Abstract
The present experiment investigated whether rats formed emergent, untrained stimulus relations in many-to-one matching-to-sample discriminations. In Phase 1, rats were trained to match two samples (triangle and horizontal stripes) to a common comparison (horizontal stripes) and two additional samples (circle or vertical stripes) to another comparison (vertical stripes). Then, in Phase 2, the rats were trained to match the one sample (triangle) to a new comparison (black) and the other sample (circle) to another comparison (white). In the Phase 3 test, half the rats (consistent group) were given two new tasks in which the sample-correct comparison relation was consistent with any emergent stimulus relations that previously may have been learned. The remaining 6 rats (inconsistent group) were given two new tasks in which the sample-correct comparison relation was not consistent with any previously learned emergent stimulus relations. Rats in the consistent group showed more accurate performance at the start of Phase 3, and faster learning to criterion in this phase, as compared with rats in the inconsistent group. This finding suggests that rats may form emergent, untrained stimulus relations between the discriminative stimuli in many-to-one matching-to-sample discriminations.
Keywords: emergent stimulus relations, many-to-one transfer effect, conditional discrimination, consistent and inconsistent group, matching to sample, lever press, rats
Urcuioli, Zentall, Jackson-Smith, and Steirn (1989) found evidence for the emergence of untrained, derived stimulus relations between stimuli in many-to-one conditional discriminations in pigeons. In Phase 1, choosing vertical lines was reinforced when the sample stimulus was either red or vertical lines, and choosing horizontal lines was reinforced when the sample stimulus was green or horizontal lines. Then, in Phase 2, the pigeons were trained to choose a circle when the sample was red and a dot when the sample was green. Finally, in Phase 3, half of the pigeons were tested with two new tasks: choose the circle (previously associated with red) when the sample stimulus was vertical lines, and choose the dot (previously associated with green) when the sample stimulus was horizontal lines (consistent group). The remaining pigeons also were tested with two new tasks: choose the dot (associated with green) when the sample stimulus was vertical lines and choose the circle (associated with red) when the sample stimulus was horizontal lines (inconsistent group). The Phase 3 test performance of pigeons in the consistent group was superior to that of pigeons in the inconsistent group. Such many-to-one transfer effects are common in pigeons (see also Urcuioli, Zentall, & DeMarse, 1995; Wasserman, DeVolder, & Coppage, 1992); however, no studies have reported such many-to-one transfer effects in rats.
Rats learn whole-reversal tasks (in which the reinforcement contingencies of all tasks trained in original learning are reversed) faster than partial-reversal tasks (in which reinforcement contingencies of only some of the tasks trained in original learning are reversed and those of the remaining tasks are maintained). This advantage has been reported using three discrimination procedures: (a) after overtraining in concurrent discrimination training (Nakagawa, 1986, 1992, 1998, 1999a, 2001, 2002a, 2002c), (b) following matching-(or nonmatching)-to-sample discrimination training (Nakagawa, 1999b, 2000a, 2002a), and (c) after same-different discrimination training (Nakagawa, 2002a, 2002b, 2002d, Experiment 5). In Phase 1 of the whole-reversal versus partial-reversal procedure in concurrent discriminations (Nakagawa, 1986, 2001), rats were trained to criterion or overtrained on two simple concurrent discriminations; for example, black (A1)+ versus white (A2)−, vertical stripes (B1)+ versus horizontal stripes (B2)−, where + and − represent reinforcement and nonreinforcement, respectively. After completing this training, they received either partial reversal (A1− versus A2+; B1+ versus B2−, or A1+ versus A2−; B2+ versus B1−) or whole reversal (A1− versus A2+; B1− versus B2+) in Phase 2. After overtraining, rats for which both discriminations were reversed took fewer sessions to reach criterion than rats for which only one discrimination of the two tasks was reversed (i.e., a whole-reversal versus partial-reversal advantage effect; see also Delius, Ameling, Lea, & Staddon, 1995; Nakagawa, 1978, 1986, 1992, 1998, 2001; Zentall, Sherburne, Steirn, Randall, Roper, & Urcuioli, 1992; Zentall, Steirn, Sherburne, & Urcuioli, 1991).
Nakagawa (1986, 1992) argued that the whole-reversal versus partial-reversal advantage effect could be explained in terms of associations between the discriminative stimuli established during training. According to Nakagawa, during the original training, for each discrimination task, rats learn that a positive stimulus is a discriminative stimulus for responding and a negative stimulus is discriminative for not responding. During overtraining, rats also form associations between the discriminative stimuli with the same type of response in concurrent discriminations. These stimulus or cue associations produce an acquired equivalence effect, giving enhanced generalization between stimuli associated with the same consequence that mediate the transfer of appropriate responding from one positive (or negative) stimulus to the other positive (or negative) stimulus in reversal learning. As a result, in whole-reversal training after overtraining, the reversal of control by any of the set of stimuli that controlled a particular response should enhance reversal of the other stimuli that previously controlled the same response. Similarly, non-reinforcement of a previously reinforced response should also transfer from that stimulus to the other stimuli that previously controlled reinforced responses. In contrast, continued training with the same nonreversed discriminations during the reversal stage would lead to interference with the reversal of other discriminations (Nakagawa, 1992). Associations between the discriminative stimuli that signal the same response assignment established during overtraining would be emergent, untrained stimulus relations between the discriminative stimuli. Thus a whole-reversal versus partial-reversal advantage indicates that emergent stimulus relations have developed between discriminative stimuli with the same outcome after overtraining in concurrent discriminations in rats. Therefore the findings of the series of experiments by Nakagawa cited above indicate that untrained stimulus relations (i.e., acquired equivalence) between discriminative stimuli emerged in rats in the original training.
Rats typically have not been used in research with matching- (or nonmatching)-to-sample discriminations because they are not visual animals. The experiments reported by Nakagawa (1993a, 2000b) found, however, that rats acquired relational rules in matching- (or nonmatching)-to-sample discriminations. Furthermore, in Experiments 1 and 2 of Nakagawa (2000a), rats in matching- (or nonmatching)-to-sample discriminations learned whole reversal, in which all 12 stimulus sets were reversed, faster than partial reversals in which 9, 6, and 3 of the 12 stimulus sets were reversed. This result again indicates that untrained stimulus relations between discriminative stimuli emerged in matching- (or nonmatching)-to-sample training in rats.
The results of Nakagawa (1993a, 1999b, 2000a, 2000b, 2002a) show that, for rats, untrained stimulus relations between stimuli emerged following matching- (or nonmatching)-to-sample discriminations that used shapes as the discriminative stimuli without rats contacting the sample and the comparison stimuli. However, none of the studies has provided evidence for the emergence of untrained stimulus relations between stimuli in many-to-one conditional discriminations in rats using the three-phase transfer design. Thus the present experiment was conducted to ask whether a stimulus could mediate the relation between two other stimuli in many-to-one conditional discriminations in rats using the three-phase transfer design (see also Urcuioli et al., 1989, 1995; Wasserman et al., 1992; Zentall, 1998). Rats were trained to criterion on four matching-to-sample discrimination tasks in Phase 1 training: All 12 rats were trained to choose one comparison (C) after two samples (A and C) and to choose a different comparison (D) after two other samples (B and D) until they reached criterion levels of accuracy. After completing Phase 1 training, they were trained to criterion in Phase 2 to choose a new comparison (E) after the sample (A) and to choose a new different comparison (F) after the other sample (B). Finally, in Phase 3, half of the 12 rats were trained on two new tasks (given C, choose E but not F [C: E+F−] and given D, choose F but not E [D: F+E−]. In these tasks, the sample-correct comparison relation was consistent with Phase 1 and Phase 2 training (consistent group). The remaining 6 rats were trained on two new tasks in Phase 3 (C: F+E− and D: E+F−) that were not consistent with Phase 1 and Phase 2 training (inconsistent group).
The findings of the series of experiments reported by Nakagawa described above are consistent with the view that rats form untrained stimulus relations (i.e., C-E and D-E) in Phase 1 and Phase 2 training. Untrained stimulus relations (C-E and D-F) that emerge as a result of training are consistent with the Phase 3 task for the rats in the consistent group but they are the opposite for the rats in the inconsistent group. Thus untrained stimulus relations that emerge as a result of training will help the performance of the consistent group in Phase 3, but will be detrimental to the performance of the inconsistent group. Thus rats in the consistent group should be more accurate, and should reach criterion faster, in Phase 3 than rats in the inconsistent group. No significant difference between groups in accuracy or sessions to criterion in Phase 3 would demonstrate that rats had not formed emergent stimulus relations between the discriminative stimuli in the prior many-to-one conditional discrimination training.
Method
Subjects
Twelve experimentally naive male Sprague-Dawley rats were used. They were about 180 days old, with an average body weight of 559 g, at the start of the experiment. Rats were handled for 5 min a day for 12 days and were maintained on a daily 2-hr feeding schedule. The amount of food in the daily ration was gradually reduced until the body weight of each rat reached 80% of its weight at the start of the experiment. Rats were housed individually with free access to water. Rats were maintained on a 2:22 hr light/dark cycle, with lights off at 7:00 p.m. Experimental sessions took place during the light phase of the cycle; that is, the experimental sessions were conducted between 5:00 p.m. and 7:00 p.m.
Apparatus
An experimental chamber 150 mm high, 225 mm wide, and 150 mm long was used in magazine training and lever-press training. A 50-mm square screen was located 50 mm above the floor with a 50-mm by 30-mm lever beside the screen, located 50 mm above the floor. There was a food tray on the opposite wall of the lever, into which a milk pellet was delivered from a feeder when rats pressed the lever.
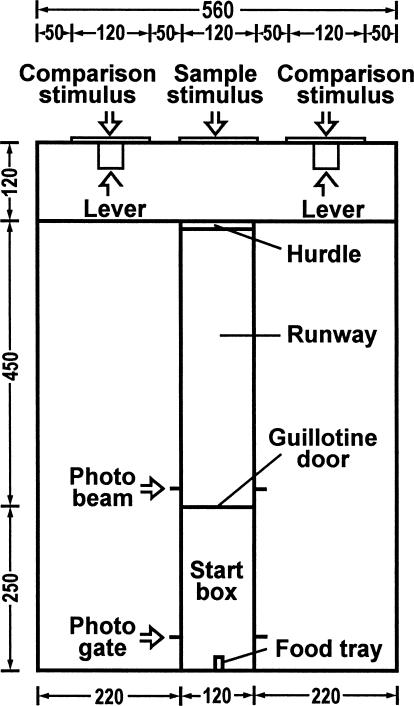
For the experiment proper, an automatic T-maze was used (Figure 1; see Experiment 2 in Nakagawa, 1993a, 1999b). The apparatus was lit throughout the experiment by a 10-W fluorescent lamp suspended 400 mm above the top of the choice chamber. The apparatus consisted of a runway (300 mm high, 120 mm wide, and 450 mm long) with a start box (300 mm high, 120 mm wide, and 250 mm long) and a choice chamber (300 mm high, 560 mm wide, and 120 mm long). A hurdle (50 mm high, 120 mm wide, and 30 mm long) was located at the end of the runway in an attempt to enhance control by the stimuli. The wall of the apparatus was medium-gray Plexiglas, and the ceiling was clear Plexiglas. The start box had a food tray in the center of the end wall into which a milk pellet was delivered from a feeder when rats made a correct response. The choice chamber contained three display screens, each 120 mm square, located 100 mm above the floor and 50 mm apart from edge to edge. There were two response levers in the choice chamber, each 40 mm square and 90 mm above the floor. These levers were located below the center of the two side screens. A guillotine door opened and closed automatically to control access to the start box. Whenever a rat interrupted a photo beam at the exit of the start box, located 30 mm from the guillotine door, stimuli were rear-projected onto the screens. The rat then was allowed to approach and press a response lever, following which it was required to return to the start box. As it approached the start box, it interrupted a photo beam 50 mm from the end wall of the start box, and the guillotine door closed behind the rat. After 10 s, the guillotine door opened automatically for the start of the next trial. The programming of events and data collection were carried out on-line using a laboratory computer. Masking of extraneous sounds was provided by white noise from a blower fan (50 db).
Fig. 1. Diagram of the T-maze used in the present experiment (units are in millimeters).
Sample stimuli were shown on the center Plexiglas screen, and comparison stimuli were shown on the side Plexiglas screens using a computer monitor (Sharp Hi-Vision® 32C-HD90). Sample stimuli came on for 4 s as soon as the rats ran through the photo beam at the exit of the start box. Both comparisons were automatically shown behind the side Plexiglas screens 1 s after the onset of the sample stimulus. When rats pressed a response lever, the comparisons disappeared. The exposure duration of the sample and comparison stimuli was controlled by the rats’ lever pressing.
Six stimuli were used: A circle (with an area of 43.00 cm2); a triangle (with an area of 43.30 cm2); vertical stripes; horizontal stripes; a black square; and a white square. Both vertical- and horizontal-stripe cards had alternating black and white lines 10 mm wide. In Phase 1 training, the circle, triangle, horizontal stripes, and vertical stripes were used as sample stimuli and vertical stripes and horizontal stripes were used as comparison stimuli. In Phase 2 training, the circle and triangle were used as sample stimuli, and the black square and white square were used as comparison stimuli. In Phase 3, vertical stripes and horizontal stripes were used as sample stimuli, and the black square and white square were used as comparison stimuli. The triangle was referred to as A, the circle as B, the horizontal stripes as C, the vertical stripes as D, the black square as E, and the white square as F.
Procedure
Rats received magazine training and lever press training in the experimental chamber for five sessions prior to the beginning of pre-training. On the last day, all rats pressed the lever at least 50 times in a 15-min session.
After completing both magazine and lever-press training, the rats received pretraining in the automatic T-maze for eight sessions, by which time they were pressing both levers at least 30 times per session. In pretraining, after the guillotine door was opened, the rats ran down the runway, pressed a response lever, and returned to the start box. By the end of pretraining, all rats returned to the start box in less than 2 s after a lever press. A medium-gray stimulus was rear-projected onto the screen during shaping and shown behind each of three Plexiglas screens during pre-training.
A trial was defined as a sequence of events, beginning when the rat left the start box after the opening of the guillotine door and continuing as it ran down the runway, pressed a response lever, and returned to the start box.
Phase 1 Training.
Twelve rats were trained for 16 trials per session on many-to-one matching-to-sample discrimination tasks (sample A, positive comparison C, negative comparison D; sample B, positive comparison D, negative C; sample C, positive C, negative D; and sample D, positive D, negative C). These four tasks are termed A: C+D−, B: D+C−, C: C+D−, and D: D+C−. Training continued until a criterion of 28 or more correct trials out of a possible 32 trials (87.5% correct) on two successive sessions had been reached. A correction procedure was used: The stimuli were removed when the rat pressed an incorrect lever, but the rat then was allowed to press the correct response lever. A correct response was a first choice of the correct comparison and an error response was a first choice of an incorrect comparison; correction responses were not used as data. The order of trials with the four tasks followed eight predetermined random sequences. The position of the correct lever also followed eight predetermined random sequences. Each random sequence was used every 8 days. Rats were given one 45-mg milk pellet accompanied by a click of the feeder when they made a correct response. Rats returned to the start box within 2 s after emitting a correct response lever (i.e., reinforcement was delayed by 2 s). The intertrial interval was 10 s.
Phase 2 Training.
After completing Phase 1 training, the rats were trained to the same criterion of 87.5% or greater correct on two successive sessions of 16 trials per session on two new matching tasks (A: E+F− and B: F+E−). Both the order of trials with both tasks and the position of the correct lever followed eight predetermined random sequences. Other aspects of the procedure were identical to those used in Phase 1 training.
Phase 3 Test.
After completing Phase 2 training, Phase 3 commenced. The rats were trained to a criterion of 14 correct trials out of a possible 16 per session on the two discrimination tasks. A less-strict criterion was used simply because trials-to-criterion was the dependent variable in Phase 3. Half of the 12 rats were trained on two new tasks (C: E+F− and D: F+E−) in which the sample-correct comparison relation was consistent with training in Phases 1 and 2 (consistent group; see Table 1). The remaining 6 rats were trained on two new tasks (C: F+E− and D: E+F−) in which the sample-correct comparison relation was not consistent with training in Phases 1 and 2 (inconsistent group; see Table 1). For each group, the order of trials and the position of the correct lever followed four predetermined random sequences. Other aspects of the procedure were identical to those used in Phase 1 training.
Table 1. Experimental treatments for each group.
The first letter of each pair represents a sample, the second letter represents the positive comparison, and the third letter represents the negative comparison in each phase.
| Group | Phase 1 | Phase 2 | Phase 3 |
| Consistent | A: C+D− | ||
| B: D+C− | A: E+F− | C: E+F− | |
| C: C+D− | B: F+E− | D: F+E− | |
| D: D+C− | |||
| Inconsistent | A: C+D− | ||
| B: D+C− | A: E+F− | C: F+E− | |
| C: C+D− | B: F+E− | D: E+F− | |
| D: D+C− |
Note. A = triangle; B = circle; C = horizontal stripes; D = vertical stripes; E = black square; F = white square.
Results
Phase 1 and Phase 2 Training
Acquisition of Phase 1 and Phase 2 performance by rats in the consistent group was compared with the acquisition performance of the rats in the inconsistent group. The mean number of sessions to reach criterion in Phases 1 and 2 was, respectively, 120.33 (SD = 17.25) and 27.50 (SD = 15.92) for rats in the consistent group, and 117.50 (SD = 16.05) and 32.50 (SD = 20.84) for rats in the inconsistent group. A two-way analysis of variance (ANOVA), using group (consistent versus inconsistent) and phase (1 versus 2), revealed that neither the effect of group nor the interaction between phase and group was significant (Fs < 1), whereas the effect of phase was significant, F (1, 10) = 75.41, p < .001. Rats reached criterion in Phase 2 more rapidly than in Phase 1.
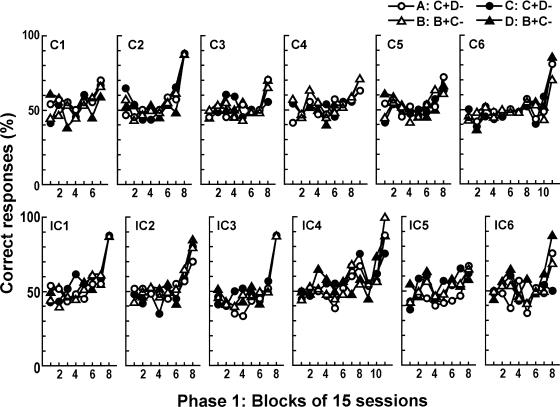
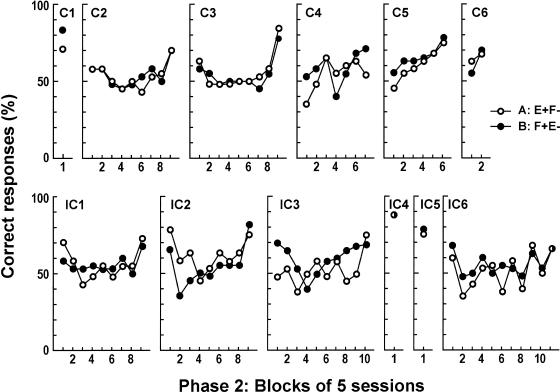
All 12 rats performed at accuracy levels well above chance on the last 32 trials in Phase 2 training, averaging 89.9% for rats in the consistent and 90.9% for rats in the inconsistent groups. Figure 2 shows mean choice accuracy for each rat in the consistent and inconsistent groups in Phase 1 training over 15-session blocks. Performance was around chance (40 to 60% correct) for many sessions before improving abruptly in Rats C2, IC1, IC3, and IC4, whereas the performance of Rats C1, C3, C4, C5, C6, IC2, IC5, and IC6 changed more gradually. Figure 3 shows mean choice accuracy for each rat in the consistent and inconsistent groups in Phase 2 training in five-session blocks and shows that performance was around chance for the first eight blocks before improving abruptly in Rats C2, C3, IC1, and IC2, whereas the performance of Rats C4, C5, IC3, and IC6 changed more gradually. Figures 2 and 3 show that performance in Phase 1 and Phase 2 training was at chance level for many sessions before reaching criterion.
Fig. 2. Mean accuracy for the 6 individual rats in both the consistent and inconsistent groups in Phase 1 training in 15-session blocks.
The number of sessions in the last block was two for Rats C2, IC1, IC3, and IC4; four for Rat IC6; five for Rats C3, C6, and IC5; six for Rat C4; and 14 for Rats C1 and C5. Symbols represent the different discrimination tasks.
Fig. 3. Mean accuracy for the 6 individual rats in both the consistent and inconsistent groups in Phase 2 training in five-session blocks.
The number of sessions in the last block was two for Rats C1, C4, IC3, and IC4 and four for Rats C3, IC2, and IC6. Symbols represent the different discrimination tasks.
The number of sessions to criterion and final accuracy on the two criterion sessions (Phases 1 and 2) or one criterion session (Phase 3) for each rat in each group are shown in Table 2.
Table 2. Number of sessions to criterion and final accuracy (% correct) for each rat in the consistent and inconsistent groups in Phases 1, 2, and 3.
| Group | Subject | Phase 1 | Phase 2 | Phase 3 |
| Consistent | C1 | 104 (90.1) | 3 (90.1) | 3 (87.5) |
| C2 | 107 (87.5) | 45 (90.1) | 2 (93.8) | |
| C3 | 110 (90.1) | 44 (87.5) | 1 (87.5) | |
| C4 | 126 (87.5) | 33 (87.5) | 5 (87.5) | |
| C5 | 120 (90.1) | 30 (90.1) | 1 (87.5) | |
| C6 | 155 (90.1) | 10 (93.8) | 1 (93.8) | |
| Inconsistent | IC1 | 107 (87.5) | 45 (90.1) | 12 (93.8) |
| IC2 | 110 (93.8) | 42 (93.8) | 8 (93.8) | |
| IC3 | 107 (87.5) | 47 (90.1) | 14 (87.5) | |
| IC4 | 152 (87.5) | 2 (87.5) | 11 (87.5) | |
| IC5 | 120 (90.1) | 5 (90.1) | 25 (87.5) | |
| IC6 | 109 (90.1) | 54 (93.8) | 30 (87.5) |
In order to examine the tendency to adopt a position bias in Phases 1 and 2, a criterion was devised: If rats chose a particular side lever (right or left) more than 13 times on the 16 daily trials, the session was scored as a positional-response session, and the number of such sessions was summed for each rat. The mean and the standard deviation of the number of sessions in which rats exhibited a positional response in Phases 1 and 2 was as follows: 40.08 (SD = 26.33) in Phase 1 and 12.92 (SD = 13.51) in Phase 2. The rats exhibited significantly more frequent position biases according to this criterion in Phase 1 sessions than in Phase 2 sessions, t (10) = 3.04, p < .02. The percentage of positional-response sessions in each phase was 33.7% in Phase 1 and 43.1% in Phase 2.
Phase 3
Two measures of performance in Phase 3 may be affected by prior discrimination training: accuracy in the first session of training, and number of trials to criterion.
Mean first-session performance in Phase 3 of rats in the consistent and inconsistent groups across trials is shown in Figure 4, and Table 3 shows performance of each rat in the consistent and inconsistent groups on each trial in the first session in Phase 3. Figure 4 and Table 3 show that 5 of the 6 rats in the consistent group made a correct response on the first trial, whereas no rat in the inconsistent group made a correct response on the first trial, only 2 rats made a correct response on the second trial, and 1 rat made a correct response on the third and on the fourth trials. Average accuracy on the first session was 84.4% and 38.5% for rats in the consistent and inconsistent groups, respectively. The mean number of correct responses in the first session was 13.50 (SD = 0.96) for rats in the consistent group and 6.17 (SD = 1.07) for rats in the inconsistent group, and this difference was significant (t [10] = 11.40, p < .001).
Fig. 4. Mean accuracy on each trial in the first session of Phase 3 for the consistent and inconsistent groups.
Table 3. Performance for individual rats in the consistent and inconsistent groups on each trial in the first session in Phase 3 (○ indicates a correct response; × indicates an error response).
| Trial | |||||||||||||||||
| Group | Subject | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| Consistent | C1 | ○ | × | ○ | ○ | × | ○ | ○ | ○ | ○ | × | ○ | ○ | ○ | ○ | ○ | ○ |
| C2 | ○ | ○ | ○ | × | ○ | ○ | ○ | ○ | ○ | × | ○ | ○ | × | ○ | ○ | ○ | |
| C3 | ○ | ○ | × | ○ | ○ | ○ | ○ | ○ | × | ○ | ○ | ○ | ○ | ○ | ○ | ○ | |
| C4 | × | ○ | ○ | ○ | × | ○ | ○ | ○ | × | ○ | ○ | × | ○ | ○ | ○ | ○ | |
| C5 | ○ | ○ | ○ | ○ | ○ | ○ | × | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | × | |
| C6 | ○ | ○ | ○ | ○ | ○ | ○ | ○ | × | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ | |
| Inconsistent | IC1 | × | × | × | × | ○ | × | × | ○ | ○ | × | ○ | ○ | ○ | × | × | ○ |
| IC2 | × | ○ | × | ○ | × | × | × | × | × | ○ | × | × | ○ | ○ | ○ | ○ | |
| IC3 | × | × | × | × | × | × | ○ | ○ | ○ | × | × | ○ | × | ○ | × | × | |
| IC4 | × | × | × | × | × | ○ | × | × | × | ○ | × | ○ | ○ | ○ | × | × | |
| IC5 | × | × | × | × | ○ | × | ○ | ○ | ○ | × | ○ | × | × | × | ○ | × | |
| IC6 | × | ○ | ○ | × | × | ○ | × | × | × | ○ | ○ | × | × | × | ○ | ○ | |
The mean number of sessions to reach criterion in Phase 3 was 2.17 (SD = 1.46) for rats in the consistent group and 16.67 (SD = 7.99) for rats in the inconsistent group. Rats in the consistent group reached criterion in Phase 3 more rapidly than did those in the inconsistent group (t [4] = 3.99, p < .05, using Welch’s [1947] method).
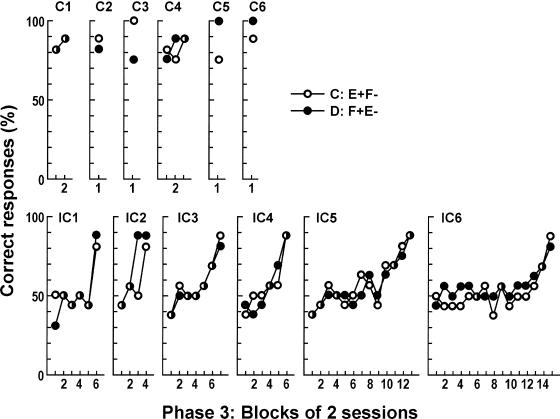
Figure 5 shows mean Phase 3 choice accuracy for each rat in both the consistent and inconsistent groups. Performance accuracy of rats in the inconsistent group was in the 40 to 60% range for a considerable number of sessions, whereas the rats in the consistent group maintained high levels of accuracy throughout Phase 3. The performance of Rat IC1 was around chance level for the first 10 sessions before improving abruptly over the final two sessions, and the performance of Rat IC2 also was around chance level for the first six sessions before improving abruptly over the final two sessions. Performance of the other rats in the inconsistent group changed gradually. Performance of Rat IC2 on the D: E+F− task (solid circle) reached 87.5% correct in the third block, whereas its performance on the C: F+E− task (open circle) remained around chance.
Fig. 5. Phase 3 mean accuracy for the 6 individual rats in both the consistent and inconsistent groups in two-session blocks.
The number of sessions in the last block was one for Rats C1, C3, C4, C6, IC4, and IC5. Symbols represent the different discrimination tasks.
Discussion
The results from Phase 3 of the present experiment showed that rats in the consistent group performed more accurately in the first session, and reached criterion significantly faster, than did those in the inconsistent group. Three consistent group rats (C3, C5, and C6) matched at high accuracy on the first session in Phase 3 (between 87.5 and 93.8%), whereas 3 inconsistent group rats (IC3, IC4, and IC5) matched at low accuracy (between 31.3 and 37.5%; see Table 3). Furthermore, 5 of the 6 consistent group rats made a correct response on the first trial in Phase 3, whereas no inconsistent group rat made a correct response on the first trial (Table 3). Thus the findings of the present experiment are consistent with the notion that an untrained, emergent relation between two stimuli (C and E) developed through their relation to a common stimulus (A) during many-to-one matching discrimination training in Phases 1 and 2.
The results of the present experiment are also similar to results reported by Urcuioli et al. (1989) using pigeons. In a comparable design, Urcuioli et al. reported that pigeons in the consistent group were more accurate in Phase 3, and reached a criterion of 80% accuracy faster than did those in the inconsistent group. In contrast, the accuracy of all inconsistent group pigeons was at or below chance level. Thus the results of the present experiment demonstrate that many-to-one transfer effects emerge in rats as well as in pigeons.
The present findings are consonant with a number of existing hypotheses that have been advanced to account for emergent transfer effect in pigeons and humans: the retrospective coding hypothesis (Zentall, Sherburne, & Urcuioli, 1995), the common coding hypothesis (Urcuioli et al., 1989; Zentall et al., 1991, 1992), the prospective coding hypothesis (Grant, 1982, 1991), and the mediated generalization hypothesis (Urcuioli, 1996; Urcuioli et al., 1995). The present results show that the purview of these theories now also extends to rats. However, in the present experimental procedures, these theories make no differential predictions, and thus the present data cannot be used to help choose among them. The question as to which of these approaches is more fruitful remains open.
Nakagawa (1986, 1992, 1993b) asserted that rats formed stimulus classes between stimuli on the basis of the shared common response. Nakagawa (1999a) found that the shared common response was a critical factor affecting stimulus-class formation in rats and found evidence that the sharing of a common response was critical in producing stimulus-class formation in rats. In Nakagawa’s (1999a) Experiment 1, rats were trained on two discriminations in a straight runway, and then trained on successive conditional discriminations in a Y maze. Group C, in which rats were required to choose the same goal box when the original positive or negative stimulus was presented at the entrance of each goal box, learned new problems faster than Group IC, in which rats were required to choose the right goal box when one of positive or negative stimuli was presented and to choose the left goal box when the other was presented. This result was found after overtraining but not after criterion training. In Experiment 2, rats were trained on two discriminations in the Y maze, and then were trained on either whole reversal (Group W, in which the two tasks were reversed) or partial reversal (Group P, in which one of the two tasks was reversed) in a straight runway. Group W learned the reversal faster than Group P after overtraining but not after criterion training. These results are consistent with the suggestion that the shared common response (i.e., the same response) is a factor that affects stimulus-class formation in rats.
The many-to-one transfer effect in rats shown here may be due to aspects of the training procedure used in the present experiment that may facilitate the development of associations between sample and correct comparison stimuli. Here the stimulus-response sequence (looking at the sample stimulus and then choosing the correct comparison stimulus by lever pressing) did not require similar responses to present the comparison stimuli and to respond to the choice alternative, as commonly arranged. In the present procedure, running down the runway produced the sample stimulus followed 1 s later by the comparison stimuli. If transfer is mediated by shared common responses, then the present procedure has two distinct observing and choice responses, whereas in the conventional procedure, the observing and choice responses are similar, so the choices may be less differentiated. These considerations, however, do not explain why pigeons show many-to-one transfer when similar observing and choice responses are required, whereas rats apparently do not. It is a testable proposition, though, that pigeons may show enhanced transfer when the observing response is differentiated from the choice response.
REFERENCES
- Delius J. D. Ameling M. Lea S. E. G. Staddon J. E. R. Reinforcement concordance induces and maintains stimulus associations in pigeons. The Psychological Record. 1995;45:283–297. [Google Scholar]
- Grant D. S. Prospective versus retrospective coding of samples of stimuli, responses, and reinforcers in delayed matching-to-sample by pigeons. Learning & Motivation. 1982;13:265–280. [Google Scholar]
- Grant D. S. Symmetrical and asymmetrical coding of food and no-food samples in delayed matching in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:186–193. [Google Scholar]
- Nakagawa E. The effect of overtraining on discrimination learning in white rats (in Japanese) Japanese Journal of Psychology. 1978;49:70–77. [Google Scholar]
- Nakagawa E. Overtraining, extinction and shift learning in a concurrent discrimination in rats. Quarterly Journal of Experimental Psychology. 1986;38B:313–326. [Google Scholar]
- Nakagawa E. Effects of overtraining reversal learning by rats in concurrent and single discriminations. Quarterly Journal of Experimental Psychology. 1992;44B:37–56. [Google Scholar]
- Nakagawa E. Matching and nonmatching concept learning in rats. Psychobiology. 1993a;21:142–150. [Google Scholar]
- Nakagawa E. Relational rule learning in the rat. Psychobiology. 1993b;21:293–298. [Google Scholar]
- Nakagawa E. Stimulus classes formation in concurrent discriminations in rats as a function of overtraining. The Psychological Record. 1998;48:537–552. [Google Scholar]
- Nakagawa E. A factor affecting stimulus classes formation in concurrent discriminations in rats. The Psychological Record. 1999a;49:117–136. [Google Scholar]
- Nakagawa E. Transfer of learning between concurrent and matching (or non-matching)-to-sample discriminations in rats. Quarterly Journal of Experimental Psychology. 1999b;52B:125–143. [Google Scholar]
- Nakagawa E. Reversal learning in conditional discriminations is not controlled by reinforcer density. The Psychological Record. 2000a;50:117–140. [Google Scholar]
- Nakagawa E. Transfer of learning between matching (or non-matching)-to-sample and same-different discriminations in rats. The Psychological Record. 2000b;50:771–805. [Google Scholar]
- Nakagawa E. Cross-modal stimulus class formation in rats. The Psychological Record. 2001;51:53–66. [Google Scholar]
- Nakagawa E. In S. P. Shohov (Ed.), Advances in Psychology Research (Vol. 11, pp. 115–148). Hauppauge, NY: Nova Science; 2002a. Whole-reversal and partial-reversal learning in conditional discriminations are not controlled by reinforcer density. [Google Scholar]
- Nakagawa E. Whole reversal versus partial reversal advantage effect on same-different discriminations in rats. The Psychological Record. 2002b;52:379–398. [Google Scholar]
- Nakagawa E. In S. P. Shohov (Ed.), Advances in Psychology Research (Vol. 13, pp. 169–187). Hauppauge, NY: Nova Science; 2002c. Cross-modal stimulus class formation in rats as function of overtraining. [Google Scholar]
- Nakagawa E. In S. P. Shohov (Ed.), Advances in Psychology Research (Vol. 15, pp. 67–110). Hauppauge, NY: Nova Science.; 2002d. The solution of two concurrent, matching- (or non-matching)-to-sample, and same-different discrimination learnings relies on a common underlying process in rats. [Google Scholar]
- Urcuioli P. J. In T. R. Zentall & P. M. Smeets (Eds.), Stimulus class formation in humans and animals (pp. 55–70). North-Holland: Amsterdam; 1996. Acquired equivalence and mediated generalization in pigeon’s matching-to-sample. [Google Scholar]
- Urcuioli P. J. Zentall T. R. DeMarse T. Transfer to derived sample-comparison relations by pigeons following many-to-one versus one-to-many matching with identical training relations. Quarterly Journal of Experimental Psychology. 1995;48B:158–178. [Google Scholar]
- Urcuioli P. J, Zentall T. R, Jackson-Smith P, Steirn J. N. Evidence for common coding in many-to-one matching: Retention, intertrial interference, and transfer. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15:264–373. [Google Scholar]
- Wasserman E. A. DeVolder C. L. Coppage D. J. Nonsimilarity based conceptualization in pigeons via secondary or mediated generalization. Psychological Science. 1992;3:374–379. [Google Scholar]
- Welch B. L. The generalization of “Student’s” problem when several different population variances are involved. Biometrika. 1947;34:28–35. doi: 10.1093/biomet/34.1-2.28. [DOI] [PubMed] [Google Scholar]
- Zentall T. R. Symbolic representation in animals: Emergent stimulus relations in conditional discrimination learning. Animal Learning & Behavior. 1998;26:363–377. [Google Scholar]
- Zentall T. R, Sherburne L. M, Steirn J. N, Randall C. K, Roper K. L, Urcuioli P. J. Common coding in pigeons: Partial versus total reversals of one-to-many conditional discriminations. Animal Learning & Behavior. 1992;20:373–386. [Google Scholar]
- Zentall T. R. Sherburne L. M. Urcuioli P. J. Coding of hedonic and nonhedonic samples by pigeons in many-to-one delayed matching. Animal Learning & Behavior. 1995;23:189–196. [Google Scholar]
- Zentall T. R. Steirn J. N. Sherburne L. M. Urcuioli P. J. Common coding in pigeons assessed through partial versus total reversals of many-to-one conditional and simple discriminations. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:194–201. [Google Scholar]