater protein knockdown in macrophages. (A) siRNA transfection scheme, which resulted in severe IPO7 deficiency as illustrated in the IPO7 Western blot assay. Each lane was loaded with 20 μg total protein. M, size marker. (B) Macrophages undergoing extended IPO7 knockdown were infected with HIV, and real-time PCR was used to quantify 2LTR circles and late cDNAs. In three independent experiments, IPO7 knockdown did not result in impaired 2LTR circle or late cDNA formation.
Macrophages undergoing extended IPO7 RNAi were infected on day 5 with VSV-G-pseudotyped HIV-1LAI, and DNA was isolated 20 h postinfection. Real-time PCR quantitation of 2LTR circles/cell and late cDNAs/cell from three independent experiments failed to find significant differences between NS control and IPO7-depleted macrophages (P > 0.1; n = 3) (Fig. 6B). Even under these conditions of severe IPO7 deficiency in macrophages, no measurable defect in RT complex nuclear import was present.
Here we have described the study of IPO7 as a mediator of HIV-1 RT complex nuclear import in HeLa cells and macrophages. Although previously described as having a function in this process (5), we could detect no impairment to HIV-1 cDNA nuclear import following depletion of IPO7 protein using RNAi. Conditions tested included serum-starved HeLa cells, primary macrophages, VSV-G-pseudotyped and wild-type enveloped virus, virus produced from IPO7-deficient cells, and intense IPO7 knockdown. Using a direct assay to measure nuclear import, such as quantitation of 2LTR circles, or indirect assays to include infection measured by expression of a GFP gene from the provirus, no defect was observed.
We chose to investigate this issue using living macrophages undergoing bona fide viral infection, which may account for the differences with previous work. Although the in vitro cell permeabilization assay has the advantage of knowing precisely which karyopherins are part of the reaction mix, its artificial nature makes it difficult to reconcile complex interactions occurring in vivo. RNAi targeting of IPO7 permits analysis in the context of a functioning cell. Were IPO7 the dominant contributor to RT complex nuclear import, a defect in nuclear import or infection should be observed upon its depletion.
Our HeLa cell data are in contrast to those of Fassati et al. (5), who found decreased HIV infection of HeLa cells undergoing IPO7 RNAi. The assay used for measurement could account for these results: flow cytometry versus manual counting. Their result could also be explained by decreased cell viability of IPO7-deficient cells under the conditions employed, although those authors indicated their attempt to optimize knockdown to prevent cell death. In one experiment, they also observed a decrease in oncoretroviral infection, likely explained by the decreased cell proliferation observed. It was suggested that the effect on HIV infection was maximal at an MOI of 0.01, but the true contribution of MOI cannot be discerned, since only one MOI was presented. Our Magi assay data (Fig. 1C) address this by showing the difference between NS control and IPO7-depleted cells to be proportionally the same over a greater-than-2,000-fold range of MOI. Regardless, the significant finding in this work is that IPO7 alone is not sufficient for HIV nuclear import in macrophages.
In conclusion, investigation of HIV-1 and SIV RT complex nuclear import in HeLa cells and macrophages following IPO7 depletion failed to detect defects in nuclear import or productive infection. HIV-1 and SIV transport to the nucleus may involve other or additional karyopherins or an unconventional mechanism.
Acknowledgments
We thank A. Fassati (University College London Medical School) for kindly providing anti-IPO7 antibody.
This work was supported by NIH grants AI32890 and AI37475 and the University of Massachusetts Center for AIDS Research (AI042845). S.P.Z. is supported by a Kirschstein National Research Service Award (T32AIO7349-15).
REFERENCES
- 1.Bauerle, M., D. Doenecke, and W. Albig. 2002. The requirement of H1 histones for a heterodimeric nuclear import receptor. J. Biol. Chem. 277:32480-32489. [DOI] [PubMed] [Google Scholar]
- 2.Bukrinsky, M. I., S. Haggerty, M. P. Dempsey, N. Sharova, A. Adzhubel, L. Spitz, P. Lewis, D. Goldfarb, M. Emerman, and M. Stevenson. 1993. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature 365:666-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukrinsky, M. I., N. Sharova, M. P. Dempsey, T. L. Stanwick, A. G. Bukrinskaya, S. Haggerty, and M. Stevenson. 1992. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc. Natl. Acad. Sci. USA 89:6580-6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elbashir, S. M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494-498. [DOI] [PubMed] [Google Scholar]
- 5.Fassati, A., D. Gorlich, I. Harrison, L. Zaytseva, and J. M. Mingot. 2003. Nuclear import of HIV-1 intracellular reverse transcription complexes is mediated by importin 7. EMBO J. 22:3675-3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallay, P., T. Hope, D. Chin, and D. Trono. 1997. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc. Natl. Acad. Sci. USA 94:9825-9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinzinger, N. K., M. I. Bukinsky, S. A. Haggerty, A. M. Ragland, V. Kewalramani, M. A. Lee, H. E. Gendelman, L. Ratner, M. Stevenson, and M. Emerman. 1994. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc. Natl. Acad. Sci. USA 91:7311-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jakel, S., W. Albig, U. Kutay, F. R. Bischoff, K. Schwamborn, D. Doenecke, and D. Gorlich. 1999. The importin beta/importin 7 heterodimer is a functional nuclear import receptor for histone H1. EMBO J. 18:2411-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis, P. F., and M. Emerman. 1994. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J. Virol. 68:510-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharkey, M. E., I. Teo, T. Greenough, N. Sharova, K. Luzuriaga, J. L. Sullivan, R. P. Bucy, L. G. Kostrikis, A. Haase, C. Veryard, R. E. Davaro, S. H. Cheeseman, J. S. Daly, C. Bova, R. T. Ellison III, B. Mady, K. K. Lai, G. Moyle, M. Nelson, B. Gazzard, S. Shaunak, and M. Stevenson. 2000. Persistence of episomal HIV-1 infection intermediates in patients on highly active anti-retroviral therapy. Nat. Med. 6:76-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song, E., S. K. Lee, D. M. Dykxhoorn, C. Novina, D. Zhang, K. Crawford, J. Cerny, P. A. Sharp, J. Lieberman, N. Manjunath, and P. Shankar. 2003. Sustained small interfering RNA-mediated human immunodeficiency virus type 1 inhibition in primary macrophages. J. Virol. 77:7174-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinberg, J. B., T. J. Matthews, B. R. Cullen, and M. H. Malim. 1991. Productive human immunodeficiency virus type 1 (HIV-1) infection of nonproliferating human monocytes. J. Exp. Med. 174:1477-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]