Abstract
Making judgments about the retrievability of information is a critical part of the metamemory processes engaged during remembering. A recent study of patients with frontal lesions suggests that ventral medial prefrontal cortex (VMPC) plays a critical role in such judgments [Schnyer, D. M., Verfaellie, M., Alexander, M. P., Lafleche, G., Nicholls, L., & Kaszniak, A. W. A role for right medial prefrontal cortex in accurate feeling of knowing judgments: Evidence from patients with lesions to frontal cortex. Neuropsychologia, 42, 957–966, 2004]. The observed impairment was thought to reflect an inability to determine the accessibility of memory contents. To further examine the neuroanatomical basis of content accessibility assessment, we used fMRI in an episodic feeling-of-knowing (FOK) paradigm. Participants were asked to make trial-by-trial predictions about the retrievability of the final word that completed studied sentences and then to select the correct completion from among alternatives. Results indicated that the VMPC is engaged during accurate FOK judgments and its activation is modulated by retrieval rating. Structural equations modeling supported the notion that VMPC, as part of a broader left hemisphere network involved in memory retrieval, monitors the output of the retrieval process. More generally, VMPC may participate in metacognitive processes that allow for the comparison of available data against an internal model.
INTRODUCTION
The attempt to retrieve information from memory is not always immediately successful. Faced with failure, a person must determine the extent to which information seems readily accessible and, therefore, whether there is sufficient reason to continue to try and retrieve it (Koriat, 2000). The cognitive mechanisms by which such metamemory judgments are made have been the focus of extensive study using the feeling-of-knowing (FOK) paradigm (Koriat, 1993; Metcalfe, 1993; Reder & Ritter, 1992).
Two theoretical views have dominated this area of research. One view has argued that FOK judgments are made primarily on the basis of the relative familiarity of the recall cue (Metcalfe, 1993; Reder & Ritter, 1992), and reflect a rapid assessment prior to engaging in a retrieval attempt. A second view has suggested that these judgments are made by engaging the retrieval process and making assessments of the perceived accessibility of memory contents (Koriat, 1993). Illustrating the role of familiarity, Reder (1987) found that increasing the familiarity of retrieval cues through priming resulted in higher FOK ratings, with no effect on actual retrieval. Illustrating the role of content accessibility, Koriat (1993) found a positive relationship between the amount of fragment information retrieved by a participant and ratings of future retrievability. This relationship was found irrespective of accuracy. Although familiarity and accessibility views have often been pitted against each other (Miner & Reder, 1994), there recently has been an attempt to integrate the two (Koriat & Levy-Sadot, 2001). Koriat and Levy-Sadot (2001) have proposed that retrieval predictions entail a cascading two-step process involving both assessments of cue-familiarity and evaluation of the accessibility of memory contents. By this view, items are initially assessed for familiarity and then either discounted (feeling-of-not-knowing) or slated for further search. Assessment of content availability consists of implementing the search or retrieval process and monitoring the results. Although that process may not always be successful, it can produce fragments of information that are then evaluated in order to come to a judgment about an item’s availability in memory (Koriat, 2000).
Although considerable knowledge has been gained about the cognitive processes that contribute to meta-memory judgments, much less is known about the functional neuroanatomy of these decisions. Research has suggested that frontal cortical structures are critically involved in predictions about memory retrieval in the FOK paradigm (Souchay, Isingrini, & Espagnet, 2000; Janowsky, Shimamura, & Squire, 1989; Shimamura & Squire, 1986), but there has as yet been little attempt to elucidate the role of specific frontal regions (or other regions) in the assessment of either cue-familiarity or accessibility of memory contents—the two processes that are thought to mediate these judgments.
In a recent FOK study in patients with damage to the frontal cortex (Schnyer et al., 2004), we found that patients whose lesion encompassed the right ventral medial prefrontal cortex (VMPC) were impaired in making predictions about their subsequent recognition performance. Because familiarity-based assessment was demonstrated to be intact in these patients, we concluded that the primary contribution of this region to metamemory judgments was in the assessment of the accessibility of memory contents. This conclusion is in line with a recent proposal by Moscovitch and Winocur (2002) that the VMPC and the anterior prefrontal cortex play an important role in the intuitive evaluation of actual stored memory contents.
The findings in patients with damage to the prefrontal cortex leave unanswered several important questions about both the neuroanatomical underpinnings of meta-memory judgments and the functional role of the VMPC in these judgments. First, lesion studies cannot delineate whether the effect of a lesion is due to damage in local gray matter or to pathways that connect distributed non-affected regions (Price, Warburton, Moore, Frackowiak, & Friston, 2001). This connectivity issue is especially important in determining the role of the VMPC, as this region contains many of the major pathways connecting the frontal cortex with the limbic and temporal regions.
Second, although lesion studies are essential for pinpointing regions that are critically involved in task performance, they fail to identify additional regions that may also contribute to performance, and they provide little evidence as to how these regions interact (Price, Mummery, Moore, Frackowiak, & Friston, 1999). The VMPC may be involved in evaluating the accessibility of memory contents, but access to these contents most likely depends on effective retrieval attempts that may be controlled by other portions of the frontal cortex in interaction with the temporal lobes (Maguire, Vargha-Khadem, & Mishkin, 2001; Grady, McIntosh, Rajah, & Craik, 1998). The examination of frontal–temporal interactions during memory retrieval judgments is critical for uncovering evidence for such a process.
The use of functional magnetic resonance imaging (fMRI) in healthy individuals provides an opportunity to address these questions. To date, only two fMRI studies have examined brain activity during FOK judgments (Maril, Simons, Mitchell, Schwartz, & Schacter, 2003; Kikyo, Ohki, & Miyashita, 2002), and only one of those has focused on new episodic learning (Maril et al., 2003). That study revealed that activation in frontal and left parietal regions was modulated by perceived ease of retrieval. Activation for items for which participants reported an FOK was less than that obtained for items that they felt they successfully recalled, but greater than that obtained for items that they felt they would not be able to recall. However, that study did not directly compare activations for accurate and inaccurate FOK judgments, and therefore, does not provide an analogue to our lesion study.
The present study uses fMRI to further elucidate the neural mechanisms underlying FOK judgments in an episodic memory task. By using a task similar to that used in our lesion study (Schnyer et al., 2004), we seek further evidence for the hypothesis that the VMPC plays a critical role in the accuracy of these judgments by engaging in the assessment of content accessibility. If this hypothesis is correct, it should also be possible to demonstrate that this region acts in concert with a larger neural network known to mediate the storage and retrieval of memory contents.
In this experiment, participants studied sentences and subsequently, while fMRI images were collected, made FOK judgments about the final word of each sentence. During the test phase, participants were presented with a sentence cue and asked to indicate the probability that they would recognize the correct word completing the sentence from five alternatives. A variable duration fixation” screen followed, and then participants selected one of the recognition alternatives presented on the screen. Performance in the test phase allowed for sorting of the FOK judgments on the basis of rating and accuracy. A schematic for the experimental sequence is presented in Figure 1.
Figure 1.
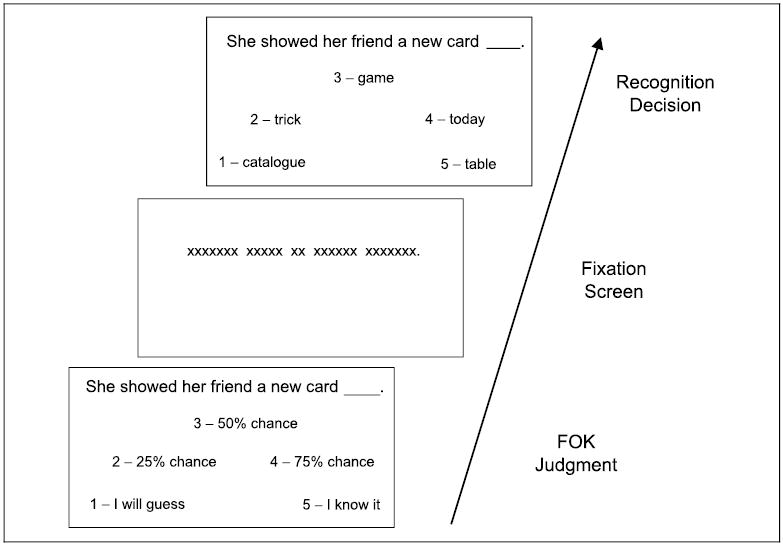
Schematic of the three screens that make up a single trial in the FOK judgment task. Both the judgment and recognition decision screens are presented until a choice is made or for a maximum of 10 sec. The fixation screen appears for a randomly determined duration of either 6, 8, or 10 sec. The three periods are separated by a 500-msec interstimulus interval.
RESULTS
Behavioral Findings
Recognition accuracy was calculated for studied items only (nonstudied items were excluded). Participants were generally accurate in their recognition decisions (mean = 0.81, SD = 0.12), but their accuracy was significantly higher on trials on which they indicated that they “knew” a sentence completion (mean = 0.91, SD = 0.09) than on trials on which they gave a 1–4 rating [mean = 0.76, SD = 0.14; t(18) = 6.59, p < .001].
Accuracy of judgment ratings was defined in the same manner as outlined in the Methods section for the fMRI modeling and was assessed using the Hamann index (Schraw, 1995). Overall accuracy, based on all ratings (1–5), was significantly above chance [mean = 0.56, SD = 0.11, t(18) = 17.66, p < .001]. Accuracy of “know” judgments (mean = 0.82, SD = 0.15) was higher than that of 1–4 ratings [mean = 0.36, SD = 0.12, t(18) = 12.77, p < .001], but the latter remained significantly above chance [t(18) = 10.70, p < .001]. The average rating given to sentence cues rated 1–4 was 2.18 (SD = 0.36). This value is significantly lower than a rating of 2.5, which would reflect an equal distribution of the four ratings [t(18) = −3.86, p < .001]. Predictive retrieval judgments that were accurate (i.e., congruent with subsequent recognition performance) on average received lower ratings (mean rating = 2.08, SD = 0.38) than those that were inaccurate [i.e., incongruent with the subsequent recognition performance; mean rating = 2.38, SD = 0.50, t(18) = 2.56, p < .05], indicating that subjects were slightly more accurate when they felt they would not recognize the target completion than when they felt they would. The average number of inaccurate and accurate 1–4 FOK judgments used in the SPM modeling were 25.2 (SD = 10.4) and 53.3 (SD = 16.2), respectively.
Inspection of reaction times for all ratings (1–5) reveals that the five “know” ratings were given faster than all other ratings (see Table 1). This was confirmed in a repeated-measures ANOVA [F(1,4) = 51.75, p < .001] and follow-up t tests (all ts > 7.87, p < .001). In addition, follow-up tests revealed differences among ratings 1–4 (1 < 4 < 3 = 2). In order to examine whether differences in reaction time contribute to the hemodynamic differences between accurate and inaccurate FOK judgments, RTs for accurate and inaccurate decisions (collapsed across ratings) were compared. This comparison revealed that accurate judgments (mean = 3202 msec, SD = 613 msec) were marginally faster than inaccurate ones [mean = 3376 msec, SD = 800 msec, t(18) = 1.99, p < .07] and therefore, any regions identified by the fMRI contrast of accurate greater than inaccurate cannot be accounted for by differences in time on task.
Table 1.
Response Latencies (in msec) and Proportion of Items Assigned to Different Groups
| Rating | Mean (SD) | % of Total Items |
|---|---|---|
| 1 | 3016 (571) | 24 |
| 2 | 3727 (867) | 11 |
| 3 | 3599 (774) | 11 |
| 4 | 3306 (770) | 11 |
| 5—”Know” | 2278 (530) | 43 |
| Inaccurate 1–4 | 3376 (800) | 39 |
| Accurate 1–4 | 3202 (613) | 18 |
fMRI Findings
Brain Regions Associated with Accurate FOK Judgments
Both accurate “know” and accurate 1–4 ratings were contrasted with inaccurate judgments. Because we assume that accurate “know” judgments additionally entail successful recall, these contrasts were performed separately; regions of overlap were considered to be specific to accurate FOK judgments, independent of recall status. Regions associated with accurate “know” judgments included multiple regions of the frontal, temporal, and parietal cortex as well as the posterior cingulate (see Figure 2A and Table 2a). In contrast, regions associated with accurate 1–4 judgments included a fairly circumscribed left hemisphere network of frontal and temporal cortical regions (see Figure 2B and Table 2b), including the medial frontal (area 10 and 11) and lateral frontal cortex (areas 6, 44, and 47), the hippocampus and parahippocampal gyrus, and the middle temporal gyrus (area 21). Two regions of the right frontal cortex were also active: the inferior frontal gyrus (area 47) and the anterior cingulate (area 32). To identify spatially overlapping locations activated by both accurate “know” and 1–4 judgments, a conjunction analysis was performed. This analysis revealed bilateral portions of the anterior inferior prefrontal cortex (aPFC; area 47) and the VMPC (area 11), the left frontal operculum (44), the lateral temporal cortex (21), and the left hippocampus (see Figure 2C and Table 2c).1 These findings reveal that accurate judgments about retrievability, regardless of retrieval success, engage specific regions of the frontal and temporal cortex, whereas posterior regions appear to be selectively involved during successful retrieval.
Figure 2.
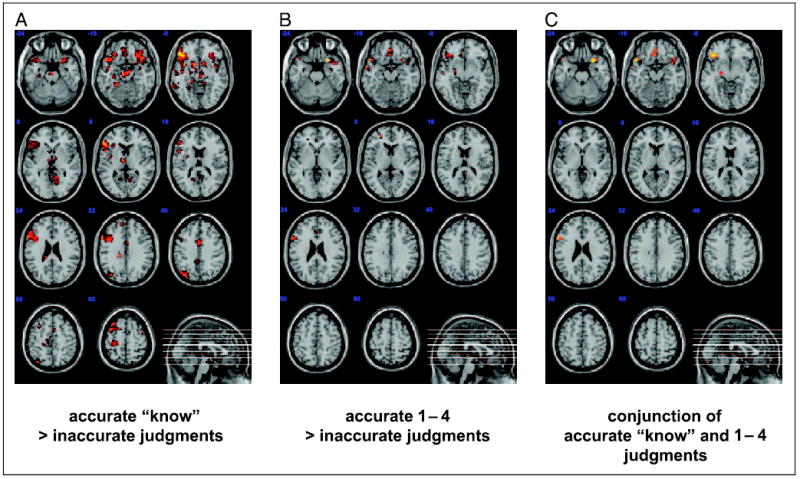
Panels show eleven 5-mm axially oriented slices of a normalized T1 structural image with overlays of significant voxels identified by the indicated contrast. All voxels shown survived the cluster correction for multiple comparisons at p < .05. (A) Accurate “know” judgments greater than all inaccurate judgments; (B) Accurate 1–4 judgments greater than all inaccurate judgments; (C) The significant conjunction between panels A and B.
Table 2.
Regions Associated with Accurate Retrieval Judgments
| Location (Brodmann) | MNI Coordinates | t Max | |||
|---|---|---|---|---|---|
| (a) “Know” > Inaccurate Judgments | |||||
| Frontal | Left Frontal Operculum (44)* | −57 | 15 | 27 | 5.75 |
| Left Fusiform Gyrus (37)* | −24 | −51 | −12 | 3.89 | |
| Left Gyrus Rectus (11)* | −3 | −30 | −15 | 4.1 | |
| Left Inferior Frontal Gyrus (45)* | −48 | 27 | 6 | 5.06 | |
| Left Inferior Frontal Gyrus (47)* | −45 | 33 | −9 | 5.61 | |
| Left Insula (48)* | −36 | 3 | −9 | 5.06 | |
| Left Medial Frontal Cortex (11) | −15 | −42 | −6 | 3.52 | |
| Left Middle Cingulate (23) | −6 | −27 | 42 | 3.09 | |
| Left Middle Frontal Gyrus (44) | −51 | 18 | 39 | 2.74 | |
| Left Middle Frontal Gyrus (8/9)* | −27 | 21 | 60 | 4.1 | |
| Left Precentral Gyrus (44/6)* | −45 | 9 | 33 | 4.39 | |
| Left Superior Medial Frontal Lobe (32) | −9 | 48 | 30 | 4.05 | |
| Left Supplemental Motor (6)* | −6 | −12 | 54 | 2.69 | |
| Right Inferior Frontal Gyrus (47) | 30 | 27 | −15 | 5.05 | |
| Right Insula (48) | 39 | 0 | −12 | 3.46 | |
| Right Middle Cingulate (23) | 3 | 3 | 39 | 4.86 | |
| Right Middle Frontal Gyrus (6) | 45 | 6 | 57 | 4.57 | |
| Right Precentral Gyrus (6) | 42 | −6 | 63 | 3.33 | |
| Right Supplemental Motor (32/6)* | 6 | 6 | 48 | 3.29 | |
| Temporal | Left Inferior Temporal Gyrus (20) | −51 | −36 | −15 | 3.37 |
| Left Superior Temporal Gyrus (48) | −39 | −12 | −9 | 4.23 | |
| Left Middle Temporal Gyrus (20/21)* | −60 | −9 | −12 | 3.96 | |
| Left Temporal Pole (38)* | −45 | 18 | −15 | 4.73 | |
| Right Superior Temporal Gyrus (21)* | 45 | −9 | −12 | 3.71 | |
| Right Temporal Pole (38) | 42 | 18 | −18 | 4.71 | |
| Medial Temporal | Left Hippocampus (35)* | −6 | −18 | −18 | 5.14 |
| Left Parahippocampus (35) | −27 | −27 | 18 | 3.35 | |
| Right Hippocampus (35) | 3 | −21 | −15 | 4.52 | |
| Posterior | Left Calcarine (17)* | −15 | −60 | 15 | 3.07 |
| Left Cerebellum | −21 | −42 | −18 | 4.87 | |
| Left Inferior Parietal (39) | −33 | −75 | 48 | 3.74 | |
| Left Middle Occipital Gyrus (19)* | −33 | −78 | 33 | 5.05 | |
| Left Postcentral Gyrus (4/48) | −33 | −30 | 69 | 3.21 | |
| Left Precuneus (23) | −6 | −45 | 39 | 3.2 | |
| Right Cerebellum (3) | 12 | −42 | −9 | 3.71 | |
| Right Lingual Gyrus (21/18) | 12 | −57 | 3 | 4.29 | |
| Subcortical | Left Caudate (25) | −3 | 9 | 3 | 3.47 |
| Left Thalamus | −6 | −12 | 3 | 4.97 | |
| Right Caudate (25) | 12 | 9 | −9 | 4.42 | |
| (b) Accurate 1–4 Judgments > Inaccurate Judgments | |||||
| Frontal | Left Inferior Frontal Gyrus (47)* | −39 | 30 | −6 | 4.27 |
| Left Middle Frontal Gyrus (44/48) | −21 | 36 | 12 | 3.89 | |
| Left Frontal Operculum/Precentral Gyrus (44/6)* | −57 | 15 | 24 | 4.49 | |
| Left Medial Frontal Cortex (11)* | 0 | 42 | −15 | 4.28 | |
| Right Anterior Cingulate Gyrus (24) | 6 | 27 | 24 | 3.18 | |
| Right Inferior Frontal Gyrus (47)* | 30 | 18 | −24 | 5.05 | |
| Medial Temporal | Left Hippocampus/Parahippocampal Gyrus (35)* | −18 | −27 | −15 | 3.18 |
| Temporal | Left Middle Temporal Gyrus (21)* | −57 | 0 | −15 | 5.13 |
| (c) Conjunction of a and b | |||||
| Frontal | Left Medial Frontal Cortex (11) | −4 | 31 | −15 | |
| Left Inferior Frontal Gyrus (47) | −38 | 29 | −8 | ||
| Right Inferior Frontal Gyrus (47) | 29 | 15 | −20 | ||
| Left Frontal Operculum (44) | −58 | 16 | 24 | ||
| Medial Temporal | Left Hippocampus | −20 | −15 | −12 | |
| Temporal | Left Middle Temporal Gyrus (21) | −57 | −12 | −12 | |
Only clusters with a significance level of p < .05 corrected are reported. Local maxima are indicated for > 8 mm apart per cluster. More than one cluster per region is indicated with an asterisk and only the greatest maxima is reported. The coordinates and their t values are at the peak voxels in each cluster. Approximate Brodmann’s area are shown in parenthesis.
A Model of Retrieval–Monitoring Interactions
Structural equations modeling (SEM) was used in order to test the proposal that within the regions associated with accurate FOK judgments revealed above, the VMPC engages in monitoring the outcome of a retrieval attempt. Clusters identified in the conjunction analysis of accurate “know” and accurate 1–4 judgments were utilized for this analysis. Based on previous empirical (for a review, see Fletcher & Henson, 2001) and modeling findings (Becker & Lim, 2003; Maguire et al., 2001), an initial model was constructed with reciprocal connections between the left inferior frontal gyrus (LIPC; area 47), the hippocampus, the middle temporal gyrus, and the left VMPC (see Figure 3 for the regions used in the model and Figure 4A for the initial model). The more lateral portion of the left frontal operculum (44) was not utilized in this network because it revealed relative levels of deactivation between conditions (mean amplitude: “know” = −0.04, FOK accurate = −0.04, FOK inaccurate = −0.09). Data from the correct 1–4 judgments were used to modify the initial model by means of stepwise alteration of pathways until a suitable AIC fit index was achieved. This resulted in a model with a unidirectional flow from the hippocampus to the LIPC to the left temporal cortex and then culminating in the VMPC (see Figure 4B). Neither monitoring output from the LIPC nor the hippocampus by the VMPC resulted in acceptable model fits (χ2 = 12.7 and 16.3, respectively, p < .01). Consistent with the notion that the VMPC monitors output from the temporal cortex, reversal of the path direction from the VMPC back to the temporal cortex resulted in a model that could be improved considerably (χ2 = 6.42, p = .09, CFI = .86, AIC = 20.42, RMSEA = .25, pclose = .11). Moreover, introduction of a bidirectional path between the VMPC and the temporal cortex did not improve the model significantly (χ2 = 1.00, df = 2, ns).
Figure 3.
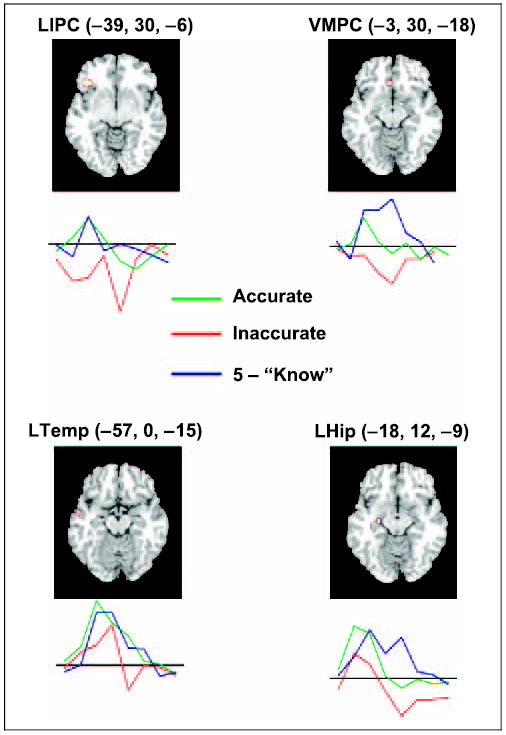
Four critical left hemisphere regions revealed by the contrast of accurate judgments > inaccurate judgments. The images and MNI coordinates indicate the local maximum. Below each region is a time-course graph of extracted signal levels used for SEM. The three conditions (accurate judgments, inaccurate judgments, and “know” trials) are indicated with separate colored lines and reflect data averaged across the identified cluster. The y-axis of this graph is normalized percent signal change and the x-axis is poststimulus time from 0 to 16 sec. All time courses are baselined to the average of −4 to 0 sec prestimulus onset. LIPC = left inferior prefrontal cortex; LHip = left hippocampus; LTemp = left temporal cortex; VMPC = ventral medial prefrontal cortex.
Figure 4.
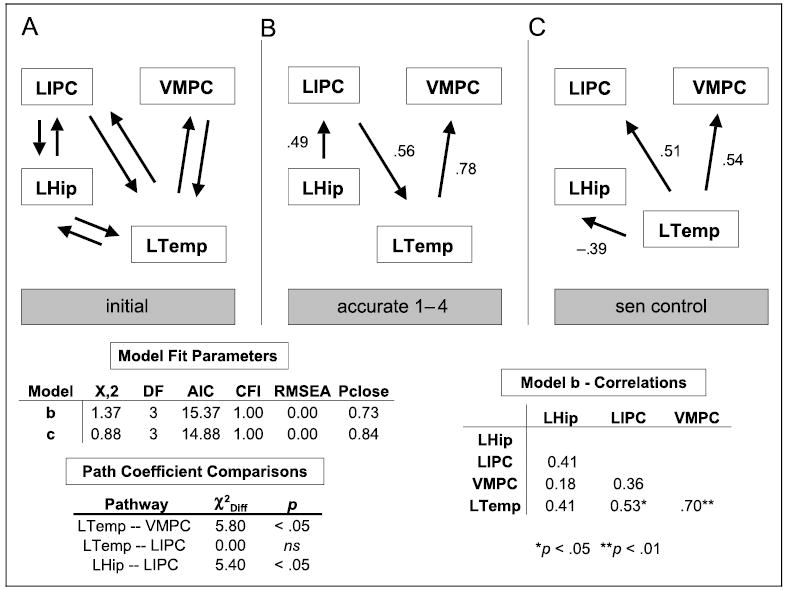
Results of SEM for regions involved in judgment accuracy. (A) The initial model utilized for SEM selection. (B) The model solution and pathways for the accurate 1–4 judgments. (C) The model solution and path coefficients for the sentence control task. Below the model schematic on the left are the fit parameters for the two emergent models and the results of path coefficients comparisons. A statistically significant chi-squared difference (χ2Diff) indicates that path coefficients are different between models B and C. On the right is the correlation matrix for the four regions used in model fitting for accurate 1–4 judgments.
In order to determine whether the resulting connectivity is specific to the monitoring demands associated with making accurate FOK judgments, values for the sentence control task, which makes low demands on monitoring, were fit to the model. The acceptable fit was considerably different from the model for 1–4 judgments, including the loss of significant connectivity between the hippocampus and the LIPC (see Figure 4C). In order to directly test the hypothesis that the link between the lateral temporal cortex and the VMPC is specific to a condition with high monitoring demands, an alternative model where the path coefficients for the high and low monitoring conditions were allowed to vary was contrasted with a constrained model where the coefficients were fixed to be equal (Buchel & Friston, 1997; McIntosh & Gonzalez-Lima, 1994). This resulted in a significantly better model fit for the alternative model (χ2 = 5.8, df = 1, p < .05), indicating an increase in the connectivity between the lateral temporal cortex and the VMPC under high monitoring conditions. Even though the pathway between the hippocampus and the LIPC was not significant in the sentence control task, this pathway was also tested for between-condition differences. There was a significant difference between the path coefficients for accurate 1–4 decisions and for the sentence control task (χ2 = 5.4, df = 1, p < .05). Finally, there was no significant between-condition difference in the path coefficients linking the LIPC and the VMPC (χ2 = 0.0, df = 1, ns), but the directionality of data flow was reversed.
Regions Modulated by 1–4 Ratings
To identify regions that were modulated by the judged level of retrievability, irrespective of the accuracy of that judgment, we identified regions whose level of activity was positively correlated with a linear parametric of 1–4 ratings. Given that FOK ratings were not correlated with rating latencies (Pearson’s correlation = .11, ns), regions identified by this parametric could not simply be a reflection of the time spent on the judgment. This analysis indicated a number of frontal, temporal, medial temporal, and posterior cortical regions that were significantly correlated with rating level (see Table 3). The frontal regions included extensive portions of the bilateral medial frontal cortex in the anterior cingulate and portions of the medial frontal gyrus that overlap with VMPC regions revealed in the accuracy network. In addition, extensive portions of the posterior cortex that were not evident in the accuracy network modulated with rating. These include the hippocampus, the parahippocampal gyrus bilaterally, and the posterior cingulate.
Table 3.
Brain Regions Linearly Modulated by 1–4 Rating
|
MNI Coordinates |
|||||
|---|---|---|---|---|---|
| Location (Brodmann) | x | y | z | t Max | |
| Frontal | Bilateral Medial Frontal Cortex (11)* | 0 | 33 | −6 | 3.53 |
| Left Orbital Frontal (10) | −12 | 48 | −9 | 2.82 | |
| Left Dorsal Medial Frontal (32) | −9 | 24 | 45 | 2.78 | |
| Left Middle Frontal (8, 9)* | −42 | 12 | 45 | 3.14 | |
| Left Superior Frontal Gyrus (8) | −18 | 27 | 54 | 2.8 | |
| Supplementary Motor Area (6) | −6 | 18 | 51 | 2.94 | |
| Right Supramaginal Gyrus (2) | 60 | −30 | 42 | 3.02 | |
| Medial Temporal | Left Parahippocampus (30, 36, 37)* | −27 | −42 | −3 | 3.49 |
| Left Hippocampus (20) | −33 | −18 | −9 | 3.02 | |
| Right Parahippocampus (27) | 18 | −33 | −9 | 2.47 | |
| Temporal | Left Middle Temporal Gyrus (37) | −54 | −60 | −3 | 3.59 |
| Right Inferior Temporal Gyrus (37) | 54 | −69 | −6 | 3.42 | |
| Left Fusiform (37) | −27 | −45 | −12 | 3.88 | |
| Posterior | Left Middle Occipital (19, 39)* | −27 | −84 | 33 | 3.43 |
| Left Precuneus (5) | −9 | −57 | 60 | 4.19 | |
| Right Precuneus (7) | 12 | −66 | 57 | 3.68 | |
| Left Posterior Cingulate (23) | −6 | −27 | 30 | 3.62 | |
| Left Inferior Parietal Lobe (40) | −45 | −42 | 54 | 3.2 | |
| Right Postcentral Gyrus (3) | 42 | −24 | 39 | 3.72 | |
| Right Inferior Parietal Lobe (2) | 45 | −39 | 54 | 2.56 | |
Only clusters with a significance level of p < .05 corrected are reported. Local maxima are indicated for > 8 mm apart per cluster. More than one cluster per region is indicated with an asterisk and only the greatest maxima is reported. The coordinates and their t values are at the peak voxels in each cluster. Approximate Brodmann’s area are shown in parenthesis.
For current purposes, we focus on the pattern of results in the VMPC, where peak amplitude values were tested for significant differences. An ANOVA of 1–4 ratings revealed a significant effect of rating [F(3,45) = 183.81, p < .001] with follow-up testing revealing significantly greater activation in the following pattern (4 > 3 > 2 > 1). When “know” ratings were added to this analysis, there was a significant quadratic fit to the data [F(1,15) = 51.73, p < .001], reflecting the fact that amplitude levels for “know” ratings did not continue the linear trend, but rather dropped off significantly. This was confirmed in follow-up testing that revealed that 2 and “know” ratings were not significantly different from each other [t(16) < 1]. Amplitudes, location, and t test results are shown in Figure 5.
Figure 5.
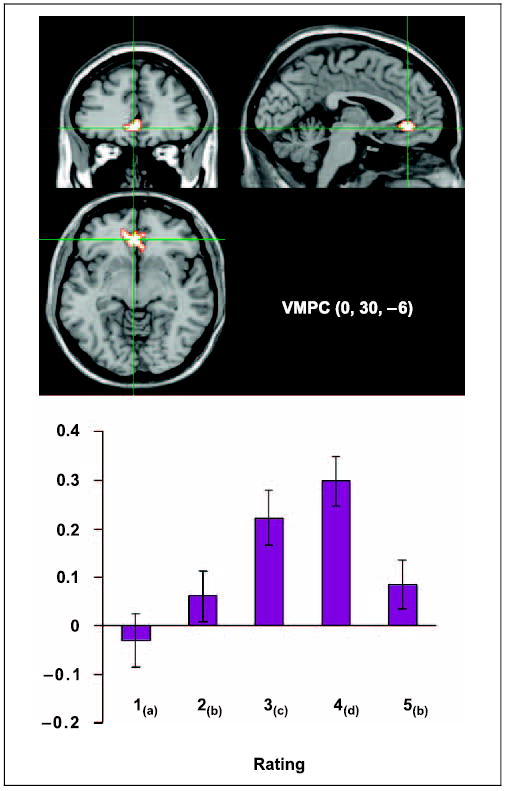
Region of the VMPC that modulates linearly with 1–4 ratings. The graph represents the model amplitude fitted in SPM99 for all ratings within the voxel identified by the group maxima (0, 30, −6). Ratings with subscripts in common are not significantly different from each other ( p < .05). The y-axis is percent signal change and error bars represent the standard error of the mean across subjects.
DISCUSSION
The results of this FOK study revealed important findings about the neuroanatomy of metamemory judgments. In particular, bilateral portions of the frontal cortex in the anterior IFC and the VMPC were activated during accurate retrieval judgments, regardless of actual recall2 or anticipated recognition of a target item. Thus, these two regions are unlikely to be associated with retrieval success, but rather, must play a role in the strategic functions that support memory retrieval (Moscovitch & Winocur, 2002; Nolde, Johnson, & Raye, 1998) or that monitor and evaluate the retrieval process (Schnyer et al., 2004).
Brain Regions Associated with Accurate Retrieval Predictions
Of the two frontal regions revealed in the conjunction analysis, the role of the IFC in memory retrieval tasks is probably best understood. Research has converged on the notion that the left IFC plays a strategic role in the retrieval process itself, through functions such as cue specification or cue selection (Buckner, 2003; Dobbins, Foley, Schacter, & Wagner, 2002; Wagner, Pare-Blagoev, Clark, & Poldrack, 2001). Such cue specification appears to depend on controlled semantic analysis. Dobbins et al. (2002) showed that a similar region of the anterior IFC was active both during encoding, when participants made one of two semantic judgments about items, and during retrieval, when they indicated which judgment had been made at encoding. They suggested that IFC activation reflects controlled semantic analysis that results in the generation of effective retrieval cues. Analogously, in the context of the current task, IFC activation during retrieval may reflect the generation of cues that are relevant to the sentence context. These cues in turn trigger memory contents on which judgments of accessibility are based.
In contrast to the role of the IFC during retrieval, the function of the VMPC in memory is less clear. Few fMRI studies of memory have demonstrated VMPC activation3 (see Maguire et al., 2001 for a notable exception), but a number of studies of episodic retrieval using positron emission tomography have demonstrated involvement of medial frontal regions, and in particular, area 11 (see Grady, 1999). One suggested role for the VMPC in memory is the intuitive assessment of the “felt-rightness” of a memory (Moscovitch & Winocur, 2002). Applied to the present study, it could be argued that the VMPC is critically involved in assessing whether information triggered by the retrieval process is contextually appropriate. This is also consistent with studies of confabulation, which have demonstrated that the VMPC is often the site of damage related to this phenomenon (Schnider & Ptak, 1999). The theoretical formulations for confabulation vary but there is broad agreement that it reflects an inability to monitor the appropriateness of retrieved memories (Schnider & Ptak, 1999). Regardless of the specific interpretation, it is clear that the functional role of the VMPC lies in monitoring memory contents rather than in supporting the retrieval process itself, as is the case for the IFC.
To further examine the notion that the VMPC serves a monitoring function during retrieval, we tested a structural equations model made up of four key components of the accuracy network: the VMPC, the IFC, the hippocampus, and the lateral temporal cortex. The latter three regions were implemented in an interactive retrieval network, on the assumption that the IFC is involved in the generation of cues that trigger memory contents, the storage and retrieval of which depends on the hippocampus and lateral temporal cortex (Buckner, 2003; Simons & Spiers, 2003). With this retrieval network as a basis, the best fitting orientation of the VMPC consisted of a directional path leading from the lateral temporal cortex to the VMPC. This solution is consistent with the known structural connectivity of these two regions through the uncinate fasciculus (Petrides & Pandya, 2002). Finally, significant differences in the functional connectivity between these two regions were observed in conditions that vary in their demands on retrieval monitoring. Although only confirmatory in nature, the resulting SEM is consistent with the notion that the VMPC monitors the retrieval process.
The resulting path directions revealed through SEM make a clear prediction as to the direction of data flow. Therefore, it could be postulated that support for the solution may be found in the time course of the fMRI data. Such an analysis using fMRI data is currently problematic and would require acceptance of a number of questionable assumptions. For example, in order to make between-region time course comparisons, it would need to be assumed that each region’s vascular response to the onset of a local process is identical. There are now data showing that this assumption may not be valid for cortical regions (Miezin, Maccotta, Ollinger, Peterson, & Buckner, 2000) and it may be even more problematic for subcortical–cortical comparisons. In addition, there is no consensus as to the proper data-analytic approach for determining time course differences, although a number of recent articles have begun to explore different techniques (Henson, Price, Rugg, Turner, & Friston, 2002; Friston et al., 1998). Future examinations of this model using methodologies with better temporal resolution will be required to further evaluate its validity.
One question raised by our findings concerns how a common mechanism can be responsible for accurate judgments that recognition will be based on guessing and for accurate judgments that recognition likely will be successful. By our view, accuracy of FOK judgments depends on the effective engagement of cue specification, content retrieval, and evaluation of the retrieved memory fragments, regardless of the ultimate determination of accessibility of appropriate content. “I will guess” judgments informed by effective implementation of this process are more likely to be accurate than those randomly determined, and can be just as accurate as those for which content was deemed to be readily accessible.
“Know” judgments, by contrast, may involve additional processes associated with successful retrieval of the target item. Contrasting with the regions commonly active for both accurate “know” and accurate 1–4 judgments, a number of posterior regions were active only during “know” judgments. These included the parahippocampal gyrus, the inferior temporal cortex, the inferior parietal lobe, and the posterior cingulate. Activation in the parahippocampal gyrus associated with successful retrieval was also observed in the previous FOK study (Maril et al., 2003). We postulate that activity in these regions reflects the reactivation of the stored memory representation. Consistent with this notion, parahippocampal (Ranganath et al., 2003; Cabeza, Rao, Wagner, Mayer, & Schacter, 2001) and posterior cingulate (Eldridge, Knowlton, Furmanski, Bookheimer, & Engel, 2000; Henson, Rugg, Shallice, Josephs, & Dolan, 1999) activation have previously been associated with successful retrieval in the context of standard memory tasks.
There was only a single region, the dorsal anterior cingulate, which was engaged during accurate 1–4 judgments but not during “know” judgments. Given the significant RT differences between these two categories of responses, it is possible that activity in this region reflects increased attentional demands (Weissman, Gopalakrishnan, Hazlett, & Woldorff, 2005).
Brain Regions Modulated by FOK Rating
Koriat (1993) has suggested that accessibility assessment is largely based on the quantity of information produced during a retrieval attempt, and this is reflected in the magnitude of FOK ratings. If the VMPC is directly involved in this computation, it follows that this region should be sensitive to the rate at which information accrues. Consistent with this notion, VMPC activation modulated linearly with 1–4 ratings. Inclusion of “know” judgments resulted in a quadratic trend, which likely reflects the fact that the retrieval process for these items was terminated early upon successful completion of the sentence cue. Importantly, the quadratic trend argues against the possibility that VMPC activation simply reflects perceived ease of retrieval, because ease of retrieval should be associated with a linear trend, reflecting continued increase in activation from “I guess” to “know” judgments (Ranganath et al., 2003).
Other portions of the accuracy network, including the medial and lateral temporal cortex, also modulated with rating, as would be predicted if FOK ratings reflect retrieval of memory contents. By contrast, IFC activation did not track with FOK ratings. If, as postulated, the IFC is critically involved in cue specification, then activity in this region would not be expected to track with ratings, as the amount of information triggered by any particular cue is unpredictable.
The lack of modulation by rating in the IFC stands in contrast to the finding of Maril et al. (2003), who observed a graded pattern of activation in the left IFC. One possible reason for the discrepancy between our finding and theirs lies in the way retrieval cues are used in the two studies. Because of the use of meaningful sentences, our study may have encouraged the generation of multiple retrieval cues, none of which were inherently predictive of retrieval success. By contrast, Maril et al. asked participants to form an image incorporating two unrelated words. Presumably, because the words are semantically unrelated, few cues other than the image would be generated, but generation of the image would be directly predictive of retrieval success. Future studies that directly manipulate the availability of retrieval cues will be needed to test this possibility.
VMPC and Meta-Level Processing
Although few fMRI studies of memory have focused on the functioning of the VMPC, this region has received considerable attention in the context of studies on the neural basis of self (e.g., Gallagher & Frith, 2003). Summarizing a broad range of neuroimaging research, Frith and Frith (1999) concluded that the VMPC may be involved in mentalizing about one’s own internal states (see also Kelley et al., 2002; Shallice, 2001). The question arises as to whether a similar focus on self can explain our findings. In making FOK judgments, participants focused on internally available information. However, if the VMPC was driven only by this internal focus, one would not expect differences in activation level as a function of accuracy or rating.
Another way to conceptualize the role of the VMPC is with reference to what Nelson and Narens (1990) have referred to as metacognition. By their view, meta-level operations involve maintaining a mental model of the relevant context and comparing the available evidence against that model. Making judgments about internal states of the self or others requires activating a model representation to which presented information is compared (Frith & Frith, 1999). Analogously, making FOK judgments requires that participants hold in mind a model about memory functioning that specifies how the retrieval of memory fragments translates into the subsequent ability to recognize the sought-after information. Ultimately, judgments of retrievability are made on the basis of weighing how retrieved memory fragments fit this model.
The idea that the VMPC participates in making judgments about the fit of data to a model is also consistent with formulations of memory confabulation (Schnider & Ptak, 1999). Patients exhibiting confabulation tell wildly inaccurate tales about their lives or current circumstances. These confabulations result from the failure to evaluate memory fragments against an internal representation of their life. Future research about the VMPC may benefit from examining the extent to which these meta-level models are fixed representations or are created on-line as a function of the available contextual information.
METHODS
Participants
Twenty-two native speakers of English (13 women, 9 men), ranging in age from 19–32 years (mean age = 23) participated in the experiment. They were remunerated US$50 for their participation. Participants had normal or corrected-to-normal vision and were free of past or current neurological disorders, brain injury, or psychiatric disability. Written informed consent was obtained from each volunteer prior to the scanning session. The Human Subjects Committees of Boston University School of Medicine, Massachusetts General Hospital, and the Department of Veteran Affairs Medical Center approved all procedures.
The results of four participants were excluded because data from two or three study/test cycles were not usable. For 5 of the 19 remaining participants, results were based on only two runs. This was due to a combination of excessive movement (n = 2), computer malfunction (n = 1), and chance performance levels (n = 2).
Materials
Stimuli consisted of 201 sentences selected from a larger set of sentences for which multiple final completion words were normed on a group of college students (Bloom & Fischler, 1980). Sentences were selected for having a minimum of five possible completions and the chosen target completion was the third most prevalent word produced (average completion for the target = 11%). The remaining four most prevalent completions were used as foils in the recognition task. Sentences were chosen or altered not to include proper names. From the 201 sentences, 150 sentences were randomly selected as experimental stimuli, with the remaining 51 sentences used as control sentences during the test phase. Three partially counterbalanced sets were constructed which rotated the experimental and control sentences. Each of these sets of materials was further subdivided into three separate study/test runs. The assignment of sets of materials was counterbalanced across participants.
Procedure
While in the MRI scanner each participant engaged in three study/test runs. During each study phase, participants silently read 40 sentences for a later memory test. Presentation was self-paced and a button press moved the display to the next sentence. No MR scanning was conducted during the study phase.
Immediately following each of the study phases, participants engaged in a test phase during which functional MR scans were obtained. Each test phase consisted of 50 experimental trials (40 studied, 10 non-studied) and 17 control trials that were randomly interspersed. On experimental trials, participants first made a retrieval judgment and then a recognition decision (see Figure 1). Each trial started with the presentation of a sentence with the final word missing. Participants were instructed to rate the likelihood that they would recognize the studied sentence completion from among five alternatives by pressing one of five response buttons (1 = guess, 2 = 25% chance, 3 = 50% chance, 4 = 75% chance, and 5 = I know it!). Prior to the first study phase, participants were given explicit instructions for making these judgments:
Only press 5 if the answer pops effortlessly to mind. If you feel that the answer is just there under the surface press 4. If you have no recollection of ever having seen the sentence then press 1. Make sure to use the full rating scale.
Participants were given up to 10 sec to make their judgment. Five hundred milliseconds after responding, a fixation screen appeared consisting of a series of Xs organized in a sentence pattern. The fixation pattern remained on the screen for a variable period of 6, 8, or 10 sec, which aided optimization of the fMRI design by providing temporal jittering between conditions. This was followed by re-presentation of the sentence fragment with five alternative sentence completions. Participants were asked to select the studied sentence completion by using one of five response keys. They had a maximum of 10 sec to respond, and the sentence frame and completions were removed from the screen 500 msec after the response.
On control trials, a complete sentence was presented. The participant was asked to read and remember the sentence for a later memory test and to indicate the position of the first three-letter word by pressing one of five response buttons. Control trials were distinguished from experimental trials by the use of a different color for stimulus presentation. The complete procedure was explained to participants and a short practice session of 20 sentences was conducted in the scanner prior to the first study/test run.
MRI Acquisition and Processing
Scanning was performed on a 3.0-T Siemens Trio whole-body MRI system with a whole-head coil. Functional data were acquired using a gradient-echo EPI pulse sequence that was optimized to reduce susceptibility artifact in the medial frontal cortex (TR = 2 sec, TE = 23 msec, 31 axial slices oriented for best whole head coverage, acquisition voxel size = 3.125 −3.125 −3 mm with a 0.3 mm interslice gap). The first four EPI volumes were discarded to allow scans to reach equilibrium. Because the trials were partially self-paced, the total number of scans acquired varied across participants. Anatomical high-resolution T1 scans were obtained during the same session. Head movement was minimized using a foam pillow, which was placed around the head and wedged into the head coil. The visual stimuli were presented with a computer-controlled LCD projector onto a back projection screen placed in the back of the scanner bore through a mirror mounted on the head coil. When necessary, vision was corrected to normal using MRI compatible plastic lenses and frame.
Data were processed using SPM99 (Welcome Department of Cognitive Neurology, London). Preprocessing of the functional MR data involved several steps. First, images were corrected for offsets in the time of acquisition by resampling all slices to match the 15th slice of the volume (approximate temporal/spatial “middle slice”). Following this, images were motion corrected across runs and then spatially normalized to an EPI template normalized in MNI stereotactic space, using both a 12-parameter affine transformation and a nonlinear transformation consisting of cosine basis functions. Finally, the images were resampled into 3 mm3 voxels, spatially smoothed with an 8-mm full width at half maximum Gaussian kernel, and rescaled to a mean signal value of 100. Individual statistical images were calculated using the general linear model for event-related designs in SPM.
Following application of a high-pass filter constructed of a basis set of cosine functions, individual events were modeled using a canonical hemodynamic response and its first-order temporal derivative. These included the judgment period for experimental trials, the judgment period for control trials, the delay period between judgment and recognition (using variable duration estimates), and the recognition period. Because the recognition period was always preceded by an extended delay during which participants’ behavior was uncontrolled, the resulting fits to that modeling are not examined further.
To evaluate hemodynamic responses during the retrieval judgment period, trials on which participants gave a 1–4 rating and trials on which participants gave a correct 5 rating (“know” judgments) were modeled separately. No judgment period reached the 10-sec timeout value and consequently all judgment periods were included in the analysis. “Know” responses were separated out because it was assumed that these responses reflect instantaneous recall, and therefore reflected both recall as well as the evaluation of that recall. Incorrect “know” judgments were separated out and modeled as a nuisance variable. Retrieval judgments rated 1–4 were evaluated by two approaches. First, judgments were modeled separately for accurate and inaccurate predictions. Accurate judgments were defined as low (1–2) ratings accompanied by an incorrect recognition decision or high (3–4) ratings accompanied by a correct recognition decision. By definition, recognition decisions to unstudied sentences were incorrect. Values from this modeling approach were also used in SEM described below. Second, all 1–4 decisions were modeled using a canonical hemodynamic response (without a temporal derivative) with the rating (1–4) entered into a first-order linearly modulated parametric estimate.
In all cases, the resulting least squares parameter estimates of the height of the modeled hemodynamic response from the modeling described above were used to construct contrast images on the basis of the canonical hemodynamic response function for each subject. These contrast images were tested against a null of no difference between contrast conditions using a one-tailed t test, the result of which served as a random effects analysis across subjects. The statistical threshold for the resulting random effects analysis was determined using a cluster-based correction for multiple comparisons (Slotnick, 2002). This calculation indicated that a 19 resampled voxel extent (513 mm3) using an individual voxel threshold of p = .01 would result in statistical maps with a corrected significance level of p < .05 (corrected for multiple voxel comparisons). The threshold used for conjunctional analysis was the same as utilized for each contrast individually (Brett, Nichols, Andersson, Wager, & Poline, 2004), p < .05, corrected.
SEM was conducted on mean signal amplitude values that were extracted using tools developed for this purpose by Poldrack (http://spm-toolbox.sourceforge.net). These tools perform peristimulus time averaging of each condition using the finite impulse response model option. Averaging is performed across a 0 to 22 sec poststimulus onset window with a 4-sec prestimulus baseline after high-/low-pass filtering and adjustment for other modeled effects. For mean activation levels used in SEM analysis, the percent signal change for all above-threshold voxels within an 8-mm radius of each of the SPM maxima (identified using the method indicated above, p < .05, corrected) was averaged across the 2 to 6 sec poststimulus onset time bin. Amplitude comparisons within regions modulated by the 1–4 rating were extracted using the parametric estimation model fit within SPM for the 1–4 ratings and the mean amplitude for the “know” trials.
SEM was performed using a maximum likelihood estimation in Amos 5.0 (SmallWaters, USA). The statistical goodness-of-fit was assessed using a χ2 test and three practical fit indices, the comparative fit index (CFI; Bentler, 1990), the root mean square error of approximation (RMSEA; Browne & Cudeck, 1992), and the Akaike information criterion (AIC; Akaike, 1973). A significant χ2 indicates that a model does not sufficiently account for the covariance matrix, however, it is highly dependent on the sample size, whereas the three fit indexes are constructed to be less influenced by sample size. The first step of model identification utilizes the AIC, which takes into account model parsimony, providing a distinct rank ordering of model fits. For the remaining indices, a general rule of thumb is that the closer the CFI is to 1 the better the model fit and fits of less than .9 can usually be substantially improved. RMSEA tests the closeness of the model fit. Models with RMSEA values of .05 or less and a pclose > .05 generally can be considered acceptable. Although small sample sizes can be problematic in SEM, the methodology has been used reliably in a number of functional imaging studies with similar samples sizes (Kilpatrick & Cahill, 2003; Maguire et al., 2001). Once an acceptable model structure is determined, sample size is less of an issue for between-condition tests of critical pathways.
Notes
Contrasting accurate retrieval judgments with the sentence control results in the same pattern of corresponding frontal regions with additional regions located in the temporal, parietal, and occipital cortex.
Although we did not directly assess spontaneous recall, we assume that “know” judgments reflect successful recall, as the instructions emphasized that these judgments should be reserved for target completions that came immediately to mind. Supporting participants’ compliance with these instructions are the fast reaction times and high recognition accuracy associated with these items.
One reason for this may be that the region of the VMPC is difficult to image effectively using fMRI, due to large susceptibility artifacts and epi distortions that reduce magnetic resonance signal in this region. The current study utilized a low TE value of 23 msec to reduce this signal dropout and distortion, and may therefore have been more successful at finding signal differences in this region.
Acknowledgments
We thank Ian Dobbins and Russ Poldrack for their helpful assistance with the fMRI analysis. This work was supported by K23MH64004 and P50 NS26985 to the Boston University and by the Medical Research Service of the Department of Veterans Affairs.
Footnotes
The data reported in this experiment have been deposited with The fMRI Data Center archive (www.fmridc.org). The accession number is 2-2004-117WD.
References
- Akaike, H. (1973). Information theory and an extension of the maximum likelihood principle. In B. N. Petrov & F. Csaki (Eds.), Proceedings of the 2nd international symposium on information theory (pp. 267–281). Budapest: Akademiai Kiado.
- Becker S, Lim J. A computational model of prefrontal control in free recall: Strategic memory use in the California Verbal Learning Task. Journal of Cognitive Neuroscience. 2003;15:821–832. doi: 10.1162/089892903322370744. [DOI] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Bloom PA, Fischler I. “Completion norms for 329 sentence contexts”. Memory and Cognition. 1980;8:631–642. doi: 10.3758/bf03213783. [DOI] [PubMed] [Google Scholar]
- Brett, M., Nichols, T., Andersson, J., Wager, T., & Poline, J.-B. (2004). When is a conjunction not a conjunction? Paper presented at the Annual Meeting of the Organization of Human Brain Mapping, Budapest.
- Browne, M. W., & Cudeck, R. (1992). Alternative ways of assessing model fit. In K. A. Bollen & J. S. Long (Eds.), Testing structural equation models (pp. 136–162). Newbury Park, CA: Sage.
- Buchel C, Friston KJ. Modulation of connectivity in visual pathways by attention: Cortical interactions evaluated with structural equations modeling. Cerebral Cortex. 1997;7:768–778. doi: 10.1093/cercor/7.8.768. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Functional–anatomic correlates of control processes in memory. Journal of Neuroscience. 2003;23:3999–4004. doi: 10.1523/JNEUROSCI.23-10-03999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Rao SM, Wagner AD, Mayer AR, Schacter DL. Can medial temporal lobe regions distinguish true from false? An event-related functional MRI study of veridical and illusory recognition memory. Proceedings of the National Academy of Sciences, USA. 2001;98:4805–4810. doi: 10.1073/pnas.081082698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: Multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: A selective role for the hippocampus during retrieval. Nature Neuroscience. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Henson RNA. Frontal lobes and human memory. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: Characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. “Interacting minds: A biological basis”. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of “theory of mind”. Trends in Cognitive Sciences. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Grady, C. L. (1999). Neuroimaging and activation of the frontal lobes. In B. L. Miller & J. L. Cummings (Eds.), The human frontal lobes: Functions and disorders (pp. 196–230). New York: Guilford.
- Grady CL, McIntosh AR, Rajah MN, Craik FIM. Neural correlates of episodic encoding of pictures and words. Proceedings of the National Academy of Sciences, USA. 1998;95:2703–2708. doi: 10.1073/pnas.95.5.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RNA, Price CJ, Rugg MD, Turner R, Friston KJ. Detecting latency differences in event-related BOLD responses: Application to words verses nonwords and initial verses repeated face presentations. Neuroimage. 2002;15:83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: An event-related fMRI study. Journal of Neuroscience. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky JS, Shimamura AP, Squire LR. Memory and metamemory: Comparisons between patients with frontal lobe lesions and amnesic patients. Psychobiology. 1989;17:3–11. [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kikyo H, Ohki K, Miyashita Y. Neural correlates for feeling-of-knowing: An fMRI parametric analysis. Neuron. 2002;36:177–186. doi: 10.1016/s0896-6273(02)00939-x. [DOI] [PubMed] [Google Scholar]
- Kilpatrick L, Cahill L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. Neuroimage. 2003;20:2091–2099. doi: 10.1016/j.neuroimage.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Koriat A. How do we know that we know: The accesibility model of the feeling of knowing. Psychological Review. 1993;100:609–639. doi: 10.1037/0033-295x.100.4.609. [DOI] [PubMed] [Google Scholar]
- Koriat A. The feeling of knowing: Some metatheoretical implications for consciousness and control. Consciousness and Cognition. 2000;9:149–171. doi: 10.1006/ccog.2000.0433. [DOI] [PubMed] [Google Scholar]
- Koriat A, Levy-Sadot R. The combined contributions of the cue-familiarity and accessibility heuristics to feelings of knowing. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:34–53. [PubMed] [Google Scholar]
- Maguire EA, Vargha-Khadem F, Mishkin M. The effects of bilateral hippocampal damage on fMRI regional activations during memory retrieval. Brain. 2001;124:1156–1170. doi: 10.1093/brain/124.6.1156. [DOI] [PubMed] [Google Scholar]
- Maril A, Simons JS, Mitchell JP, Schwartz BL, Schacter DL. Feeling-of-knowing in episodic memory: An event-related fMRI study. Neuroimage. 2003;18:827–836. doi: 10.1016/s1053-8119(03)00014-4. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F. Structural equations modeling and its application to network analysis in functional brain imaging. Human Brain Mapping. 1994;2:2–22. [Google Scholar]
- Metcalfe J. Novelty monitoring, metacognition, and control in a composite holographic associative recall model: Implications for Korsakoff amnesia. Psychological Review. 1993;100:3–22. doi: 10.1037/0033-295x.100.1.3. [DOI] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Peterson SE, Buckner RL. Characterizing the hemodynamic response: Effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Miner, A. C., & Reder, L. M. (1994). A new look at feeling of knowing: Its metacognitive role in regulating question answering. In J. Metcalfe & A. P. Shimamura (Eds.), Metacognition: Knowing about knowing (pp. 47–70). Cambridge: MIT Press.
- Moscovitch, M., & Winocur, G. (2002). The frontal cortex and working with memory. In D. T. Stuss & R. T. Knight (Eds.), Principles of frontal lobe function (pp. 188–209). New York: Oxford University Press.
- Nelson, T. O., & Narens, L. (1990). Metamemory: A theoretical framework and new findings. In G. H. Bower (Ed.), The psychology of learning and motivation: Advances in research and theory (Vol. 26, pp. 125–173). San Diego: Academic Press.
- Nolde, S. F., Johnson, M. K., & Raye, C. L. (1998). The role of prefrontal cortex during tests of episodic memory. [DOI] [PubMed]
- Trends in Cognitive Sciences, 2, 399–406. Petrides, M., & Pandya, D. N. (2002). Association pathways of the prefrontal cortex and functional observations. In D. T. Stuss & R. T. Knight (Eds.), Principles of frontal lobe function. Oxford: Oxford University Press.
- Price CJ, Mummery CJ, Moore C. J. Frakowiak, R. S, Friston, K. J. “Delineating necessary and sufficient neural systems with functional imaging studies of neuropsychological patients”. Journal of Cognitive Neuroscience. 1999;11:371–382. doi: 10.1162/089892999563481. [DOI] [PubMed] [Google Scholar]
- Price CJ, Warburton EA, Moore CJ, Richard SJ, Frackowiak SJ, Friston KJ. “Dynamic diaschisis: Anatomically remote and context-sensitive human brain lesions”. Journal of Cognitive Neuroscience. 2001;13:419–429. doi: 10.1162/08989290152001853. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D’Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2003;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Reder LM. “Strategy selection in question answering”. Cognitive Psychology. 1987;19:90–137. [Google Scholar]
- Reder LM, Ritter FE. What determines initial feeling of knowing? Familiarity with question terms, not with the answer. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1992;18:435–451. [Google Scholar]
- Schnider A, Ptak R. Spontaneous confabulators fail to suppress currently irrelevant memory traces. Nature Neuroscience. 1999;2:677–681. doi: 10.1038/10236. [DOI] [PubMed] [Google Scholar]
- Schnyer DM, Verfaellie M, Alexander MP, Lafleche G, Nicholls L, Kaszniak AW. A role for right medial prefrontal cortex in accurate feeling of knowing judgments: Evidence from patients with lesions to frontal cortex. Neuropsychologia. 2004;42:957–966. doi: 10.1016/j.neuropsychologia.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Schraw G. Measures of feeling-of-knowing accuracy: A new look at an old problem. Applied Cognitive Psychology. 1995;9:321–332. [Google Scholar]
- Shallice T. Theory of mind and the prefrontal cortex. Brain. 2001;124:247–248. doi: 10.1093/brain/124.2.247. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Squire LR. Memory and metamemory: A study of feeling-of-knowing phenomenon in amnesic patients. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1986;12:452–460. doi: 10.1037//0278-7393.12.3.452. [DOI] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long term memory. Nature Reviews: Neuroscience. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Slotnick, S. D. (2002). Cluster_threshold_alpha. Version retrieved December 2003, from http://www.wjh.harvard.edu/~slotnick/scripts.htm Boston.
- Souchay C, Isingrini M, Espagnet L. Aging, episodic memory feeling-of-knowing, and frontal functioning. Neurospychology. 2000;14:299–309. doi: 10.1037//0894-4105.14.2.299. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Gopalakrishnan A, Hazlett CJ, Woldorff MG. Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cerebral Cortex. 2005;5:229–237. doi: 10.1093/cercor/bhh125. [DOI] [PubMed] [Google Scholar]


