Abstract
Background:
The tyrosine kinase Fyn previously has been shown to play a key role in mediating acute tolerance to ethanol. Recently, we found that the compartmentalization of Fyn to the NR2B subunit of the NMDA receptor (NMDAR) in the hippocampus regulates Fyn phosphorylation of NR2B in response to ethanol, which mediates the acute tolerance of NMDAR to ethanol inhibition in hippocampal slices. In this study we determined, first, whether acute tolerance to ethanol inhibition is mediated via NR2B-containing NMDARs in vivo and, second, whether the increase in acute sensitivity to ethanol in the Fyn−/− mice influences ethanol consumption or ethanol’s conditioned rewarding effects.
Methods:
A loss of righting reflex test was used to study the acute/sedative effects of ethanol after intraperitoneal injections of sedative doses of ethanol. Conditioned place preference was used to study the rewarding properties of ethanol. The two-bottle choice protocol was used to measure oral ethanol self-administration and preference as described previously.
Results:
We found that systemic injection of the NR2B-containing NMDAR selective antagonist, ifenprodil, abolished the differences between Fyn+/+ and Fyn−/− mice in sensitivity to the acute sedative effects of ethanol. Moreover, we found that Fyn−/− and Fyn+/+ mice did not differ in their voluntary ethanol consumption or in the rewarding properties of ethanol.
Conclusions:
Our results suggest that the interaction between Fyn and NR2B mediates the acute sedative effects of ethanol, and that alteration in acute ethanol sensitivity does not necessarily correlate with levels of ethanol consumption or the rewarding properties of ethanol.
Keywords: Fyn, NR2B, Phosphorylation, Ethanol, Acute Sensitivity, Self-Administration
FYN KINASE IS a member of the Src family of protein tyrosine kinases and is expressed in the adult brain (Bare et al., 1993; Zhao et al., 2000). Fyn plays an important role in processes linked to synaptic plasticity via its interactions with the ionotrophic glutamate receptor channel, the N-methyl-d-aspartate receptor (NMDAR). NMDAR-dependent long-term potentiation (LTP) in the CA1 region of the hippocampus is blunted in mice in which the Fyn gene is deleted (Fyn−/−) (Grant et al., 1992); however, LTP was rescued when the wild-type Fyn gene was introduced into the Fyn−/− mice (Kojima et al., 1997). Subsequent studies further suggested that Fyn is important for the induction of LTP (Lu et al., 1998, 1999). NMDAR is a major target for ethanol (Allgaier, 2002; Kumari and Ticku, 2000). It has been shown that deletion of the Fyn gene eliminates the development of acute tolerance to ethanol inhibition in hippocampal slices taken from Fyn−/−mice compared with Fyn+/−littermates, and the duration of the loss of righting reflex (LORR) after ethanol administration was longer for Fyn−/−mice than for Fyn+/− mice (Miyakawa et al., 1997). These findings suggest that the duration of the LORR may depend on the degree of acute tolerance that occurs after ethanol administration. Furthermore, these results suggest that the interaction between Fyn and the NMDAR is important for mediating ethanol-induced behaviors.
Among the processes that regulate NMDAR channel function is tyrosine phosphorylation of the long intracellular tail of the NR2B subunit by Src family protein tyrosine kinases, including Fyn (Nakazawa et al., 2001; Yaka et al. 2002, 2003b), and this phosphorylation enhances channel function (Yaka et al., 2002, 2003b). We and others found that acute exposure of slices to ethanol, as well as systemic injection of ethanol to mice, results in increased NR2B phosphorylation in the hippocampus that is mediated by Fyn (Miyakawa et al., 1997; Yaka et al., 2003a). Recently, we found that compartmentalization of Fyn to the NR2B subunit of the NMDAR in the hippocampus regulates the enhanced Fyn phosphorylation of NR2B in response to acute ethanol administration. We also found that the Fyn-dependent increase in NR2B phosphorylation induced by ethanol mediates the acute tolerance to ethanol inhibition in hippocampal slice preparation (Yaka et al., 2003a). Our results suggest that the absence of Fyn-mediated phosphorylation of the NR2B subunit, and hence an absence of acute tolerance to ethanol’s effects at this receptor subtype, mediate the increase in ethanol-induced sedation found in Fyn−/− mice. If this is so, then blockade of acute tolerance at this receptor with the NR2B-selective antagonist, ifenprodil, should alter the LORR in mice expressing the Fyn gene (and hence expressing acute tolerance) but not in mice with no Fyn expression. Therefore, in the present study we tested whether blockade of NR2B-containing NMDARs alters ethanol’s acute effects in the Fyn+/+ and Fyn−/− mice.
Previous studies have demonstrated that deletion of specific genes increases acute sensitivity to ethanol and that this increase was associated with a decrease in ethanol consumption (Hodge et al., 1999; Phillips et al., 1998) and vice versa (Thiele et al., 2002). These studies suggest that alteration in acute sensitivity to ethanol can predict ethanol intake. Because Fyn−/− mice are more sensitive to ethanol’s acute effects, we hypothesized that deletion of the Fyn gene will decrease ethanol intake and ethanol’s rewarding properties. Therefore, we compared voluntary ethanol intake and ethanol-induced conditioned place preference (CPP) in Fyn−/− mice and Fyn+/+ littermate controls.
We found that inhibition of NR2B-containing NMDARs resulted in an increased sensitivity to the acute sedative effects of ethanol in Fyn+/+ but not in Fyn−/− mice. However, Fyn+/+ and Fyn−/− mice did not differ in their voluntary ethanol consumption and preference or in the conditioned rewarding properties of ethanol.
MATERIALS AND METHODS
Animals
Experimental protocols involving the use of animals were approved by the Gallo Research Center Institutional Animal Care and Use Committee and met National Institutes of Health guidelines. Mating pairs of Fyn−/− (C57/Bl6;129s) male and C57/Bl6 female mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were mated in-house to generate F1 Fyn+/− offspring. F1 heterozygote mice then were mated to generate F2 Fyn−/− mice and their corresponding Fyn+/+ littermate controls, and these mice are termed as Fyn+/+ (C57) and Fyn−/− (C57). The Fyn−/− and Fyn+/+ mice primarily used in the study were generated from F2 of Fyn−/− (C57) mice that were mated with pure 129svj wild-type mice (Jackson Laboratories), and these mice are termed as Fyn+/+ (129) and Fyn−/− (129). The genotyping of mice was determined by reverse transcription-polymerase chain reaction analysis of products derived from tail DNA. Only F2 Fyn−/− and Fyn+/+ male mice were used in these studies. The age of the individuals ranged from 8 to 10 weeks at the start of each experiment. After weaning, mice were randomly (with respect to the genotype) housed in groups of five individuals in Plexiglas cages. Food and water were available ad libitum. Animals were kept in conditions of constant temperature (23°C) and humidity (50%) in a light-dark cycle (lights on from 6:00 AM to 6:00 PM).
Loss of Righting Reflex
Ethanol (3.6 or 4.0 g/kg, 20% solution) was injected intraperitoneally, and individual mice were placed immediately in a clean Plexiglas cage. For ifenprodil experiments, mice were injected intraperitoneally with ifenprodil (3.0 mg/kg) followed 30 min later by ethanol (4.0 g/kg, intraperitoneally). After the mice lost the righting reflex, they were put on their backs in their home cage. The duration of LORR was defined as the time from the loss of the righting reflex to the time at which it was regained. Recovery was defined as the time at which mice could right themselves three times in 1 min after being placed on their backs. The behavioral room was illuminated with a soft light, and external noise was attenuated.
Voluntary Ethanol Consumption
Oral ethanol self-administration and preference were examined using a two-bottle choice protocol as described previously (Crabbe et al., 1996; Phillips et al., 1998). Mice were housed individually, and 1 week later, mice were given access to two bottles, one containing ethanol in tap water and the other containing tap water alone. Two-bottle drinking sessions were conducted 23 hr per day, 7 days per week. During the course of the exposure period, ethanol concentration was increased gradually from 3% to 20% (3%, 6%, 10%, and 20%) with 4 days of access at each concentration. Each day, the mice were weighed and placed in individual holding chambers while the fluids were replaced on the home cage. Fluid levels were recorded at the beginning and end of 23 hr fluid access periods. The position (left or right) of each solution was alternated daily to control for side preferences.
Conditioned Place Preference
The rewarding effects of ethanol were assessed using the CPP paradigm (Care et al., 1989). The testing apparatus consisted of two 16.8 × 12.7 cm compartments with distinct visual and tactile cues (i.e., one with white-colored walls and stainless steel mesh flooring, the other with black-colored walls and flooring consisting of stainless steel rods placed on 8 mm centers; Med Associates, Lafayette, IN). A 2.8 × 12.7 cm center compartment, with gray walls and solid plastic flooring, connected the two compartments. The center compartment was equipped with two computer-controlled guillotine doors that provided access to one or both of the conditioning compartments. On the first day of testing (habituation session), all mice received a saline injection and were given access to both conditioning compartments for 5 min. Over the next 8 days (conditioning sessions), animals received alternating intraperitoneal injections (one injection per day) of either saline or ethanol (2.0 g/kg; 20% solution) immediately before placement within either the black-walled or the white-walled conditioning compartment for 5 min. A 2-day training-free period intervened after the first 4 days of conditioning. Saline- and ethanol-paired environments were counterbalanced within each group as well as across genotypes. On the final (test) day, animals were placed in the center chamber and given access to both compartments for 30 min, and the amount of time spent in the ethanol- and saline-paired compartments was measured electronically by photo beams placed 1.2 cm apart in the conditioning compartments.
Blood Ethanol Concentration
Blood samples of 50 μl were obtained from the tail vein of Fyn+/+ (129) and Fyn−/− (129) mice at the time indicated in the figure legends. Blood ethanol concentration was determined using the Analox AM1 Analyzer (Analox Instruments, North Yorkshire, UK).
Statistical Analysis
Differences in duration of LORR and ethanol intake were analyzed by two-way repeated measure ANOVA with post hoc Tukey’s test for multiple comparisons or by using Student’s t test when comparison was made between ifenprodil preinjected Fyn+/+ and Fyn−/− mice that were injected with ethanol alone. Ethanol preference was calculated as the ratio of the amount of ethanol consumed relative to total fluid consumed. Ethanol CPP was measured by calculating a CPP difference score from the test day data [(time on ethanol-paired side) − (time on saline-paired side)]. For CPP data analysis, Student’s t test was used.
RESULTS
Increased Sensitivity of Fyn−/− mice to the Acute/Sedative Effects of Ethanol
First, we compared the acute sedative effects of ethanol in Fyn−/− mice and their wild-type littermate controls. In mice, high doses of acute ethanol cause sedation (Finn et al., 1994). Therefore, we determined ethanol sensitivity by measuring the LORR after systemic injection of sedative doses of ethanol. As shown in Fig. 1A, the duration of LORR after administration of 3.6 and 4.0 g/kg ethanol was significantly longer for Fyn−/− (129) mice than for Fyn+/+ (129) littermate controls. These results suggest that increased sensitivity to the acute sedative effects of ethanol is due to deletion of the Fyn gene. As shown in Fig. 1B, the difference in the duration of sleep time between Fyn−/− (129) and Fyn+/+ (129) mice was not due to differences in ethanol metabolism.
Fig. 1.
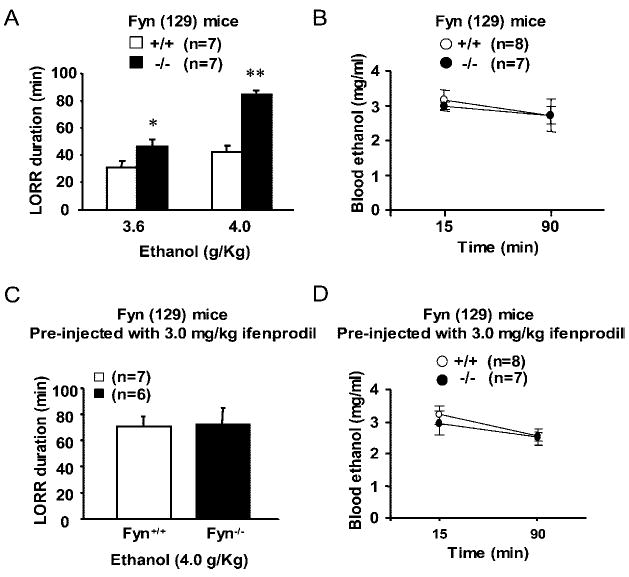
(A–B) Increased sensitivity to acute sedative effects of ethanol in Fyn−/− mice. (A) Mice were bred on a primarily 129 background. Sensitivity was evaluated by measuring the duration of LORR after administration of sedative doses of ethanol. Fyn−/− mice (black bars) showed a dose-dependent increase in the duration of LORR compared with Fyn+/+ control mice (white bars) [F(1,17)(genotype) = 8.0, p < 0.01; F(2,34)(dose) = 19.2 p < 0.001; F(dose × genotype), not significant]. (B) Blood ethanol levels were determined 15 and 90 min after intraperitoneal injection of 4.0 g/kg ethanol. There was no significant difference in ethanol clearance between Fyn+/+ and Fyn−/−. (C–D) Ifenprodil abolishes differences in sensitivity to acute sedative effects of ethanol. (C) Sensitivity was evaluated as described in A. Fyn−/− (129) mice and their wild-type littermate controls were injected intraperitoneally with ifenprodil (3.0 mg/kg) 30 min before a second injection of ethanol (4.0 g/kg), and the duration of LORR was measured. There was no significant difference in the sensitivity to ethanol in Fyn−/− (black bar) compared with Fyn+/+ mice (white bar) (t test, p = 0.94). (D) Fyn+/+ and Fyn−/− mice were treated as in C, and blood ethanol levels were determined 15 and 90 min after intraperitoneal injection of 4.0 g/kg ethanol. There was no significant difference in ethanol clearance between Fyn+/+ and Fyn−/−. The number of animals tested is shown in parentheses on the top of each histogram. Data are presented as mean ± SEM, and groups were compared by two-way repeated-measures ANOVA. *p = 0.05 and **p = 0.01 relative to wild-type littermates, Tukey’s test.
The Increase in Ethanol Sensitivity in Fyn−/− Mice Is Mediated Via NR2B-Containing NMDA Receptors
Our previous results suggest that the Fyn-mediated enhancement of NR2B-containing NMDA receptor currents mediates acute tolerance to ethanol inhibition (Yaka et al., 2003a), and this may be the mechanism that accounts for the reduced ethanol-induced sedation in Fyn+/+ mice compared with Fyn−/− mice. Therefore, we hypothesized that blocking the NR2B-containing NMDARs should normalize the differences in ethanol LORR between the two genotypes. Fyn−/− (129) and Fyn+/+ (129) littermate controls were injected intraperitoneally with ifenprodil, a selective antagonist for NR2B-containing NMDARs (Williams, 1993), at a concentration (3.0 mg/kg) that does not produce sedation. Thirty minutes later, ethanol was injected intraperitoneally at 4.0 g/kg, and the duration of LORR was measured. As predicted, preinjection of ifenprodil increased the duration of LORR in Fyn+/+ (129) mice to the same levels measured in the Fyn−/− (129) animals (Fig. 1C). Moreover, there was no significant difference in the duration of LORR in the Fyn−/− mice that were preinjected with ifenprodil compared with those that were tested with ethanol alone (Fig. 1A versus Fig. 1C; p = 0.8, t test). As shown in Fig. 1D, preinjection of mice with ifenprodil did not affect blood alcohol concentrations. Taken together, these results suggest that activation of NR2B-containing NMDARs mediates the acute desensitization to intoxicating doses of ethanol.
Deletion of the Fyn Gene Did Not Affect Voluntary Ethanol Intake or Preference
We evaluated oral ethanol self-administration by allowing mice continuous access to two drinking bottles, one containing water and the other containing an ascending range of ethanol concentrations. As shown in Fig. 2A, Fyn−/− (129) mice consumed the same amounts of ethanol as their wild-type littermate controls, and there was no difference in the preference for ethanol in Fyn−/− (129) and Fyn+/+ (129) mice (Fig. 2). These results suggest that increased sensitivity to the acute effects of ethanol does not inversely correlate with alteration in ethanol consumption.
Fig. 2.
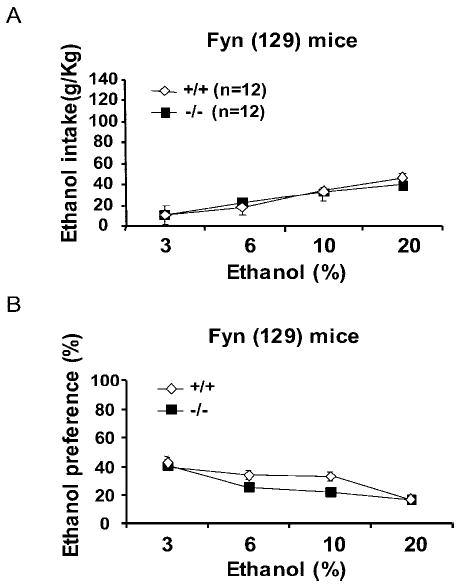
No differences in ethanol intake and preference were detected in Fyn−/− mice compared with Fyn+/+ mice. (A–B) Mice were bred on a primarily 129 background. (A) Voluntary ethanol intake (g/kg) plotted as a function of ethanol concentration. Fyn−/− mice (black squares) consumed the same amounts of ethanol as Fyn+/+ mice (white diamonds). (B) Ethanol preference calculated as milliliters of ethanol divided by total milliliters (water + ethanol) consumed. Fyn−/− mice demonstrated no significant alteration in ethanol preference compared with Fyn+/+ littermates. The number of animals tested is shown in parentheses on the top of each histogram. Data are presented as mean ± SEM, and groups were compared by two-way repeated-measures ANOVA.
Deletion of Fyn Did Not Alter the Rewarding Properties of Ethanol
To further test whether deletion of the Fyn gene alters ethanol’s behavioral effects, we tested mice for ethanol-induced CPP. As shown in Fig. 3, both Fyn+/+ (129) and Fyn−/− (129) mice developed a significant preference for the ethanol-paired chamber, and there was no difference in the magnitude of the preferences between the genotypes. These results suggest that ethanol-induced CPP is not affected by deletion of Fyn kinase in these mice.
Fig. 3.
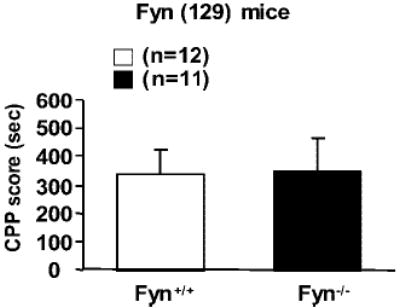
Ethanol-induced CPP is not altered in Fyn−/− compared with Fyn+/+mice. Mice were bred on a primarily 129 background. Conditioning was performed with 2.0 g/kg ethanol (intraperitoneally) as described in “Materials and Methods.” Both Fyn−/− and Fyn+/+ show significant CPP (p < 0.01, paired t test); however, there was no significant difference in the net time that Fyn+/+ mice (white bar) and Fyn−/− mice (black bar) spent in the ethanol-paired compartment (t test, p = 0.81). The number of animals tested is shown in parentheses.
Genetic Background Does Not Contribute to the Duration of Sleep Time or to Ethanol Consumption in Fyn−/− and Fyn+/+ Mice
Finally, to determine whether the enhancement in ethanol sensitivity is due solely to the deletion of the Fyn gene and not due to an interaction between the gene and the genetic background, the LORR and ethanol consumption experiments were repeated in Fyn−/− and Fyn+/+mice with a greater proportion of C57 in their background (see “Methods”). As shown in Fig. 4, the duration of the LORR after ethanol administration was significantly longer for Fyn−/− (C57) compared with Fyn+/+ (C57) littermate control mice (Fig. 4A). Ethanol consumption (Fig. 4B) and preference (Fig. 4C) were not significantly different between Fyn−/− (C57) and Fyn+/+ (57) mice. Therefore, genetic background is not likely to contribute to these ethanol-induced behavioral phenotypes in Fyn−/−and Fyn+/+ mice.
Fig. 4.
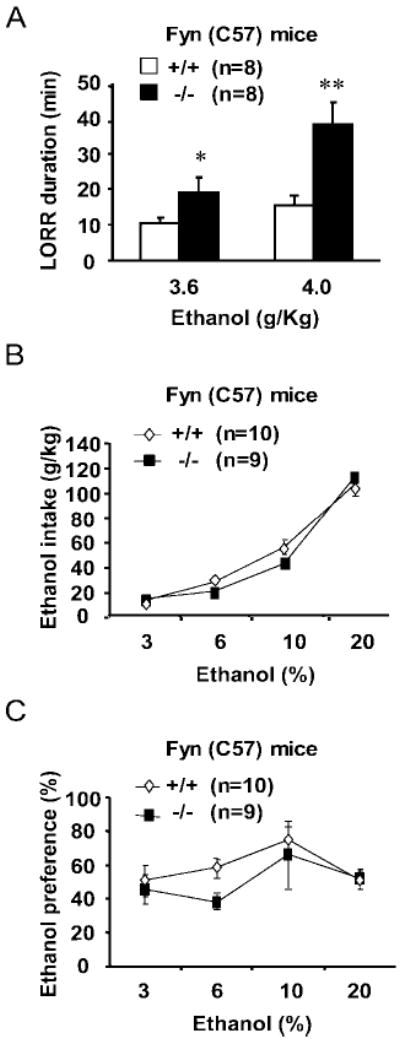
Fyn−/− and Fyn+/+ mice bred on a primarily C57 background show similar LORR and ethanol consumption and preference phenotypes as Fyn(129) mice. (A) Ethanol sensitivity was evaluated by measuring the duration of LORR after administration of sedative doses of ethanol. Fyn−/− mice (black bars) showed a dose-dependent increase in the duration of LORR compared with Fyn+/+ control mice (white bars) when mice were bred on a primarily C57 background [F(1,14)(genotype) = 10.3, p < 0.006; F(1,11)(dose) = 15.0, p < 0.003; F(dose × genotype), not significant]. (B) Voluntary ethanol intake (g/kg) plotted as a function of ethanol concentration as in Fig. 2. (C) Ethanol preference was calculated as in Fig. 2. Fyn−/− mice demonstrated no significant alteration in ethanol consumption or preference compared with Fyn+/+ littermates. The number of animals tested is shown in parentheses on the top of each histogram. Data are presented as mean ± SEM, and groups were compared by two-way repeated-measures ANOVA. *p = 0.05 and **p = 0.01 relative to wild-type littermates, Tukey’s test.
DISCUSSION
In the present study we confirmed that Fyn−/− mice are more sensitive to the acute sedative effects of ethanol compared with their wild-type littermates. We found that acute ethanol injection in Fyn−/− mice generated using homologous recombination insertion (Grant et al., 1992) caused prolonged LORR, similar to that found in Fyn−/− mice generated by LacZ insertion into the Fyn gene (Miyakawa et al., 1997). The increase in ethanol-induced sedation in Fyn−/− mice is unlikely to be related to altered ethanol metabolism because there were no genotype-dependent differences in blood ethanol levels after systemic ethanol injection. Importantly, we found that systemic administration of the NMDA-NR2B antagonist, ifenprodil, ameliorated the difference in the sensitivity to the acute sedative effects of ethanol observed between the Fyn+/+ and Fyn−/− mice, and this was also not due to changes in ethanol metabolism. However, we found that Fyn+/+ and Fyn−/− mice did not differ in their voluntary ethanol consumption and preference or in the rewarding properties of ethanol.
Acute ethanol exposure inhibits NMDAR function, whereas chronic ethanol treatment results in up-regulation of NMDAR function (Kumari and Ticku, 2000; Samson and Harris, 1992). NMDARs are heteromers composed of an obligatory NR1 subunit and combinations of different modulatory NR2 (A–D) subunits (Sucher et al., 1996). The subunit diversity affects the pharmacological and physiologic properties of NMDA receptors and, hence, gives rise to receptor heterogeneity. Studies on recombinant NMDARs with a defined subunit composition revealed that ethanol inhibition of NR2A- or NR2B-containing NMDARs coexpressed with NR1 is greater than ethanol inhibition of NR2C- or NR2D-containing NMDARs (Kuner et al., 1993; Masood et al., 1994; Sucher et al., 1996). Previous studies have demonstrated that NR2B-containing NMDARs mediate several forms of ethanol-induced behaviors (Malinowska et al., 1999; Miyakawa et al., 1997; Stork et al., 2002). However, the molecular mechanisms and region specificity by which ethanol modulates NMDAR responses remain poorly understood. Recently, we demonstrated that the brain region-specific compartmentalization of Fyn determines NMDAR sensitivity to ethanol. We found that ethanol-induced NR2B phosphorylation accounts for the development of acute tolerance to ethanol inhibition in the hippocampus (Yaka et al., 2003a). In the present study, we found evidence that Fyn-mediated ethanol-induced NR2B phosphorylation also determines the degree of ethanol-induced sedation in vivo. We found that inhibition by ifenprodil of NR2B-containing NMDARs in wild-type mice produced the same degree of ethanol sedation as observed in the Fyn−/− mice. Hence, in the presence of ethanol, when NR2B-containing NMDAR-mediated channel activity is reduced in the wild-type mice by the antagonist, these subjects resemble the knock-out mice. These results strongly suggest that Fyn interaction with the NR2B subunit of the NMDA receptor mediates the degree of sedation induced by acute ethanol exposure. In addition, our results provide further support to the hypothesis that sensitivity of NMDAR to ethanol underlies at least some of ethanol’s behavioral effects (Tabakoff and Hoffman, 1996); however, whether the acute sedative effects of ethanol are directly mediated by the hippocampus remains to be determined. In addition, other molecules are likely to also contribute to the modulation of NMDA receptor activity by ethanol. For example, Alvestad et al. (2003) showed that acute exposure of the hippocampal CA1 region to ethanol results in the activation of a tyrosine phosphatase that leads to the reduction of NR2B phosphorylation and NMDAR activity. It therefore would be of interest to test the in vivo activities of a tyrosine phosphatase inhibitor to determine whether its effect correlates with the in vitro findings.
Human studies have shown that lower sensitivity to modest doses of alcohol is associated with a significant increase in the risk of future alcoholism (Schuckit, 1994). Single gene mutations in mice are used widely to investigate the influence of specific gene products on traits relevant to alcohol abuse and alcoholism, which include sensitivity, reward, and self-administration (Hoffman et al., 2001). Many of these studies suggest that increased sensitivity to the acute sedative effects of ethanol is correlated with reduced voluntary ethanol consumption and vice versa (Hodge et al., 1999; Phillips et al., 1998; Thiele et al., 2002). For example, it has been demonstrated that neuropeptide Y-deficient mice are less sensitive to the acute sedative effects of ethanol and consume more ethanol compared with their wild-type littermates. However, mice overex-pressing neuropeptide Y are more sensitive to ethanol’s acute effects and consume less ethanol (Thiele et al., 1998, 2002). In contrast, our results suggest that alteration in acute sensitivity to ethanol in deletion mutant mice does not necessarily predict corresponding changes in ethanol consumption or ethanol’s rewarding properties.
In contrast to our results, Stork et al. (2002) recently showed that mice overexpressing the Fyn gene voluntarily consumed less ethanol compared with their wild-type controls, suggesting that Fyn deletion may lead to enhanced ethanol consumption. The differences in our results compared with the findings of Stork et al. can be explained by the different conditions used to determine voluntary ethanol consumption. Whereas Stork et al. used continuous access to 10% ethanol for only 3 days, in our study ethanol concentration was gradually increased with 4 days of free access for each concentration. This standard protocol may reduce the initial taste aversion that many rodents display to ethanol as well as reduce the contribution of neophobia or olfactory and gustatory factors. Interestingly, when we repeated Stork et al.’s experiment and tested ethanol consumption in Fyn−/− (129) and Fyn+/+ (129) mice by exposing the mice directly to 10% ethanol, the Fyn−/− (129) mice consumed more ethanol compared with their littermate controls (data not shown). However, as mentioned previously, these results are likely to be related to many factors that control fluid consumption other than the pharmacological effects of ethanol.
Recently, Boehm et al. (2003) reported that deletion of the Fyn gene increased the duration of the LORR and that ethanol consumption was reduced in the Fyn−/− compared with Fyn+/+ littermate controls. The differences between our findings and those of Boehm et al. (2003) could be due to differences in breeding strategies. Both our lines of mice were bred with inbred mice (129svj and C57bl6), whereas Boehm et al. used the hybrid B6129SF2/J. Regardless of the observed differences between studies, together they clearly demonstrate the contribution of the Fyn kinase gene to ethanol’s behavioral effects.
In conclusion, this study suggests that the interaction of Fyn with the NR2B subunit of NMDAR is important for mediating ethanol’s acute sedative effects and that mechanisms mediating these acute effects of ethanol may be different from those mediating ethanol’s rewarding properties or consumption. Therefore, ethanol sensitivity in one behavioral paradigm does not necessarily correlate with ethanol sensitivity in the other.
Footnotes
Supported by funds from the State of California for medical research on alcohol and substance abuse through the University of California, San Francisco (DR, PHJ) and National Institute on Alcohol Abuse and Alcoholism Grant R01AA/MH13438-O1A1 (DR).
References
- Allgaier C. Ethanol sensitivity of NMDA receptors. Neurochem Int. 2002;41:377–382. doi: 10.1016/s0197-0186(02)00046-3. [DOI] [PubMed] [Google Scholar]
- Alvestad RM, Grosshans DR, Coultrap SJ, Nakazawa T, Yamamoto T, Browning MD. Tyrosine dephosphorylation and ethanol inhibition of N-methyl-D-aspartate receptor function. J Biol Chem. 2003;278:11020 –11025. doi: 10.1074/jbc.M210167200. [DOI] [PubMed] [Google Scholar]
- Bare DJ, Lauder JM, Wilkie MB, Maness PF. p59fyn in rat brain is localized in developing axonal tracts and subpopulations of adult neurons and glia. Oncogene. 1993;8:1429 –1436. [PubMed] [Google Scholar]
- Boehm SL, II, Peden L, Chang R, Harris RA, Blednov YA. Deletion of the fyn-kinase gene alters behavioral sensitivity to ethanol. Alcohol Clin Exp Res. 2003;27:1033–1040. doi: 10.1097/01.ALC.0000075822.80583.71. [DOI] [PubMed] [Google Scholar]
- Care GD, Fibiger HC, Phillips AG (1989) Conditioned place preference as a measure of drug reward, in The Neuropharmacological Basis of Reward (Liebman JM, Cooper SJ eds), pp 264 –319. Oxford University Press, Oxford, UK.
- Crabbe JC, Phillips TJ, Feller DJ, Hen R, Wenger CD, Lessov CN, Schafer GL. Elevated alcohol consumption in null mutant mice lacking 5-HT1B serotonin receptors. Nat Genet. 1996;14:98 –101. doi: 10.1038/ng0996-98. [DOI] [PubMed] [Google Scholar]
- Finn DA, Syapin PJ, Bejanian M, Jones BL, Alkana RL. Temperature dependence of ethanol depression in mice: dose response. Alcohol Clin Exp Res. 1994;18:382–386. doi: 10.1111/j.1530-0277.1994.tb00029.x. [DOI] [PubMed] [Google Scholar]
- Grant SG, O’Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science (Wash DC) 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, Wang D, Sanchez-Perez AM, Messing RO. Supersensitivity to allosteric GABAA receptor modulators and alcohol in mice lacking PKCepsilon. Nat Neurosci. 1999;2:997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- Hoffman PL, Yagi T, Tabakoff B, Phillips TJ, Kono H, Messing RO, Choi DS. Transgenic and gene “knockout” models in alcohol research. Alcohol Clin Exp Res. 2001;25:60S–66S. doi: 10.1097/00000374-200105051-00011. [DOI] [PubMed] [Google Scholar]
- Kojima N, Wang J, Mansuy IM, Grant SG, Mayford M, Kandel ER. Rescuing impairment of long-term potentiation in fyn-deficient mice by introducing Fyn transgene. Proc Natl Acad Sci USA. 1997;94:4761–4765. doi: 10.1073/pnas.94.9.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M, Ticku MK. Regulation of NMDA receptors by ethanol. Prog Drug Res. 2000;54:152–189. [PubMed] [Google Scholar]
- Kuner T, Schoepfer R, Korpi ER. Ethanol inhibits glutamate-induced currents in heteromeric NMDA receptor subtypes. Neuroreport. 1993;5:297–300. doi: 10.1097/00001756-199312000-00029. [DOI] [PubMed] [Google Scholar]
- Lu YF, Kojima N, Tomizawa K, Moriwaki A, Matsushita M, Obata K, Matsui H. Enhanced synaptic transmission and reduced threshold for LTP induction in fyn-transgenic mice. Eur J Neurosci. 1999;11:75–82. doi: 10.1046/j.1460-9568.1999.00407.x. [DOI] [PubMed] [Google Scholar]
- Lu YM, Roder JC, Davidow J, Salter MW. Src activation in the induction of long-term potentiation in CA1 hippocampal neurons. Science (Wash DC) 1998;279:1363–1367. doi: 10.1126/science.279.5355.1363. [DOI] [PubMed] [Google Scholar]
- Malinowska B, Napiorkowska-Pawlak D, Pawlak R, Buczko W, Gothert M. Ifenprodil influences changes in mouse behaviour related to acute and chronic ethanol administration. Eur J Pharmacol. 1999;377:13–19. doi: 10.1016/s0014-2999(99)00393-3. [DOI] [PubMed] [Google Scholar]
- Masood K, Wu C, Brauneis U, Weight FF. Differential ethanol sensitivity of recombinant N-methyl-D-aspartate receptor subunits. Mol Pharmacol. 1994;45:324 –329. [PubMed] [Google Scholar]
- Miyakawa T, Yagi T, Kitazawa H, Yasuda M, Kawai N, Tsuboi K, Niki H. Fyn-kinase as a determinant of ethanol sensitivity: relation to NMDA-receptor function. Science (Wash DC) 1997;278:698 –701. doi: 10.1126/science.278.5338.698. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T, Yamamoto T. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2001;276:693–699. doi: 10.1074/jbc.M008085200. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Brown KJ, Burkhart-Kasch S, Wenger CD, Kelly MA, Rubinstein M, Grandy DK, Low MJ. Alcohol preference and sensitivity are markedly reduced in mice lacking dopamine D2 receptors. Nat Neurosci. 1998;1:610 –615. doi: 10.1038/2843. [DOI] [PubMed] [Google Scholar]
- Samson HH, Harris RA. Neurobiology of alcohol abuse. Trends Pharmacol Sci. 1992;13:206 –211. doi: 10.1016/0165-6147(92)90065-e. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184 –189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Stork O, Kojima N, Stork S, Kume N, Obata K. Resistance to alcohol withdrawal-induced behaviour in Fyn transgenic mice and its reversal by ifenprodil. Brain Res Mol Brain Res. 2002;105:126 –135. doi: 10.1016/s0169-328x(02)00400-x. [DOI] [PubMed] [Google Scholar]
- Sucher NJ, Awobuluyi M, Choi YB, Lipton SA. NMDA receptors: from genes to channels. Trends Pharmacol Sci. 1996;17:348 –355. [PubMed] [Google Scholar]
- Tabakoff B, Hoffman PL. Alcohol addiction: an enigma among us. Neuron. 1996;16:909 –912. doi: 10.1016/s0896-6273(00)80113-0. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Koh MT, Pedrazzini T. Voluntary alcohol consumption is controlled via the neuropeptide Y Y1 receptor. J Neurosci. 2002;22:RC208. doi: 10.1523/JNEUROSCI.22-03-j0006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Marsh DJ, Ste Marie L, Bernstein IL, Palmiter RD. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature (Lond) 1998;396:366 –369. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Yaka R, He DY, Phamluong K, Ron D. Pituitary adenylate cyclase-activating polypeptide (PACAP(1–38)) enhances N-methyl-D-aspartate receptor function and brain-derived neurotrophic factor expression via RACK1. J Biol Chem. 2003b;278:9630 –9638. doi: 10.1074/jbc.M209141200. [DOI] [PubMed] [Google Scholar]
- Yaka R, Phamluong K, Ron D. Scaffolding of Fyn kinase to the NMDA receptor determines brain region sensitivity to ethanol. J Neurosci. 2003a;23:3623–3632. doi: 10.1523/JNEUROSCI.23-09-03623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaka R, Thornton C, Vagts AJ, Phamluong K, Bonci A, Ron D. NMDA receptor function is regulated by the inhibitory scaffolding protein, RACK1. Proc Natl Acad Sci USA. 2002;99:5710 –5715. doi: 10.1073/pnas.062046299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Cavallaro S, Gusev P, Alkon DL. Non-receptor tyrosine protein kinase pp60c-src in spatial learning: synapse-specific changes in its gene expression, tyrosine phosphorylation, and protein-protein interactions. Proc Natl Acad Sci USA. 2000;97:8098 –8103. doi: 10.1073/pnas.97.14.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]


