Abstract
Tadpoles (Xenopus laevis) have a lateral line system whose anatomical structure has been described, but whose functional significance has not been closely examined. These experiments tested the hypothesis that the lateral line system is involved in rheotaxis. Tadpoles in developmental stages 47–56 oriented toward the source of a water current. Orientation was less precise after treatment with cobalt chloride or streptomycin, but was similar to that of untreated animals after exposure to gentamicin. In no current conditions, tadpoles exhibited a characteristic head-down posture by which they held themselves in the water column at an angle around 45°. This body posture became significantly less tilted in the presence of water current. Treatment with cobalt chloride or streptomycin increased the angle of tilt close to that seen in no current conditions, while gentamicin treatment tended to decrease tilt angle. The data are consistent with anatomical and physiological findings that tadpole neuromasts are similar to superficial, but not canal, neuromasts in fishes, and they suggest that the lateral line system is involved in both directional current detection and current-related postural adjustments in Xenopus.
Keywords: African clawed frog, Lateral line, Rheotaxis, Tadpole, Xenopus
Introduction
Rheotaxis, orientation with respect to a water current, is a behavior characteristic of many different species of fishes (Montgomery et al. 1997; Baker and Montgomery 1999; Vogel and Bleckmann 2000; Kanter and Coombs 2003). Detection of water currents is clearly of relevance for aquatic species, aiding in such important behaviors as station holding, localization and identification of objects (such as food), schooling, and upstream migration.
There has been some debate over which sensory system mediates rheotaxis. Initially, visual cues were believed to be paramount in rheotaxis, as orientation of some species of pelagic fish was more accurate when a fixed visual external reference point was provided (Arnold 1974). More recent research using benthic, semipelagic, and cryopelagic species and conducted in the absence of visual cues (Montgomery et al. 1997; Baker and Montgomery 1999) suggest that the lateral line system is also crucial for providing information on the direction of water currents. Treatment with cobalt chloride (CoCl2), which acts as a competitive calcium channel blocker (Karlsen and Sand 1987), affects both superficial and canal neuromasts of the fish lateral line, and increases the behavioral threshold for rheotaxis (Montgomery et al. 1997; Baker and Montgomery 1999). Treatment with the aminoglycoside antibiotic streptomycin, which damages hair cells in both canal and superficial neuromasts (Wersäll and Flock 1964), also increases rheotactic threshold. In contrast, treatment with the aminoglycoside antibiotic gentamicin sulfate, which damages ciliary bundles of canal neuromasts while leaving those of superficial neuromasts unaffected (Song et al. 1995), has little effect on rheotaxis. If superficial neuromasts are selectively ablated, then rheotaxis is again disrupted (Montgomery et al. 1997; Baker and Montgomery 1999). These data suggest that rheotaxis in fishes is mediated predominately by superficial neuromasts, consistent with the role of these organs in sensing net velocity (Coombs and Janssen 1990). Canal neuromasts, on the other hand, sense net acceleration and are involved in orienting responses to higher-frequency disturbances (Coombs et al. 2001).
Little is known about the behavioral function of the lateral line system in aquatic amphibians. Adult African clawed frogs, Xenopus laevis, turn towards the source of surface water waves, but the degree to which this behavior depends on an intact lateral line is not clear (Elepfandt 1982; Claas et al. 1993; Claas and Münz 1996). In some experiments, good behavioral accuracy was maintained even after large numbers of peripheral neuromasts were lesioned (Elepfandt 1982), while in other experiments, performance decreased significantly after lesion-induced damage (Görner et al. 1984; Claas et al. 1993). Inactivation of the entire lateral line system or of groups of neuromasts with CoCl2 resulted in decreased accuracy of the turning response, although the behavior was not abolished completely in all animals (Claas et al. 1993; Claas and Münz 1996). The lateral line organs of adult Xenopus have been characterized as similar to superficial neuromasts of teleost fish on the basis of their anatomy and physiological responses (Kroese et al. 1978; Elepfandt 1996). This then predicts that, in Xenopus, streptomycin and gentamicin should have differing effects on rheotaxis and on orientation to low- and high-frequency water disturbances.
Larval Xenopus possess a lateral line system that undergoes structural changes across metamorphic development (Shelton 1970, 1971; Mohr and Görner 1996; Winklbauer 1989). Little is known about the behavioral changes that parallel these anatomical rearrangements. A functioning lateral line system is necessary for regulating the geometry of tadpole schools (Katz et al. 1981). If the neuromasts are pharmacologically blocked with streptomycin, tadpoles will position themselves closer to each other than they do under control conditions (Lum et al. 1982). Shelton (1971) observed that Xenopus tadpoles orient towards a water current source, but concluded that this behavior was not mediated by the lateral line, as animals with severed lateral line nerves were “able to face the current in a normal fashion.” However, no criteria for what constituted “normal fashion” were provided, tadpole developmental stage was not specified, rate of current flow was not specified, and quantitative data were not presented. Shelton (1971) did find, however, that turning responses to localized jets of current were abolished in animals with severed lateral lines. Given the importance of the lateral line system in mediating rheotaxis in fishes and wave localization in adult Xenopus, this apparent lack of lateral line influence on tadpole orientation towards a water source is surprising, and worth further investigation.
Under static pressure conditions in a laboratory, Xenopus tadpoles typically hang suspended in the water column, floating head downward (). They maintain this position by swimming downward against their own buoyancy (Wassersug and Souza 1990). It is not clear what sensory systems are involved in mediating this posture, or how this posture changes under conditions of water flow. Van Bergeijk (1959) attributed this “hydrostatic balancing mechanism” to the functioning of the bronchial diverticula, which anatomically link the lungs and the saccule in the inner ear. Wassersug and Souza (1990) argued that the ability of tadpoles to hang suspended in this manner indicates that they are aware of their position and the direction of water movement, but they refuted the hypothesis that the bronchial diverticula mediated this behavior. If this posture relies on sensing of water movement, then it is possible that the lateral line system, rather than or in addition to the inner ear, is involved in its maintenance.
Predicting how and why Xenopus tadpole orientation and position in the water column might change in the presence of current is the main purpose of this experiment. It is possible that the hydrostatic balancing reflex of Van Bergeijk (1959) and the orientation to jets of current observed by Shelton (1971) are mediated by different sensory systems; if so, the separate contributions of these different systems may be observed in response to pharmacological treatments that affect one system rather than another. The goal of this experiment is to examine the possible sensory bases of sensitivity to current flow using methods previously established in research on fish rheotaxis (Montgomery et al. 1997; Baker and Montgomery 1999; Kanter and Coombs 2003), with the goal of developing a behavioral measure of sensory maturation in these larval animals.
Materials and methods
Animals
Xenopus laevis tadpoles (stages 37–66; Nieuwkoop and Faber 1994) and adults (4–5 cm snout-vent length) were obtained from NASCO (Fort Atkinson, Wis., USA). Animals were housed in aquaria filled with aerated water and fed frog brittle ad libitum. The colony room was maintained on a 12:12 light:dark cycle with an ambient temperature of 22–25°C. Each animal was tested in only one pharmacological condition (drug or no drug). A subset of animals, both untreated and drug-treated, was tested in both current and no current conditions. After testing, tadpoles were euthanized by immersion in 0.15% tricaine methanesulfonate (MS-222) for 15 min; a subset of animals was preserved in 0.4% paraformaldehyde in 1×PBS (pH 7.4) for anatomical verification of neuromast damage.
Equipment
Animals were tested in a rectangular Plexiglas laminar flow tank (105×15×20 cm), based on the design of Vogel and LaBarbera (1978), and similar to that used by Kanter and Coombs (2003). The tank was designed to produce a constant, laminar water flow of adjustable velocity, produced by a rotating propeller motor with adjustable control, mounted on a separate platform at the downstream end of the tank. Water flowed through the tank by means of two circular openings at each end, connected together by a circular (10 cm diameter) PVC return tube. This sort of motor not only pushes the water axially, but it also induces a circular component of flow (Vogel and LaBarbera 1978). Two Plexiglas collimators were installed in series at the upstream end of the tank to prevent turbulence in the flow and to keep it moving in a straight manner. Tadpoles were placed into a test area (15×76 cm) bounded by the second collimator and by a mesh screen on the downstream side (to prevent the animals from being drawn into the motor). A 1 cm×1 cm grid was attached to the back of the tank for angular reference. Prior to each testing session, the tank was filled to a depth of 15 cm with room temperature water treated with calcium-free aquarium conditioner.
Flow rates were estimated by dropping food coloring into a 14-cm section of the tank at the approximate location of tadpoles from the tank bottom, and videotaping the dye’s movement as it traveled from one video frame to the next. For pilot experiments, tadpoles were tested under two flow rates, 1 and 2 cm s−1. Tadpoles were often unable to hold their position in the current at higher rates. There was no behavioral difference in rheotactic response under these two conditions, so a flow rate of 1 cm s−1 was used for actual testing.
Procedure
Experiments were conducted once in 2000 and replicated in 2002, with some modifications to the procedure. In 2000, tadpoles were placed individually into the tank when current was already flowing, allowed to acclimate for 1 min, and then data were recorded for 5 min. Each animal was tested only once, in no current, untreated (current, no drug) or one of three drug conditions (between subjects design). To account for the role of visual cues produced by room illumination, different animals were tested under ambient light and under red light (25-W light bulbs), to which the retina of Xenopus is not sensitive (Jaeger and Hailman 1976; Elepfandt 1996). In 2002, individual or pairs of tadpoles were placed into the tank with no current flow, allowed to acclimate for 2 min, and then current was turned on. Behavior was monitored for 3–5 min after current onset. Untreated and drug-treated animals were tested under both no current and current conditions, providing both between subjects and within subjects comparisons. All trials were conducted under red light. Behavior was videotaped (3–14×magnification) using a Panasonic Palmcorder IQ (model PV-IQ405) camera, mounted on a tripod approximately 30 cm from and perpendicular to the long side of the tank.
In one group of tadpoles, the lateral line system was chemically altered by exposure to CoCl2, using a procedure adapted from Karlsen and Sand (1987). Individual tadpoles were immersed for 1 h in a 1 mmol l−1, calcium free solution of CoCl2 (adjusted with 0.5 N NaOH to produce a final pH 7.2). This concentration and exposure time is less than half that used in behavioral studies of fish rheotaxis (3 h exposure to 2 mmol l−1; Montgomery et al. 1997; Baker and Montgomery 1999). Karlsen and Sand (1987) recommended a concentration of 0.1 mmol l−1 for exposures of 12–24 h. Because Co2+ is highly toxic (Janssen 2000), its potency is an important issue to consider. The concentration we used was chosen from pilot studies in which tadpole behavior in the absence of current flow was monitored after exposure to different concentrations of CoCl2 for different exposure times. At concentrations higher than the 1 mmol l−1 dose, or after longer exposure times to this dose, tadpoles began to swim erratically or lay flat on the tank floor; several also died. At the concentration we used, the behavior of treated tadpoles in their home tanks was qualitatively indistinguishable from that of untreated animals. In addition, concentrations were adjusted downward to account for the smaller size of tadpoles (15–30 mm) compared to adult fish (>40 mm used by Montgomery et al. 1997). After immersion in the solution for 1 h, tadpoles were rinsed off for 1 min in distilled water, and then introduced into the flow tank. To examine the potentially reversible effects of cobalt treatment, a subset of these animals was tested again after a 1-week recovery time. A second group of tadpoles was exposed to streptomycin treatment, at the same concentration (0.13 g l−1, pH 6.5) used by Lum et al. (1982) in their studies of Xenopus schooling behavior. Animals were immersed for 1 h in this solution, and were rinsed off in distilled water for 1 min before testing. Montgomery et al. (1997) exposed fish to a solution of 0.5 g l−1 streptomycin for 3 h. Because Lum et al. (1982) reported a significant increase in the distance between tadpoles in schools after only 18 min of exposure, we opted not to use the longer exposure time used by Montgomery et al. (1997) and to keep the streptomycin exposure time equal to the CoCl2 exposure time. A third group of tadpoles was exposed to a 0.004 g l−1 solution of gentamicin sulfate for 20 h, a concentration twice as potent as that used by Montgomery et al. (1997) but for less than half the exposure time. Concentration and exposure time were chosen on the basis of pilot experiments as described above. A subset of untreated animals was taken outside the home tank and immersed in aquarium water for 20 h as a control.
Data analysis
Videotaped data from each animal were digitized and analyzed using Dazzle MGI Videowave software (version 4.0) running on a Pentium 4 computer. Data were cut into 1-s-long “clips”, made every 10 s throughout the observation interval, which were then exported using Adobe Premiere 6.0 into KA Video software (Bob Schleihauf, San Francisco State University, version 5.91). Data were extracted using the program KA2D by two individuals unaware of both the hypothesis being tested and of any drug treatment condition.
Two different behavioral measures were obtained for each animal under each condition. These were orientation angles (the direction the tadpole faced in the tank, regardless of its degree of tilt) and tilt angle (the angle at which the tadpole hung suspended in the water column). Both angles were measured by reference to two fixed points on the tadpole’s body: the eye and the gut (intestines). Xenopus tadpoles at the stages of development tested here are transparent; on the digitized images, the eye and the intestines both appeared black and easily distinguishable (Fig. 1). The orientation angle was defined as the direction that the tadpole faced with respect to the current source. It was estimated as 0° (facing directly upstream with the long axis of the body parallel to the stream direction), 90° (facing either long sides of the tank, perpendicular to the current flow), and 180° (facing directly away from the source of the current). Because a vertical (overhead) camera was not used, it was not possible to ascertain with great precision if the animal was facing, for example, a 30° angle to the source of the current in the horizontal plane. In practice, orientation angles of up to 45° away from the current source were coded as 0°, from 45 to 134° as 90°, and from 135 to 225° as 180°. Thus, if only one of the tadpole’s eyes was visible on the videotape, orientation angle was coded as either 0 or 180°, depending on the position of the snout with respect to the current flow. If both eyes were visible, or if neither eye but the tail was visible, then orientation angle was coded as 90° (no distinction was made between orientations of 90° and 270°). For each individual animal, angle estimates were taken every 10 s for the duration of the observation period, and these angles were then averaged across the entire observation period to give one overall mean angle of orientation for that animal. Mean orientation angles for an individual could thus vary between 0 and 180°, such that angles greater than 0° indicate further deviation from “perfect” rheotaxis and greater variability in orientation throughout the observation interval. In addition, data were analyzed as the percent of animals in each condition facing towards the current source within some angular deviation, consistent with the quantification procedure used by Montgomery et al. (1997). Montgomery et al. (1997) used angular deviations of 0, 20 and 45°, depending on species; we chose the 45° criterion for our measure.
Fig. 1.
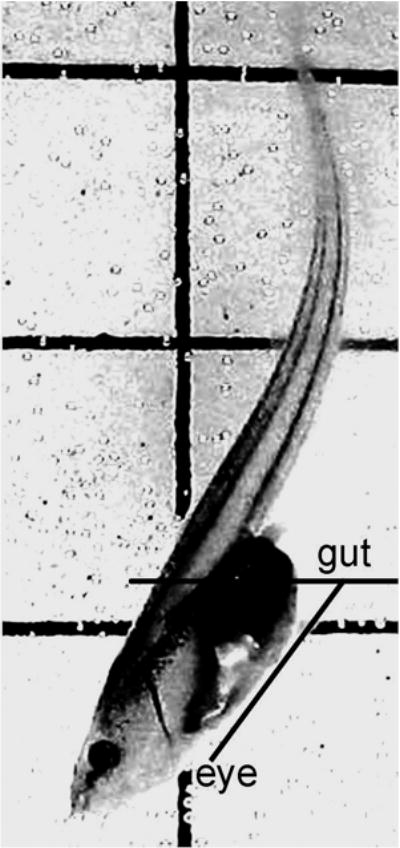
Digital image of a Xenopus laevis tadpole (stage 52) in the flow tank, in no current conditions. Grid lines on the back wall of the tank are used as references to assist measurements of tilt angle. To determine tilt angle, two lines were drawn through the digitized image of the tadpole, using tools in KA video software. One line was the diagonal connecting the center of the eye point to the center of the gut point, both of which appear black. This line was made to intersect a horizontal line drawn through the center of gut point, parallel to the reference grid lines and extending in the direction at which the animal’s snout pointed. The angle between these two lines was automatically measured in the software program, and termed the tilt angle
The tilt angle was defined as the angle between the horizontal plane and the straight line between the eye and intestine (Fig. 1). This angle varies between +0° (animal lying flat in the water or on the bottom of the tank) and +90° (animal’s body forms a perfect vertical line, with its snout perpendicular to the tank bottom). If the animal’s body forms a perfect vertical line with its snout perpendicular to the surface of tank, the tilt angle would be −90°. Tilt angles were measured every 10 s, and then averaged across the entire observation period to give one mean angle of tilt per animal.
For data collected in 2000, measurements were made for current conditions only, beginning 1 min after current onset for a duration of 5 min. For 2002 data, data for each animal were analyzed separately in each of two time periods—before the water current was turned on (2 min; pre-current) and after the water current was turned on (3–5 min; post-current). To parallel the analyses of the 2000 data, the first data point was taken 1 min after current onset; however, untreated animals adopted a stable orientation angle within the first 30 s of current onset, and there was no statistical difference between orientation angles at 30 s and 1 min. Results presented here are combined across the two replications of the experiment. Pre-current data from 2002 experiments were excluded from analyses of group effects so that data from an individual animal were represented only once (no current or current). Within-group analyses comparing behavior between pre-current and current conditions were performed on 2002 data only. Results from the 20-h control group did not differ from those of the other controls, so these data were combined for analyses. Statistical analyses (nonparametric tests to account for differences in variances between groups) were performed using SPSS version 11.5. Significance levels of post-hoc tests were adjusted using the Bonferroni procedure.
Confocal microscopy
The otic capsules of two tadpoles per treatment group were microdissected and irrigated with 0.9% saline solution to flush out the calcium carbonate crystal overlying the saccule to minimize autofluorescence. The skin was removed from the same animals with a scalpel in order to visualize any damage to the peripheral neuromasts. The samples were cold shocked in a freezer and then incubated in 175 μl of 0.9% saline and 20 μl horse serum for 20 min. The tissue was incubated with 5 μl Alexa Fluor 594 phalloidin (A-12381, Molecular Probes) for 20 min to visualize actin in the hair cells. Samples were whole-mounted on depression slides with Prolong Antifade (P-7481, Molecular Probes). A Zeiss 410 confocal laser scanning microscope with a krypton-argon laser was used to collect digital images (magnification 40×and 63×) of the fluorescent-labeled tissue, using the software program Renaissance (2.0.20, Microcosm). Images were stored as TIFF files and imported into Adobe Photoshop for illustrations. Intensity of label was quantified using ImageJ software by an individual unaware of the treatment condition.
Results
Rheotaxis was examined in untreated (normal) tadpoles at developmental stages 37–66 and adults. Because only animals within developmental stages 47–56 showed a pronounced tilting posture, drug treatments were confined to this group.
Orientation angle
All untreated tadpoles showed an unconditioned rheotactic response, orientation toward the source of the current, upon being subjected to current flow. Young tadpoles (stages 37–45; n = 18) showed a mean orientation angle of 25° in current conditions; 15 of these animals exhibited a stable orientation angle of 0° while the other three remained oriented within 45° of the current source throughout the observation period.
For untreated tadpoles between stages 47 and 56, mean orientation angle differed significantly between no current and current conditions (Mann–Whitney U = 71.5, P < 0.001; Table 1). All tadpoles tested with current were oriented within 45° of the source of the current throughout the observation period. Visual (diffuse illumination) cues did not have a significant effect on orientation. There were no differences in orientation angle under red light and ambient light conditions.
Table 1.
Orientation behavior of tadpoles (stages 47–56) in five different experimental groups
| Group | Orienting (%) | Mean orientation angle (range) | Median interval to orient | n |
|---|---|---|---|---|
| No current | 14 | 85 (0–180) | – | 28 |
| Untreated | 100 | 3 (0–36) | 1 | 28 |
| Cobalt | 62 | 33 (0–180) | 2 | 36 |
| Streptomycin | 45 | 37 (0–180) | 3 | 21 |
| Gentamicin | 76 | 14 (0–90) | 1 | 25 |
Each animal was tested only once. The untreated, cobalt-treated, streptomycin-treated and gentamicin-treated animals were all tested under current conditions. Tadpoles tested under no current conditions received no drug treatments. The percentage of animals in each group orienting within 45° of the current source is shown as % orienting. Mean orientation angle for each animal was calculated from 10 s intervals for a total of 3–5 min and then averaged across the entire group of animals in a particular group. Orientation angle and its range are in degrees. Orientation angle varied significantly between the five groups (see text). For each animal, the first observation interval (30-s bins) at which orientation remained at 0° for at least four intervals was calculated, and then used to calculate median interval to orient for that group. n number of tadpoles per group
Metamorphic climax tadpoles (stages 56–66; n = 15) were tested in both no current and current conditions. Under no current conditions, mean orientation angle was 115.5°, compared to 23.7° under current; this difference was statistically significant (Wilcoxon signed-ranks test: z = 2.8, P = 0.005). Two of the 15 animals were oriented within 45° of the current source before current onset, and 12 were oriented within 45° of the current source after current onset.
In contrast, adults (n = 24) did not exhibit rheotaxis to the current flow used in these experiments; mean orientation angle was 100.7° in no current and 92.5° in current conditions, a difference that was not statistically significant.
A subset of animals (stages 47–56) was tested first in no current and then in current conditions. The change in orientation angle for two animals in each of four groups (untreated, three drug treatments) is shown in Fig. 2 both before and after current was turned on. For untreated animals (Fig. 2a), orientation angle varied between 0 and 180° in the pre-current condition, but decreased to 0°, indicating orientation toward the source of the current, by the first observation interval after current onset, and remained stable throughout the observation period. All 16 animals in this group showed this effect. Changes in orientation angle for drug-treated animals (two in each group) before and after current onset are shown in Fig. 2b (cobalt-treated group), 2c (streptomycin-treated group), and 2d (gentamicin-treated group). Tadpoles in all drug groups were less likely to maintain a stable orientation angle (defined as holding the same position for a minimum of four 30-s intervals) over the observation period than were untreated animals, even though most of the drug-treated animals were able to orient towards the current source in at least some part of the observation period. Mean orientation angle differed significantly between pre-current and current conditions (Friedman test: X2(1) = 42.1, P < 0.001; Table 2). Post-hoc testing revealed no significant difference in mean orientation angle in pre-current conditions between the four groups. After current was turned on, mean orientation angle became closer to 0° for all groups, but differed significantly between groups (X2(3) = 8.8, P < 0.05). Orientation angle was highest for the cobalt-treated and the streptomycin-treated groups and lowest for the untreated and gentamicin-treated groups.
Fig. 2a–d.
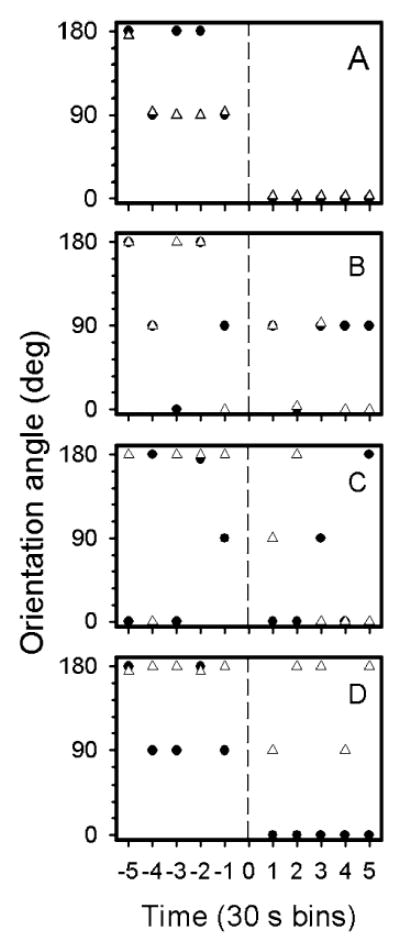
Change in orientation angles for tadpoles tested both in no current and current conditions. The vertical dashed line at time 0 depicts time of current onset. Pre-current times are indicated by negative numbers and post-current times are indicated by positive numbers. Each individual data point represents means of observations averaged in 30-s bins (1 s every 10 s), beginning 1 min after current onset for a total time of 2.5 min. a Untreated animals; b cobalt-treated; c streptomycin-treated; d gentamicin-treated. Data from two animals are shown in each treatment condition (closed circles and open triangles), and were chosen to represent the variability in the responses. Symbols that overlap are o3set slightly for clarity
Table 2.
Orientation angle and tilt angle (both in degrees) for a subset of animals tested under both pre-current and current conditions
| Group | Pre-current
|
Current
|
|||
|---|---|---|---|---|---|
| Orientation angle | Tilt angle | Orientation angle | Tilt angle | n | |
| Untreated | 109 | 48.6 | 2 | 18.8 | 16 |
| Cobalt | 121 | 44.2 | 34 | 36.5 | 14 |
| Streptomycin | 87 | 39.3 | 29 | 27.1 | 14 |
| Gentamicin | 107 | 33.8 | 16 | 10.9 | 15 |
There is no statistical difference in orientation angle and tilt angle between the four groups in the pre-current condition. The introduction of current significantly influenced orientation for all groups, and decreased tilt angle for the untreated and gentamicin-treated groups
For the entire sample of animals, the proportion of tadpoles facing within 45° of the current source over the observation period differed significantly between the five groups (no current, untreated, three drug groups, with data from an individual animal contributing to only one group; X2(4) = 52.3, P < 0.0001; Table 1). This difference was due primarily to the behavior of the untreated group, in which all animals met the criterion. When data from the three drug groups were analyzed separately, there was no significant difference in the proportion of animals facing within 45° of the current, although the streptomycin-treated group was least likely to meet the criterion while the gentamicin-treated group was the most likely. This indicates a higher frequency of position change in the streptomycin-treated group. Drug-treated animals tended to respond more slowly to current onset than did untreated animals. The number of 30-s observation intervals needed to maintain a stable orientation position within 45° of the current was calculated for each animal, with the criterion that a stable position was one in which the animal remained for a minimum of four intervals. Table 1 shows the median number of intervals required to reach this criterion for all four groups tested in current. The untreated and the gentamicin-treated groups both adopted a stable orientation at a median interval of one, while the cobalt- and streptomycin-treated groups did not adopt a stable position until a median interval of two and three, respectively.
Mean orientation angle also differed significantly between the five groups (X2(4) = 45.5, P < 0.001; Table 1). When comparing the four groups of animals tested in current conditions only, orientation angle also differed significantly (X2(3) = 17.9, P < 0.001). The cobalt-treated and the streptomycin-treated groups both showed significantly larger mean orientation angles (P < 0.05), indicating less precise orientation to the current source, than did the untreated group. There was no difference in mean orientation angle between the untreated group and the gentamicin-treated group.
A group of animals subjected to cobalt treatment was retested after a 1-week recovery. There was no difference in orientation angle between these animals and the untreated drug group at this time point, indicating that the effects of cobalt treatment had been reversed.
Tilt angle
The ability of Xenopus to hang suspended in the water column varied with developmental stage and with presence of current. Young tadpoles (stages 37–45) tended to lay flat, either near the bottom of the tank or within the water column. Metamorphic climax tadpoles (stages 56–66) also tended to lay flat (mean tilt angle in no current conditions of 9.9°), although they appeared to be more tilted at the beginning of climax than near the end. Tadpoles between stages 47 and 56 showed a pronounced body tilt, and tilt angle differed between no current and current conditions. Tilt angle was significantly greater under no current conditions than when current was introduced (U = 116, P < 0.001; Fig. 3). There were no differences in tilt angle between tadpoles tested under red light and ambient light conditions. Adult Xenopus did not hang suspended in the water column, but lay flat at the bottom of the tank.
Fig. 3.
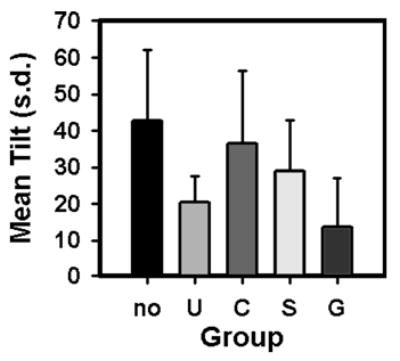
Mean (±SD) tilt angle for five groups of tadpoles. No no current condition, U untreated condition, C cobalt-treated condition, S streptomycin-treated condition, G gentamicin-treated condition. Tadpoles in groups U, C, S, and G were all tested under current. Each tadpole was tested only once, and contributed only one data point to these graphs. Non-parametric analysis of variance showed significant differences in tilt angle between the five groups
Changes in tilt angle were examined in a subset of animals tested under both pre-current and current conditions (Table 2). There was a significant difference in mean tilt angle between these two conditions (Friedman test: X2(1) = 16.9, P < 0.001). In the pre-current condition, mean tilt angle did not vary significantly between the four groups, but was highest in untreated animals and lowest in the gentamicin-treated animals (this difference was marginally significant, P < 0.1). After current was turned on, mean tilt angle decreased significantly in both the untreated and the gentamicin-treated groups (P < 0.05), although there was no significant difference in tilt between these two groups.
Between subjects analyses showed a significant change in tilt angle between the five groups (no current, untreated, three drug treatments; X2(4) = 41.3, P < 0.001; Fig. 3). Tilt angle was highest in the no current group and lowest in the gentamicin group. Post hoc testing revealed significant differences in mean tilt angle between the untreated (current) group and the cobalt-treated group (P < 0.001) and between the untreated and no current groups (P < 0.001). Tilt angle in the cobalt-treated group was significantly higher than in the gentamicin-treated group (P < 0.001). Tilt angle was also higher in the streptomycin-treated group than in either the gentamicin-treated group (P < 0.05) or the no current group (P < 0.05). Tilt angle was significantly lower in the gentamicin-treated group than in the no current group (P < 0.001), but did not differ significantly between the gentamicin and the untreated groups. These comparisons suggest that animals treated with cobalt reacted to the introduction of current in a manner significantly different than those in untreated or gentamicin-treated groups. In response to current, tadpoles in this group not only adopted a more tilted body posture, but they also tended to hang such that their snouts touched the tank floor while the rest of the body tilted upwards. The proportion of animals in each group showing such a posture in at least four observation intervals differed significantly (X2(3) = 31.5, P < 0.001), with 48.5% of the animals in the cobalt-treated group, 25% of the animals in the streptomycin-treated group, and none of the animals in untreated or gentamicin-treated groups showing this behavior.
In contrast to the behavior of the cobalt-treated group, tadpoles exposed to gentamicin not only showed a less pronounced body tilt than animals in other drug groups, but they also tended to lay flat on the tank bottom. The proportion of animals laying flat in at least four observation intervals varied significantly between the four groups tested in current (X2(3) = 11.7, P < 0.01). In the gentamicin-treated group, 44% of the animals showed this posture, while 7% of the untreated group, 17% of the cobalt-treated group, and 30% of the streptomycin-treated group also lay flat during some part of the observation period.
Visualization of hair cells
Figure 4 shows examples of confocal images of the saccular macula in the otic capsules of untreated and drug-treated tadpoles. Label can be seen in the saccule of untreated, cobalt-treated, and streptomycin-treated animals. In contrast, the saccule of the gentamicin-treated animal contains bare patches in which hair cells are not visible. Intensity of fluorescence was less in the gentamicin-treated saccules than in the other samples (statistical tests were not performed because of the small sample size). Figure 5 shows confocal images of the skin in the area of the head of one tadpole treated with gentamicin and one animal treated with CoCl2. There is less fluorescence seen in the sample from the cobalt-treated animal. The intensity of fluorescence in the gentamicin-treated animals was similar to that of the untreated animals (we were unable to obtain high quality images of the skin from streptomycin-treated animals).
Fig. 4a–d.
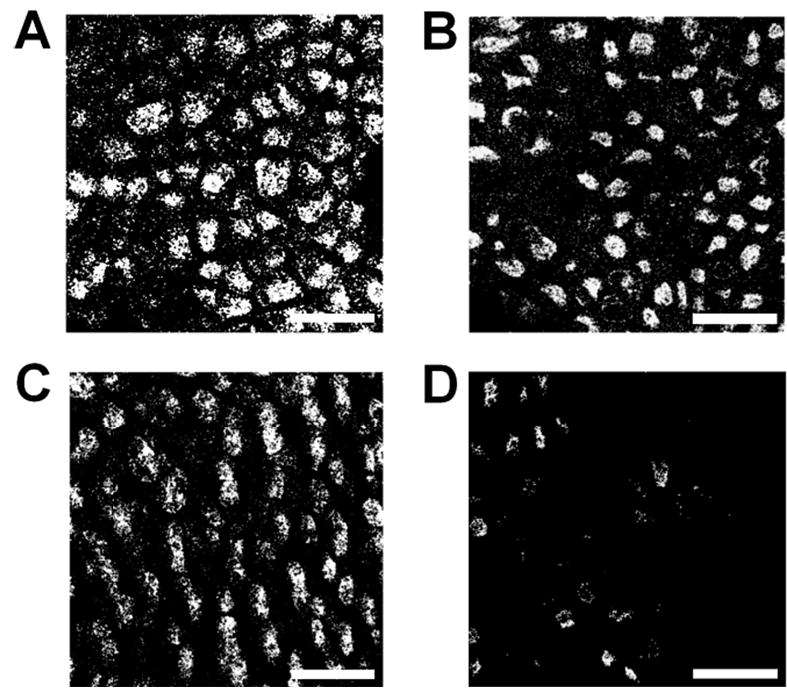
Confocal images through the saccule of phalloidin-labeled otic capsules of four different tadpoles. Image magnification is 40×. In original images, actin-rich stereocilia fluoresce red. Digitized images were imported into Adobe Photoshop and adjusted for similar values of brightness/contrast/intensity. Intensity of fluorescence was quantified using ImageJ software and expressed as normalized (to background) values. a Untreated, normalized intensity 2704; b cobalt-treated, 2455; c streptomycin-treated, 2782; d gentamicin-treated, 710. Scale bar: 50 μm
Fig. 5a–b.
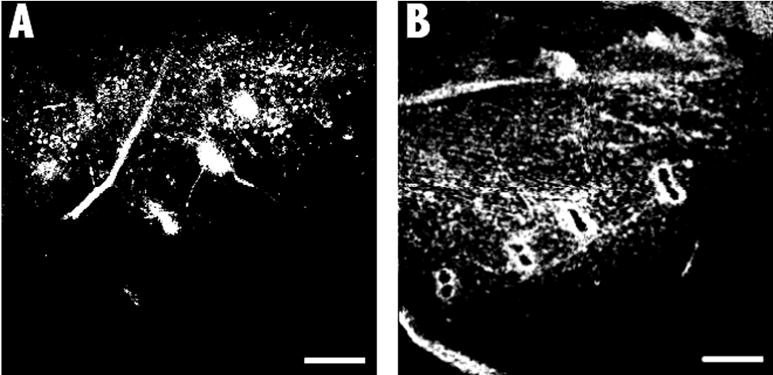
Confocal images of phalloidin-labeled skin specimens of two different tadpoles. Image magnification is 40×. Files were imported into Adobe Photoshop and adjusted to have similar intensity. Intensity of fluorescence was quantified using ImageJ software and expressed as normalized (to background) values. a Gentamicin-treated, normalized intensity 2389. b Cobalt-treated, intensity 1546. Scale bar: 200 μm
Discussion
The results of these experiments show that Xenopus tadpoles between stages 47 and 56 exhibit positive rheotaxis, orientation towards the source of a water current. Tadpoles respond to the onset of current flow rapidly, typically within 1 min (the first interval at which data were quantified). Directional orientation in response to the current flows used here is also observed in younger tadpoles (stages 37–46) and in metamorphic climax tadpoles (stages 57–66) but not in adult frogs. Exposure to CoCl2 or to streptomycin decreases the accuracy of the rheotactic response, while exposure to gentamicin produces less of an effect. Data also show that tadpoles between stages 47 and 56 normally hang suspended in the water column at a pronounced body tilt. The degree of body tilt in normal animals is reduced by the introduction of water current; however, in animals treated with either CoCl2 or streptomycin, tilt angle increases towards that seen under no current conditions while gentamicin treatment tends to decrease degree of body tilt. These behavioral results may provide a window to the structural and functional maturation of tadpole sensory systems at various stages of development.
Tadpoles oriented in response to current flows of 1 cm s−1. Above a flow rate of 2 cm s−1 they could not hold their position in the current. At rates lower than 1 cm s−1, tadpole orientation became less consistent, although present in the responses of some individuals. Although we did not explicitly measure rheotactic threshold, our data suggest that it is around 1 cm s−1, which is the lowest flow rate at which we could consistently induce the orienting response in untreated tadpoles. The estimated threshold for rheotaxis in Xenopus tadpoles is within the range of that found for torrentfish (Cheimarrichthys fosteri, 0.5 cm s−1), Antarctic fish (Pagothenia borchgrevinki, 2 cm s−1), blind cave fish (Astyanax fasciatus, 3 cm s−1) and mottled sculpin (Cottus bairdi, 4 cm s−1; Montgomery et al. 1997; Kanter and Coombs 2003). Shelton (1971) reported that individual lateral line afferent fibers in tadpoles can respond to current velocities as low as 0.5 cm s−1 and Bauknight et al. (1976) found that these fibers do not increase firing rate to flow rates greater than 1.4 cm s−1. Our behavioral data showing consistent orientation to a rate of 1 cm s−1 but inconsistent response to rates higher than 2 cm s−1 appear to reflect these physiological results, and support the hypothesis that the lateral line system mediates rheotaxis in tadpoles.
Role of lateral line cues in mediating rheotaxis
In several species of fishes, the superficial neuromasts of the lateral line system have been shown to be crucial in mediating rheotaxis (Montgomery et al. 1997; Baker and Montgomery 1999; Kanter and Coombs 2003). In these animals, treatment with either CoCl2 (Karlsen and Sand 1987) or streptomycin (Kroese et al. 1978) abolishes or reduces the amount of rheotaxis (defined as the number of fish orienting within some angular deviation from the current source). These drug treatments are believed to affect the functioning of both superficial and canal neuromasts (Montgomery et al. 1997; Baker and Montgomery 1999). In contrast, treatment with gentamicin, which affects canal but not superficial neuromasts (Song et al. 1995), does not significantly affect the amount of rheotaxis. These conclusions can be tested by studying Xenopus tadpoles, whose neuromast morphology and physiological responsiveness are similar to those of superficial neuromasts in fishes (Kroese and van der Brecken 1982; Shelton 1970). We then expect to find that rheotaxis is similar in untreated and gentamicin-treated groups, and less precise in cobalt and streptomycin-treated groups. Results of our experiments (Table 1) confirm that rheotaxis is significantly more variable in animals treated with either CoCl2 or streptomycin compared to untreated animals. Mean orientation angle is higher, median time to orient is longer, and fewer animals are consistently oriented within 45° of the current in these two drug groups. Treatment with gentamicin does not significantly increase mean orientation angle or median time to orient, but it does decrease the overall proportion of tadpoles facing within 45° of the current source over time, consequently reducing the consistency of the directional response. Because gentamicin does not produce the same pattern of change as either CoCl2 or streptomycin, it appears that these pharmacological treatments differentially affect rheotaxis. These results are generally consistent with those reported in fishes (Montgomery et al. 1997; Baker and Montgomery 1999), but with some important qualifications.
Although rheotactic behavior in tadpoles is significantly reduced by treatment with either CoCl2 or streptomycin, it is not completely eliminated. Even after treatment with cobalt, 62% of animals remained oriented within 45° of the current source, and the median latency to orient for all animals was the second observation interval. The failure to suppress orientation to the current under these drug treatments may be attributed to several factors. First, flow rates were relatively low, around the estimated rheotactic threshold for untreated animals. In fishes, the degree of rheotaxis increases with flow rate (Montgomery et al. 1997; Baker and Montgomery 1999; Kanter and Coombs 2003). If we had tested tadpoles at higher flow rates, on the basis of the fish work, we would expect the drug treatments to produce greater effects. Second, the effects of drug treatments are likely dose dependent. Because cobalt can be toxic in high doses (Janssen 2000), we administered dosages that did not qualitatively interfere with normal behavior of tadpoles in their home tanks. This is supported by the data (Table 2) showing in pre-current conditions, the orientation behavior of drug-treated tadpoles in the flow tank did not differ from that of untreated animals. Higher dosages may have produced a more pronounced change in rheotactic behavior but could also have interfered with other aspects of the animals’ behavior, and may have introduced a more pronounced stress or decline in motivation to respond (Janssen 2000). Montgomery et al. (1997) argued that fish need only a few residual neuromasts in order to assess current direction. Although we were able to verify neuromast damage after cobalt treatment in a small sample, it appears that sufficient neuromasts remained unaffected by the drug doses we administered to mediate the behavioral response (although we could not obtain a large enough sample size to confirm this anatomically). Third, tadpole neuromasts may not be as sensitive to these pharmacological treatments as are those of fishes at any dose.
The results of these behavioral experiments impact our understanding of the morphological maturation of the lateral line system in Xenopus tadpoles. Shelton (1970) proposed that the Xenopus lateral line is functional by at least stage 54. Because tadpoles as young as stage 37 showed significant orientation to the water current, our data suggest that this system is functional earlier than proposed by Shelton (1970). The lateral line system reorganizes at metamorphosis (Shelton 1970; Winklbauer 1989; Mohr and Görner 1996), such that the location and distribution of stitches (groups of neuromasts) and their maximal plane of sensitivity change. Neuromasts in tadpoles are more superficially located than those in postmetamorphic toads, where cilia no longer protrude above the skin surface. These structural changes imply that despite being previously characterized as anatomically similar to the superficial neuromasts of fishes (Kroese et al. 1978; Elepfandt 1996), in the adult toad, neuromasts may function more like the canal neuromasts of fishes, being sensitive to acceleration of water movements. Rheotaxis may thus not be a displayed behavior in adult Xenopus, in which neuromasts may become more specialized for prey capture. It is also possible, however, that the neuromasts in the adult have a higher threshold than those of the tadpole for detecting water currents, and that the adult could exhibit rheotaxis to stronger current flows.
Role of other sensory cues
Experiments on rheotaxis in fishes have pointed to the roles of visual and odor cues in mediating this behavior. Under the testing conditions used here, visual cues do not appear to be important for mediating rheotaxis in Xenopus tadpoles. Untreated tadpoles oriented just as consistently when tested under red light as when tested under ambient conditions. Moreover, most tadpoles were tested individually, and so could not derive position information from conspecifics. There were no significant differences in rheotaxis behavior between tadpoles tested individually and those tested in pairs. Under natural conditions, adult Xenopus are active at night and inhabit murky water, making it unlikely that vision is used to detect objects at great distances (Elepfandt 1996). Mathis et al. (1988) found that Xenopus are hyperopic in water, also indicating that vision is not highly developed for image formation. Little is known about the behavioral development of visual sensitivity in tadpoles over metamorphosis. Katz et al. (1981) observed that Xenopus tadpoles (stage not specified) can maintain their orientation in schools even in the dark, although visual information does enhance the precision of schooling. Our data suggest that rheotactic behavior can occur in the absence of visual information; however, because drug-treated animals were tested under red light only, our data do not rule out the possibility that rheotaxis can be influenced by visual cues when the lateral line is inactivated.
Odor cues may also influence rheotaxis in fishes (Montgomery et al. 1997; Baker and Montgomery 1999) and detection of water waves in adult Xenopus (Claas et al. 1993). Manipulation of odors was not performed in this experiment, and care was taken to rinse the experimental tank between conditions to minimize the inadvertent introduction of chemical cues. Although we cannot explicitly rule out the possibility that odors may facilitate detection of water waves in tadpoles, it is unlikely that such cues influenced rheotaxis in this particular design. Moreover, anatomical studies suggest that nuclei of the olfactory nervous system are not fully developed until metamorphic climax stages (Byrd and Burd 1991; Nezlin and Schild 2000). Our data show that tadpoles between stages 47 and 55, when the olfactory system is not anatomically mature, orient as precisely as those in metamorphic climax stages.
Inner ear function and behavioral responses
Van Bergeijk (1959) and Wassersug and Souza (1990) noted that Xenopus tadpoles tend to hang at a downward facing posture in the water column, although they did not specify developmental stage. Our data show that this downward facing posture is a characteristic of tadpoles between stages 47 and 56, being absent in younger animals and declining throughout metamorphic climax stages. This posture, which we term the tilt angle, varied between no current and current conditions in untreated animals. Under current flow, the tilt was significantly less pronounced. This may be produced by the necessity to maintain a stable position in response to the current. Treatment with CoCl2 or with streptomycin increased tilt angle in response to current close to levels seen in no current conditions. Moreover, some cobalt-treated animals showed a more pronounced tilt than seen even in the no current group, and they adopted a posture in which their snouts touched the tank floor while their bodies were held upright at angles approaching 90°. This is consistent with the hypothesis that when the lateral line is inactivated, animals need a tactile reference point in order to maintain their position in and orientation towards the current. These data also suggest that the “hydrostatic balancing mechanism” (Van Bergeijk 1959) of Xenopus tadpoles is influenced by the functioning of the peripheral neuromasts.
Our data also suggest that the downward tilted posture of Xenopus tadpoles may be related to the functioning of the inner ear, although they do not explicitly speak to the possible role of the bronchial diverticula in maintaining this posture. In both pre-current and current conditions, the mean tilt angles shown by tadpoles treated with gentamicin were lower than those seen in the untreated or other drug-treated groups. Moreover, in response to current, a large proportion of gentamicin-treated animals lay flat on the tank floor, but still oriented toward the current source. This variability in the response, with some animals laying flat and others being tilted at angles somewhat below that of untreated animals, indicate individual differences in susceptibility to gentamicin, but overall the data suggest that gentamicin affected the animals’ balance, particularly under current conditions. Although sample size is small, the saccule of gentamicin-treated animals fluoresced less intensely than those of untreated, cobalt-treated or streptomycin-treated animals. Assuming that intensity of fluorescence is related to number of healthy hair cells, this suggests that the behavioral effects following gentamicin treatment are related to damage of saccular hair cells. Gentamicin treatment did not significantly affect all measures of rheotaxis, although it did reduce the proportion of animals facing within 45° of the current. It is possible that the 30% of streptomycin-treated animals that lay flat on the tank floor for at least some portion of the observation interval also suffered some damage to the saccule, but this could not be verified.
Studies of rheotaxis in fishes have generally assumed that pharmacological treatments that affect superficial and canal neuromasts do not affect the functioning of the inner ear. Karlsen and Sand (1987) reported that CoCl2 treatment affected the mechanosensitivity of the lateral line in the roach (Rutilus rutilus) without affecting microphonic activity from the utricle. Streptomycin, when administered systemically or directly into the inner ear, is vestibulotoxic and cochleotoxic in mammals (reviews: Garetz and Schacht 1996; Bagger-Sjoback 1997). This aminoglycoside also blocks transduction channels in saccular hair cells in adult bullfrogs (Kroese et al. 1989). Gentamicin, administered intramuscularly, selectively damages type I hair cells in the utricle and lagena of fish (Yan et al. 1991), and, like streptomycin, damages vestibular and cochlear hair cells in mammals (Bagger-Sjoback 1997). There have been no reports in fishes or mammals of damage to the inner ear when aminoglycosides are administered by immersion. Tadpoles have cartilaginous otic capsules and skulls, which may be more easily permeated by these agents than are the bony otic capsules and skulls of fishes; moreover, the permeable skin of anurans renders them extremely sensitive to environmental agents.
Although the anatomical maturation of the Xenopus inner ear across metamorphosis has been examined, little is known about its functional maturation. By stage 47, the saccule and the utricle are anatomically distinct, and separate recesses for the amphibian and basilar papilla are seen (Nieuwkoop and Faber 1994). The eighth nerve enters the otic capsule at this stage. By stage 50/52, gross morphogenesis of all component parts of the inner ear is complete (Paterson 1960; Bever et al. 2003). Rapid growth in hair cell numbers in both the saccule and the amphibian papilla occur between stages 47 and the end of metamorphic climax (Diaz et al. 1995). It is interesting to note that the onset of the tilted body posture in untreated tadpoles is correlated with the appearance of a distinct saccule, but that rheotaxis can be observed before this time. Future studies should aim to selectively block the inner ear while leaving the lateral line system intact in order to further separate the functioning of these two systems in tadpoles.
Acknowledgments
This research was supported by NIH grant NS28565 and the Rhode Island Space Grant Consortium. We thank A. Barnstable, R. Brown, N. Catanzaro, K. DeLucia, S. Horowitz, W. Rice, and R. Simmons for assistance. Experimental protocols comply with the Principles of Animal Care, publication No. 86-23 of the National Institutes of Health, and were approved by the Brown University Institutional Animal Care and Use Committee.
References
- Arnold GP. Rheotropism in fishes. Biol Rev. 1974;49:515–576. doi: 10.1111/j.1469-185x.1974.tb01173.x. [DOI] [PubMed] [Google Scholar]
- Bagger-Sjoback D. Effect of streptomycin and gentamicin on the inner ear. Ann N Y Acad Sci. 1997;830:120–129. doi: 10.1111/j.1749-6632.1997.tb51884.x. [DOI] [PubMed] [Google Scholar]
- Baker CF, Montgomery JC. The sensory basis of rheotaxis in the blind Mexican cave fish, Astynax fasciatus. J Comp Physiol A. 1999;184:519–527. [Google Scholar]
- Bauknight RS, Strelioff D, Honrubia V. Effective stimulus for the Xenopus laevis lateral-line hair cell system. Laryngoscope. 1976;86:1836–1844. doi: 10.1002/lary.5540861208. [DOI] [PubMed] [Google Scholar]
- Bever MM, Jean YY, Fekete DM. Three-dimensional morphology of inner ear development in Xenopus laevis. Dev Dyn. 2003;227:422–430. doi: 10.1002/dvdy.10316. [DOI] [PubMed] [Google Scholar]
- Byrd CA, Burd GD. Development of the olfactory bulb in the clawed frog, Xenopus laevis:a morphological and quantitative analysis. J Comp Neurol. 1991;314:79–90. doi: 10.1002/cne.903140108. [DOI] [PubMed] [Google Scholar]
- Claas B, Münz H. Analysis of surface wave direction by the lateral line system of Xenopus: source localization before and after inactivation of different parts of the lateral line. J Comp Physiol A. 1996;178:253–268. doi: 10.1007/BF00188167. [DOI] [PubMed] [Google Scholar]
- Claas B, Münz H, Görner P. Reaction to surface waves by Xenopus laevis Daudin: are sensory systems other than the lateral line involved? J Comp Physiol A. 1993;172:759–765. doi: 10.1007/BF00195401. [DOI] [PubMed] [Google Scholar]
- Coombs S, Janssen J. Behavioral and neurophysiological assessment of lateral line sensitivity in the mottled sculpin, Cottus bairdi. J Comp Physiol A. 1990;167:557–567. doi: 10.1007/BF00190827. [DOI] [PubMed] [Google Scholar]
- Coombs S, Braun CB, Donovan B. The orienting response of Lake Michigan mottled sculpin is mediated by canal neuromasts. J Exp Biol. 2001;204:337–348. doi: 10.1242/jeb.204.2.337. [DOI] [PubMed] [Google Scholar]
- Diaz ME, Varela-Ramirez A, Serrano EE. Quantity, bundle types and distribution of hair cells in the sacculus of Xenopus laevis during development. Hear Res. 1995;91:33–42. doi: 10.1016/0378-5955(95)00159-x. [DOI] [PubMed] [Google Scholar]
- Elepfandt A. Accuracy of taxis response to water waves in the clawed toad (Xenopus laevis Daudin) with intact or with lesioned lateral line system. J Comp Physiol A. 1982;148:535–545. [Google Scholar]
- Elepfandt A (1996) Sensory perception and the lateral line system in the clawed frog Xenopus laevis In: Tinsley RC, Kobel HR (eds) The biology of Xenopus Clarendon Press, Oxford, pp 97–120
- Garetz SL, Schacht J (1996) Ototoxicity: of mice and men. In: Van de Water TR, Popper AN, Fay RR (eds) Clinical aspects of hearing. Springer, Berlin Heidelberg New York, pp 116–154
- Görner P, Moller P, Weber W. Lateral-line input and stimulus localization in the African clawed toad Xenopus sp. J Exp Biol. 1984;108:315–328. [Google Scholar]
- Jaeger RG, Hailman JP. Ontogenetic shift of spectral phototactic preferences in anuran tadpoles. J Comp Physiol Psychol. 1976;90:930–945. doi: 10.1037/h0077275. [DOI] [PubMed] [Google Scholar]
- Janssen J. Toxicity of Co2+: implications for lateral line studies. J Comp Physiol A. 2000;186:957–960. doi: 10.1007/s003590000148. [DOI] [PubMed] [Google Scholar]
- Kanter MJ, Coombs S. Rheotaxis and prey detection in uniform currents by Lake Michigan mottled sculpin (Cottus bairdi) J Exp Biol. 2003;206:59–70. doi: 10.1242/jeb.00056. [DOI] [PubMed] [Google Scholar]
- Karlsen HE, Sand O. Selective and reversible blocking of the lateral line in freshwater fish. J Exp Biol. 1987;133:249–262. [Google Scholar]
- Katz LC, Potel MJ, Wassersug RJ. Structure and mechanisms of schooling in tadpoles of the clawed frog, Xenopus laevis. Anim Behav. 1981;29:20–33. [Google Scholar]
- Kroese ABA, van den Bercken J. Effect of ototoxic antibiotics on sensory hair cell functioning. Hear Res. 1982;6:183–197. doi: 10.1016/0378-5955(82)90053-3. [DOI] [PubMed] [Google Scholar]
- Kroese ABA, van der Zalm JM, van der Bercken J. Frequency response of the lateral line organ of Xenopus laevis. Pflugers Arch. 1978;375:167–175. doi: 10.1007/BF00584240. [DOI] [PubMed] [Google Scholar]
- Kroese ABA, Das A, Hudspeth AJ. Blockage of the transduction channels of hair cells in the bullfrog’s sacculus by aminoglycoside antibiotics. Hear Res. 1989;37:203–217. doi: 10.1016/0378-5955(89)90023-3. [DOI] [PubMed] [Google Scholar]
- Lum AM, Wassersug RJ, Potel MJ, Lerner SA. Schooling behavior of tadpoles: a potential indicator of ototoxicity. Pharmacol Biochem Behav. 1982;17:363–366. doi: 10.1016/0091-3057(82)90093-4. [DOI] [PubMed] [Google Scholar]
- Mathis U, Schaeffel F, Howland HC. Visual optics in toads (Bufo americanus) J Comp Physiol A. 1988;163:201–213. doi: 10.1007/BF00612429. [DOI] [PubMed] [Google Scholar]
- Mohr C, Görner P. Innervation patterns of the lateral line stitches of the clawed frog, Xenopus laevis, and their reorganization during metamorphosis. Brain Behav Evol. 1996;48:55–69. doi: 10.1159/000113186. [DOI] [PubMed] [Google Scholar]
- Montgomery JC, Baker CF, Carton AG. The lateral line can mediate rheotaxis in fish. Nature. 1997;389:960–963. [Google Scholar]
- Nezlin LP, Schild D. Structure of the olfactory bulb in tadpoles of Xenopus laevis. Cell Tissue Res. 2000;302:21–29. doi: 10.1007/s004410000208. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J (1994) Normal table of Xenopus laevis (Daudin). Garland Publishing, New York
- Paterson NF. The inner ear of some members of the Pipidae (Amphibia) Proc Zool Soc Lond. 1960;134:509–546. [Google Scholar]
- Shelton PMJ. The lateral line system at metamorphosis in Xenopus laevis (Daudin) J Embryol Exp Morphol. 1970;24:511–524. [PubMed] [Google Scholar]
- Shelton PMJ. The structure and function of the lateral line system in larval Xenopus laevis. J Exp Zool. 1971;178:211–232. doi: 10.1002/jez.1401780207. [DOI] [PubMed] [Google Scholar]
- Song J, Yan HY, Popper AN. Damage and recovery of hair cells in fish canal (but not superficial) neuromasts after gentamicin exposure. Hear Res. 1995;91:63–71. doi: 10.1016/0378-5955(95)00170-0. [DOI] [PubMed] [Google Scholar]
- Van Bergeijk WA. Hydrostatic balancing mechanism of Xenopus larvae. J Acoust Soc Am. 1959;31:1340–1347. [Google Scholar]
- Vogel D, Bleckmann H. Behavioral discrimination of water motions caused by moving objects. J Comp Physiol A. 2000;186:1107–1117. doi: 10.1007/s003590000158. [DOI] [PubMed] [Google Scholar]
- Vogel S, LaBarbera M. Simple flow tanks for research and teaching. Biosci. 1978;28:638–643. [Google Scholar]
- Wassersug RJ, Souza KA. The bronchial diverticula of Xenopus laevis: are they essential for hydrostatic assessment? Naturwissenschaften. 1990;77:443–445. doi: 10.1007/BF01135948. [DOI] [PubMed] [Google Scholar]
- Wersäll J, Flock A. Suppression and restoration of the microphonic output from the lateral-line organ after local application of streptomycin. Life Sci. 1964;3:1151–1155. doi: 10.1016/0024-3205(64)90132-8. [DOI] [PubMed] [Google Scholar]
- Winklbauer R. Development of the lateral line system in Xenopus. Prog Neurobiol. 1989;32:181–206. doi: 10.1016/0301-0082(89)90016-6. [DOI] [PubMed] [Google Scholar]
- Yan HY, Saidel WM, Chang JS, Presson JC, Popper AN. Sensory hair cells of a fish ear: evidence of multiple types based on ototoxicity sensitivity. Proc R Soc Lond B. 1991;245:133–138. doi: 10.1098/rspb.1991.0099. [DOI] [PubMed] [Google Scholar]


