Abstract
Several researchers have suggested that conditioning history may have long-term effects on fixed-interval performances of rats. To test this idea and to identify possible factors involved in temporal control development, groups of rats initially were exposed to different reinforcement schedules: continuous, fixed-time, and random-interval. Afterwards, half of the rats in each group were studied on a fixed-interval 30-s schedule of reinforcement and the other half on a fixed-interval 90-s schedule of reinforcement. No evidence of long-term effects attributable to conditioning history on either response output or response patterning was found; history effects were transitory. Different tendencies in trajectory across sessions were observed for measures of early and late responding within the interreinforcer interval, suggesting that temporal control is the result of two separate processes: one involved in response output and the other in time allocation of responding and not responding.
Keywords: fixed interval, schedule history, temporal control, lever press, rats
In fixed-interval (FI) reinforcement schedules, a response is reinforced if it occurs after a fixed time has elapsed since the previous reinforcer. Under such circumstances, it is generally expected that, regardless of previous conditioning, FI responding will approach the same steady-state pattern of responding: a pause after a reinforcer, followed by an accelerated or a constant response rate until the next reinforcer (Baron & Leinenweber, 1994; Dews, 1970; Ferster & Skinner, 1957; Gentry, Weiss, & Laties, 1983; Schneider, 1969). Several authors have suggested, however, that conditioning history may affect some of the properties of responding observed under current FI contingencies (Freeman & Lattal, 1992; Johnson, Bickel, Higgins, & Morris, 1991; LeFrancois & Metzger, 1993; Weiner, 1964).
Research findings about the effects of conditioning history on pattern of responding have not been conclusive. Some studies reported a relatively constant rate throughout the interval on an FI schedule when a fixed ratio (FR) was programmed as the history reinforcement schedule (Urbain, Poling, Millam, & Thompson, 1978; Wanchisen, Tatham, & Mooney, 1989). In contrast, both Baron and Leinenweber (1995) and Freeman and Lattal (1992) found that on FI schedules most of the patterns consisted of a pause after a reinforcer followed by an abrupt change to a constant response rate (break and run) regardless of the schedule history. In addition, Cole (2001) provided evidence indicating that the reported effects on FI response pattern could be the result of insufficient training on the FI schedule. He evaluated the effects on FI performance of exposure to a schedule in which only interresponse times (IRTs) greater than 20 s were reinforced (a differential reinforcement of low rate [DRL] 20-s schedule), to an FR 40, or to both schedules in succession. Afterwards, no differences in the steady-state response pattern after prolonged exposure (90 sessions) to an FI 30-s reinforcement schedule were observed. All rats showed a break-and-run or a scalloped pattern (a pause after a reinforcer, followed by an increasingly higher response rate as the interval progressed).
The second property of responding for which history effects have been analyzed is overall response rate (i.e., mean session response rate). Some evidence suggests that FI produces sustained higher overall rates after variable-ratio (VR) or FR schedules than following no history or DRL training (Johnson et al., 1991; LeFrancois & Metzger, 1993; Urbain et al., 1978). However, Baron and Leinenweber (1995) and Cole (2001) provided evidence that such history effects tend to disappear under extended training on FI.
The tendency for response output and response pattern to converge into similar final measures, regardless of initial differences, indicates that the steady-state rate and patterns of responding usually reported on FI schedules may be reached through different routes or trajectories. It has been suggested that the analysis of behavioral trajectories, or behavior changes from the first to the last sessions of training, may help to determine the processes involved in temporal control in periodic schedules (cf. Machado, 1997; Machado & Cevik, 1998). However, reports of the development of responding in FI schedules as the experiment progresses are scarce.
In the case of response rate, the evidence suggests that the pattern of change across sessions is related to the previous conditioning history: After DRL, response rate on FI starts low and increases across sessions until it stabilizes at an intermediate level (Cole, 2001). When FR or VR precede the FI presentation, response rate starts high and decreases across sessions until it also stabilizes at an intermediate level (Baron & Leinenweber, 1995; Cole, 2001).
For response pattern, some researchers have reported a transition from undifferentiated responding throughout the interreinforcer interval (IRI) during the first sessions to a steady-state response pattern mostly characterized by the predominance of intervals with a break-and-run pattern (Baron & Leinenweber, 1995; see also Baron & Leinenweber, 1994; Ferster & Skinner, 1957; Machado & Cevik, 1998; Schneider, 1969). Typically, the behavioral trajectory to the FI steady-state response pattern may be described as involving a decrease in response rate during the first half of the IRI and an increase during the second half across sessions (Machado & Cevik, 1998). Machado and Cevik provided evidence indicating that those changes in opposite directions occur at different rates across sessions, thus suggesting that they evolve from two different processes. Notwithstanding, there is scarce information on the trajectory of the response pattern as the experiment progresses, and inferences about the factors involved in the development of temporal control need further evaluation.
The present experiment addresses the effects of conditioning history and the processes involved in temporal control acquisition. The performance of rats exposed to FI reinforcement schedules across sessions was analyzed after different conditioning histories. This design allowed us to evaluate possible sustained effects of conditioning history on FI performance and to find evidence about the factors involved in the trajectory of progress toward stability in measures of initial and terminal IRI responding. Three conditioning-history schedules were programmed: continuous reinforcement (FR 1), random interval (RI), and fixed time (FT), with FT and RI providing the same reinforcer rate as the subsequent FI schedule. With this arrangement, no change in reinforcer rate occurs when the initial history schedule is either FT or RI, whereas reinforcer rate decreases when going from FR 1 to FI scheduling. Initial response rate, however, is expected to be lower with FT than with FR 1 or RI, so the course to steady-state can be evaluated when starting from different initial response levels. In addition, we extended the analyses to a longer FI value than the usual 30 s used in previous research on the effects of conditioning history.
Method
Subjects
The subjects were 30 experimentally naive male Wistar rats, bred in a local colony. Rats were approximately 90 days old at the beginning of the experiment and were maintained at 80% of their free-feeding weights throughout the experiment. They were individually housed in a vivarium with free access to water and under a 12:12 hr light/dark cycle.
Apparatus
Four similar experimental chambers (350 mm deep by 305 mm wide by 365 mm high) were each equipped with a response lever and a liquid dipper. The lever was 48 mm wide and extended 20 mm into the chamber. It was located on the front wall 70 mm above the chamber floor and 75 mm from the left wall, below one stimulus lamp, and was connected to a microswitch that required approximately 0.3 N to operate. A 3-W lamp mounted behind a translucent screen located at the center of the ceiling provided general illumination of the chamber. The reinforcer, which consisted of 3-s access to a mix of tap water with condensed milk in a 2:1 volume to volume proportion, was delivered into a 0.05-mL solenoid-operated dipper that could be accessed through a cylindrical opening located at the center of the front wall 20 mm above the grid floor.
The experimental chamber was enclosed in a sound-attenuating chest. A ventilating fan masked external noises. A personal computer with a MED interface using MED STATE® software controlled the experimental operations and recorded data in real time, with a resolution to 0.1 s.
Procedure
After being trained to drink from the dipper, rats were randomly assigned to one of six groups of 5 rats each. Groups differed in the particular combination of conditions received in two consecutive phases. In the first phase, an FR 1, an FT, or an RI reinforcement schedule was in effect with two groups of rats in each condition. In the second phase, one group of rats in each condition of the first phase was exposed to an FI 30-s reinforcement schedule and the other to an FI 90-s reinforcement schedule. Time requirements on the groups exposed to the FT and RI schedules in the first phase matched the forthcoming FI in the second phase.
The first phase was in effect for 30 sessions and the second phase was scheduled for 60 sessions, but some sessions were lost because electrical failures produced incomplete sessions and data loss. Table 1 shows the number of sessions per phase. Each session was terminated by the delivery of the 46th reinforcer. Sessions started with the dipper in the lower position and the chamber light on. When the schedule requirement was met, the liquid dipper was raised and the chamber light was off for 3 s. Groups starting with an FT schedule experienced the temporal regularity of reinforcer delivery but were not allowed to respond because the lever was not available. When the lever was introduced in the second phase, no particular shaping of the response was attempted so as to avoid interfood intervals shorter than the programmed ones. To equate groups, no shaping was attempted with the FR 1 or RI schedule requirements. Except for Rat B-8, which required about 10 sessions, all of these rats started responding by the first or second session of the first phase. Rats initially exposed to the FT schedule started responding between the first and the third session of the target FI schedule.
Table 1. Number of sessions per subject on the history schedule (Phase 1) and on the target FI schedule (Phase 2).
| Group | Rat | Phase |
|
| 1 | 2 | ||
| FR 1-FI 30 | LR-1 | 30 | 60 |
| LR-2 | 30 | 60 | |
| LR-3 | 30 | 60 | |
| LR-4 | 30 | 60 | |
| LR-19 | 30 | 60 | |
| FT 30-FI 30 | S-21 | 30 | 58 |
| S-22 | 30 | 59 | |
| S-23 | 30 | 58 | |
| S-24 | 30 | 56 | |
| S-25 | 30 | 59 | |
| RI 30-FI 30 | LR-6 | 30 | 60 |
| LR-7 | 30 | 60 | |
| LR-9 | 30 | 60 | |
| LR-10 | 30 | 60 | |
| LR-18 | 30 | 60 | |
| FR 1-FI 90 | B-6 | 30 | 58 |
| B-7 | 30 | 58 | |
| B-8 | 30 | 59 | |
| B-9 | 30 | 52 | |
| B-10 | 30 | 60 | |
| FT 90-FI 90 | LR-11 | 30 | 58 |
| LR-12 | 30 | 60 | |
| LR-15 | 30 | 59 | |
| LR-16 | 30 | 59 | |
| LR-17 | 28 | 60 | |
| RI 90-FI 90 | B-1 | 30 | 58 |
| B-2 | 30 | 59 | |
| B-3 | 30 | 59 | |
| B-4 | 30 | 54 | |
| B-5 | 30 | 49 | |
Results
Response Pattern
Three measures were calculated: postreinforcer pause (PRP), response rate within equal successive intervals of the IRI, and a curvature index. All analyses excluded data obtained before the first reinforcer in a session. For the FT–FI rats, all analyses started with the first day in which the rats responded on the FI schedule.
Figures 1, 2, and 3 show the daily mean PRP for each rat from the FR 1, FT, and RI history groups, respectively. Rats initially submitted to FR 1 (Figure 1) and RI (Figure 3) reinforcement schedules produced similar results. Typically, the mean PRP obtained in the first session on FI was short and sometimes close to zero, and except for the RI 30–FI 30 rats (Figure 3, left column), then increased rapidly up to about the 10th to the 15th session. Afterwards, the mean PRP either stabilized or kept increasing, but at a lower rate. For rats in the RI 30–FI 30 group, PRP increased gradually in a negatively accelerated fashion until reaching a similar level to that of the comparable FR 1 and FT rats.
Figure 1. Mean postreinforcer pause in consecutive sessions on the target FI 30-s schedule (left column) and on the target FI 90-s schedule (right column) for rats with an FR 1 schedule history.
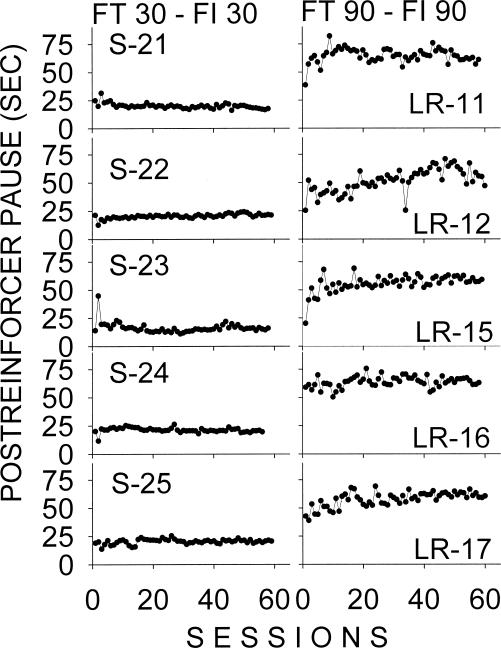
Figure 2. Mean postreinforcer pause in consecutive sessions on the target FI 30-s schedule (left column) and on the target FI 90-s schedule (right column) for rats with an FT schedule history.
History FT and target FI were comparable.
Figure 3. Mean postreinforcer pause in consecutive sessions on the target FI 30-s schedule (left column) and on the target FI 90-s schedule (right column) for rats with an RI schedule history.
History RI and target FI were comparable.
Among rats previously submitted to the FT 30-s and FT 90-s schedules (Figure 2), a steady PRP was reached within a few sessions following introduction of the FI, more clearly for FI 30-s group. The mean PRP for rats previously submitted to FT 90 s was more variable and with some tendency to increase over the sessions (see Rats LR-12, LR-15, and LR-17).
To facilitate comparisons, data were averaged across rats for each group. Data for Rat B-8 of the FR 1–FI 90 group were excluded from this and all subsequent averaged and statistical analyses because of an atypically low response rate throughout the IRI. This pattern clearly deviated from that observed in all the other rats. Figure 4 presents the daily mean group PRP for the FR 1, FT, and RI history groups. The resulting group plots were representative of the individual rats of each group and agreed with descriptions based upon individuals. First, there was a similar sharp increase in PRP in the initial sessions among groups with prior exposure to FR 1 (filled circles) and RI (open squares) reinforcement schedules, excepting the RI 30–FI 30 group. Second, groups with prior exposure to the FT schedules (filled triangles) started with a longer PRP and attained a relatively steady PRP faster than the comparable FR 1 or RI groups.
Figure 4. Mean group postreinforcer pause in consecutive sessions for FR 1–FI (filled circles), FT–FI (filled triangles), and RI–FI (open squares) groups on the FI 30-s (upper graph) and on the FI 90-s schedule (lower graph).
Considering all groups during the last sessions, Figure 4 shows that the PRP was longer on the FI 90-s schedules than on the FI 30-s schedules and that no sizable differences due to history appeared. These observations were confirmed when individual mean PRP (log transformed to correct for heterogeneity of variance) from the last five sessions of each subject was submitted to a repeated measures analysis of variance (ANOVA) using session as a within factor, and history and FI schedule as between factors. Only a main effect for FI was significant, F(1, 23) = 535.26, p < .00001: Mean PRP for the FI 90-s groups was about 3 times longer than that of the FI 30-s groups (see Table 2 for details).
Table 2. Mean and standard deviation for the postreinforcer pause averaged across the last five sessions for each group.
| Group | N | M(s) | SD |
| FR 1-FI 30 | 5 | 20.44 | 2.37 |
| FT 30-FI 30 | 5 | 18.92 | 2.60 |
| RI 30-FI 30 | 5 | 17.83 | 2.45 |
| FR 1-FI 90 | 4 | 59.42 | 8.81 |
| FT 90-FI 90 | 5 | 59.25 | 3.80 |
| RI 90-FI 90 | 5 | 58.65 | 8.41 |
Figures 5, 6, and 7 present within-interval response patterns for the FR 1, FT, and RI history groups, respectively. To represent changes in FI patterning as the FI training progressed, Sessions 1, 10, 20, 40 and the last session of the FI training were selected for presentation. The FI was divided into 10 equal time bins, and the average rate per session during each bin was computed, thus obtaining one response rate curve per rat per session.
Figure 5. Mean response rate in successive tenths of the interval for rats with an FR 1 schedule history, for Sessions 1, 10, 20, 40, and the last session, on the target FI 30-s schedule (left column) and on the target FI 90-s schedule (right column).
Figure 6. Mean response rate in successive tenths of the interval for rats with an FT schedule history, for Sessions 1, 10, 20, 40, and the last session, on the target FI 30-s schedule (left column) and on the target FI 90-s schedule (right column).
Figure 7. Mean response rate in successive tenths of the interval for rats with an RI schedule history, for Sessions 1, 10, 20, 40, and the last session, on the target FI 30-s schedule (left column) and on the target FI 90-s schedule (right column).
Changes across sessions may be grouped into three stages. In the first stage, the different history groups show two contrasting tendencies in response pattern during the first session. Most rats in the FR 1 and RI groups (Figures 5 and 7) showed a gradual response rate decrease as time bins increased, beginning with the first bin or after a previous increase from the first to the second bin. Only Rat B-8 (Figure 5) showed an increase in response rate across time bins. In contrast, rats previously exposed to FT (Figure 6) showed a zero or close to zero rate in the first four to five bins and a slight tendency to increase in the following bins.
In the second stage, the response pattern for the RI and FR 1 groups changed by the 10th session. Responding in the first two to four time bins practically disappeared, and the general within-interval pattern of responding assumed a sigmoidal shape for the majority of the rats. The FT–FI groups increased responding in the second half of the interval while responding in the first half remained at a low or zero rate. The response pattern was similar to that of the FR 1 and RI groups, although positively accelerated patterns occurred in a couple of FI 30-s rats (S-21 and S-24).
In the third stage, after the 10th or the 20th session, no further changes were observed in the first five or so bins of most rats of all groups, but response rate tended to increase further in later bins. By the last session, the response-rate function was either positively accelerated or sigmoidal for most rats. There were no substantial differences in response pattern among the different groups at the end of training.
The patterns shown in Figures 5, 6, and 7 are well represented in Figure 8, which presents response-rate curves averaged across rats for each group: temporally undifferentiated response-rate curves in the initial session, a decrease in rate in the first bins by the 10th session, reaching a zero or close to zero rate by the 20th session, and rate increases during the last bins from the 20th session on.
Figure 8. Mean group response rate in successive tenths of the interval, for Sessions 1, 10, 20, 40, and the last session, for FR 1 (upper graphs), FT (middle graphs), and RI (lower graphs) conditioning histories, on the target FI 30-s schedule (left column) and on the target FI 90-s schedule (right column).
To obtain a quantitative evaluation of how the response-rate curves changed from the first to the last sessions of training, a polynomial quadratic function was fitted to each daily response-rate curve for each rat. Figure 9 presents the session-by-session mean group quadratic coefficient during all FI sessions. Positive values indicate that response rate was a positively accelerated function of time across the interval; negative values indicate that response rate was a negatively accelerated function of time across the interval; and a value of zero represents a constant rate across the interval. Constant response rates (quadratic coefficients of zero; groups FR 1–FI 90, FT 30–FI 30, and FT 90–FI 90) or negatively accelerated response rates (negative coefficients; groups FR 1–FI 30, RI 30–FI 30, and RI 90–FI 90) across the IRI were observed on the very first session or over the first five or six sessions. All groups showed an increasing tendency in the degree of positive acceleration as the FI sessions continued (higher and higher positive quadratic coefficients). After about the 45th session, a tendency for the quadratic coefficient to stabilize was observed suggesting that all groups converged on a similar, positively accelerated response-rate curve. The last suggestion was supported by a repeated measures ANOVA of the individual quadratic coefficients obtained in the last five sessions for each rat, using session as a within factor and history and FI as between factors. None of the possible within or between comparisons proved significant (p > .05).
Figure 9. Mean group quadratic coefficient in consecutive sessions on the target FI 30-s schedule (left column) and on the target FI 90-s schedule (right column).
Mean Response Rate
Previous studies used overall response rate to assess the effects of prior history: The total number of responses in a session is divided by session time, omitting reinforcer time. As a consequence, the time base contains the PRP, and the obtained overall rate becomes sensitive to the duration of the PRP. For this reason, we also calculated the running rate, dividing number of responses in a session by the time from the first response to reinforcer delivery cumulated over the session.
Figures 10, 11, and 12 present the overall rate for the last five sessions on the history schedule, and running and overall rates for all sessions on the FI 30 s (left columns) and FI 90 s (right columns) for all rats in the FR 1–FI, FT–FI, and RI–FI groups. For both FI values of the FR 1 history group (Figure 10), overall response rate tended to stabilize after a few sessions. In fact, overall rates in the final sessions were about the same as those obtained in the first few sessions for most rats. The running rate started at about the same level as the overall rate, but increased with a negatively accelerated tendency across sessions. A notable exception was Rat B-8 on the FI 90-s schedule, for which overall and running rate occurred at an atypical low level and slowly decreased across sessions.
Figure 10. Mean overall rate for the last five sessions of each rat on the FR 1 history schedule (data points before the break) and mean overall and running rates in all sessions on the target FI 30-s schedule (left column) and on the target FI 90-s schedule (right column).
Figure 11. Mean overall rate for the last five sessions of each rat on the FT history schedule (data points before the break) and mean overall and running rates in all sessions on the target FI 30-s schedule (left column) and on the target FI 90-s schedule (right column).
Figure 12. Mean overall rate for the last five sessions of each rat on the RI history schedule (data points before the break) and mean overall and running rates in all sessions on the target FI 30-s schedule (left column) and on the target FI 90-s schedule (right column).
Because the lever was retracted on the FT history schedule, response rate necessarily was zero previous to the introduction of the FI schedule (Figure 11). Data points begin with the first session in which the rats started responding. The overall response rate slowly increased across sessions, with a tendency to stabilize in later sessions. Running rate started at a similar level to overall rate but, by the second or third session, began to increase to higher levels.
For rats with RI history (Figure 12), overall response rate reached a stable level on both FI schedules after a few sessions. Again, in both groups of rats, overall and running rate started at similar levels but overall rate tended to decrease or remain at the level obtained in the first few sessions, whereas running rate grew to higher levels. The overall rates at the introduction of the FI schedules were similar to those in the last sessions of the prior RI schedule. When the overall rate generated by the RI was relatively high, it was followed by a similar rate when the FI was introduced, but rapidly decreased to a moderate level after a few sessions of the FI training. This is most clearly observed in Rat LR-10 on the FI 30 s and, to a lesser extent, in Rats LR-9 and LR-18 on FI 30 s, and Rat B-1 on the FI 90 s.
Figure 13 presents response rate averaged across rats for each group. Typically, for the FR 1–FI and RI–FI groups, overall response rate tended to stabilize after a few sessions on the FI, whereas in the FT–FI groups a slight tendency to increase across sessions was observed. In contrast, running rate tended to increase as experience on the FI schedule increased. By the last sessions, the only differences on both measures were associated with FI value, not with conditioning history. To corroborate these observations, repeated measures ANOVA tests were run for each measure, with the individual means of the last five sessions for each rat as a within factor, and history and FI as between factors. Only main effects for FI value were found for the overall rate (log transformed to correct for heterogeneity of variances; F[1, 23] = 18.23, p < .001) and for the running rate (F[1, 23] = 13.34, p < .002). Table 3 presents central tendency details. FI 30-s groups produced higher overall and running rates than the FI 90-s groups.
Figure 13. Mean group overall rate (open symbols) for the last five sessions of each group on the FR 1 (circles), FT (triangles), and RI (squares) history schedules (data points before the break) and mean group overall and running (closed symbols) rates in all sessions on the target FI 30-s schedule (upper graph) and on the target FI 90-s schedule (lower graph).
Table 3. Mean (presses per minute) and standard deviation for overall and running response rate averaged across the last five sessions for each group.
| Group | N | Overall rate |
Running rate |
||
| M | SD | M | SD | ||
| FR 1-FI 30 | 5 | 35.57 | 13.86 | 101.17 | 39.52 |
| FT 30-FI 30 | 5 | 36.31 | 19.03 | 90.67 | 28.11 |
| RI 30-FI 30 | 5 | 39.45 | 9.92 | 97.06 | 31.25 |
| FR 1-FI 90 | 4 | 26.73 | 10.23 | 76.47 | 8.99 |
| FT 90-FI 90 | 5 | 17.28 | 3.57 | 50.21 | 11.08 |
| RI 90-FI 90 | 5 | 19.51 | 4.43 | 57.29 | 16.92 |
Discussion
The present experiment indicates that conditioning history does affect the performance of rats on FI schedules, but that the effects are transient. By the final sessions, effects of the FI schedule on response rate and PRP were clear, although the mean response pattern was similar on the two FI schedules. In particular, our findings are consistent with the findings of Baron and Leinenweber (1995) and of Cole (2001) in that the effects of different conditioning histories on measures of rate and pattern of responding tended to dissipate with continued exposure to FI schedules (see Figures 4, 8, 9, and 13 for summarized results). The former authors programmed VR schedules with different arrangements, whereas the latter programmed FR and DRL schedules as conditioning histories, and both measured the possible effects on a target FI 30-s schedule. Our results extend those findings to other conditioning histories and to a longer FI.
Previous evidence supporting differential effects of conditioning history on response rate (Johnson et al., 1991; LeFrancois & Metzger, 1993; Urbain et al., 1978; Wanchisen et al., 1989) and response pattern (Wanchisen et al., 1989) seems to be the consequence of an insufficient number of sessions on the FI schedule (Cole, 2001). The training conditions before the FI presentation may affect only the particular route to the steady-state performance. This possibility was examined for the present experiment, beginning with response pattern.
FI schedules engender a distinctive temporal pattern of responding described as either scalloped, break-and-run, or a mixture of the two (Ferster & Skinner, 1957; Gentry et al., 1983; Schneider, 1969), in which the IRI is clearly partitioned into a period of not responding and a period of responding beginning at about halfway into the interval. It has been suggested that this temporally differentiated pattern evolves from an undifferentiated one observed in the initial sessions of the FI presentation (Baron & Leinenweber, 1994). Our findings on average within-interval response-rate curves (Figures 5, 6, 7, and 8) and curvature index (Figure 9) agree with this view. Additionally, our evidence throws some light on the factors involved in the process culminating in a temporally differentiated pattern of responding.
First, in the groups initially exposed to FR 1 or RI reinforcement schedules (Figures 5, 7, and 8), responding in the initial part of the interval (of about one half to two thirds of the interval) reached an extremely low or zero rate by the 20th session, with no further changes. However, response rate in the terminal part of the interval was still increasing up to at least the 40th session. This difference in the speed with which the initial and terminal rates change was observed by Machado and Cevik (1998). As noted by these authors, the difference in speed of change suggests that two processes are involved in shaping response patterning under periodic schedules.
Two-process hypotheses have been proposed previously by Schneider (1969), Shull, Guilkey, and Witty (1972), and Palya (1993). The idea of two processes also is supported by research demonstrating that response rate can be affected without altering the duration of the PRP as long as the IRI remains constant (Elsmore, 1971; Farmer & Schoenfeld, 1964; Killeen, 1969; Morgan, 1970; Neuringer & Schneider, 1968; Shull, 1970; Shull et al., 1972). These findings have favored the conclusion that temporal factors associated with the IRI are involved in determining the PRP size whereas response-reinforcer contingencies determine the response rate. The present experiment suggests one way in which IRI and response-reinforcer contingencies contribute to shape the temporally differentiated pattern with exposure to the FI.
The temporally differentiated response pattern of pause responding develops across sessions as the rat experiences both reinforcer periodicity and the response-reinforcer dependency. Because under pretraining conditions the rat usually experiences an FR 1 schedule, at the introduction of the FI the schedule control is mainly exerted by the response-reinforcer contingency and no temporally differentiated patterns are observed. In the FT–FI groups of the present experiment, the rats were first exposed to the temporal contingency, thus reversing the usual training sequence (Figure 6). When the FI was introduced, response rates during the initial segment of the intervals were very low or zero and did not noticeably change across sessions for either FI. The response rates in the terminal segment of the IRIs increased with FI exposure. Two facts suggest that the temporal contingency was the main variable operating from the first few sessions: First, PRP was similar in the first few sessions to the PRP observed in the last sessions on the FI; second, PRP was larger on the FI 90-s than on the FI 30-s from the first session (Figures 2 and 4).
Groups with FR 1 and RI history schedules showed a different pattern of results: First, after a short PRP in the initial session on the FI, PRP increased in a negatively accelerated fashion up to the last sessions (Figures 1, 3, and 4). Second, mean response rate was relatively constant throughout the IRI during the initial session, but as the experiment progressed, response rate declined after reinforcer delivery and increased as the time to the next reinforcer approached (Figures 5 and 7). Because the PRP has been regarded as an important indicator of temporal control (cf. Higa, Thaw, & Staddon, 1993), the differences between FT–FI and the FR 1–FI and RI–FI groups suggest that PRP was sensitive to the temporal factors involved in FI schedules and that the effects of temporal factors were facilitated by prior exposure to the FT schedule. It remains to be seen whether such facilitation is related to the temporal distribution of reinforcers or to the mere noncontingent delivery of the reinforcer in the FT schedule.
Complementing the previous analyses, response output reflected the transition in response-reinforcer contingencies from the history to the FI schedule. An FR 1 to an FI schedule transition represents a reduction in reinforcer frequency and a consequent reduction in responding, which was observed in the FR 1–FI groups (Figure 10). The transition from noncontingent to contingent reinforcer delivery should have the effect of increasing response rate, as in the FT–FI groups (Figure 11). Finally, in the RI–FI groups reinforcer frequency remains the same, but a higher rate of responding might be expected on the schedule providing reinforcers at variable intervals than at regular intervals. However, this was not always observed, because in some cases overall rate was similar under both schedules (Figure 12). Typically, overall rate remained stable after a few sessions on both FI schedules. In contrast, the running rate tended to increase across sessions in all groups, indicating that the increased temporal control exerted as the experiment progressed determined the reallocation of responding to the latter segments of the IRI.
The evidence obtained in the present experiment suggests that IRI and response contingency are the main factors involved in determining the pause-responding pattern in FI schedules (cf. Shull et al., 1972). The different rates of change across sessions of early and late responding within the IRI (Figure 8), and the tendency for PRP and running rate to increase across sessions (Figures 4 and 13), are consistent with the two-process dynamic hypothesis (Machado, 1997) and with session-to-session analyses of FI responding undertaken by Machado and Cevik (1998). However, our results have some further implications: Although the experiment was not devised to test dynamic models like those proposed by Higa and Staddon (1997) and Machado, it shows how initial conditions lead to particular trajectories that end in the steady-state temporal control. Our evidence suggests that initial properties of responding interact with the restrictions imposed by the FI contingencies. The transition from conditioning history to FI training may involve changes in reinforcer rate, in reinforcer distribution, or in both rate and distribution. Depending on the nature of the changes, the development of steady-state temporal allocation of responding and not responding under FI may be accelerated or decelerated. For the same reason, development of the steady-state response rate may be delayed or facilitated. These are facts that dynamical models will have to consider in the future.
Acknowledgments
This research was supported by grant 37802-H from the Consejo Nacional de Ciencia y Tecnología. Appreciation is expressed to Dr. John Staddon for his suggestions and comments.
REFERENCES
- Baron A, Leinenweber A. Molecular and molar aspects of fixed-interval performance. Journal of the Experimental Analysis of Behavior. 1994;61:11–18. doi: 10.1901/jeab.1994.61-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron A, Leinenweber A. Effects of a variable-ratio conditioning history on sensitivity to fixed-interval contingencies in rats. Journal of the Experimental Analysis of Behavior. 1995;63:97–110. doi: 10.1901/jeab.1995.63-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M.R. The long-term effect of high- and low-rate responding histories on fixed interval responding in rats. Journal of the Experimental Analysis of Behavior. 2001;75:43–54. doi: 10.1901/jeab.2001.75-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dews P.B. The theory of fixed-interval responding. In: Schoenfeld W.N, editor. The theory of reinforcement schedules. New York: Appleton-Century-Crofts; 1970. pp. 43–61. [Google Scholar]
- Elsmore T.F. Independence of postreinforcement pause length and running rate on fixed-interval pacing reinforcement schedules. Psychonomic Science. 1971;23:371–372. [Google Scholar]
- Farmer J, Schoenfeld W.N. Effects of a DRL contingency added to a fixed-interval reinforcement schedule. Journal of the Experimental Analysis of Behavior. 1964;7:391–399. doi: 10.1901/jeab.1964.7-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster C.B, Skinner B.F. Schedules of reinforcement. New York: Appleton-Century-Crofts; 1957. [Google Scholar]
- Freeman T.J, Lattal K.A. Stimulus control of behavioral history. Journal of the Experimental Analysis of Behavior. 1992;57:5–15. doi: 10.1901/jeab.1992.57-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry G.D, Weiss B, Laties V.G. The microanalysis of fixed-interval responding. Journal of the Experimental Analysis of Behavior. 1983;39:327–343. doi: 10.1901/jeab.1983.39-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa J.J, Staddon J.E.R. Dynamical models of rapid temporal control in animals. In: Bradshaw C.M, Szabadi E, editors. Time and behaviour: Psychological and neurobehavioural analyses. Amsterdam: Elsevier; 1997. pp. 1–40. [Google Scholar]
- Higa J.J, Thaw J.M, Staddon J.E.R. Pigeons' wait-time responses to transitions in interfood-interval duration: Another look at cyclic schedule performance. Journal of the Experimental Analysis of Behavior. 1993;59:529–541. doi: 10.1901/jeab.1993.59-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L.M, Bickel W.K, Higgins S.T, Morris E.K. The effects of schedule history and the opportunity for adjunctive responding on behavior during a fixed-interval schedule of reinforcement. Journal of the Experimental Analysis of Behavior. 1991;55:313–322. doi: 10.1901/jeab.1991.55-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen P. Reinforcement frequency and contingency as factors in fixed-ratio behavior. Journal of the Experimental Analysis of Behavior. 1969;12:391–395. doi: 10.1901/jeab.1969.12-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeFrancois J.R, Metzger B. Low-response-rate conditioning history and fixed-interval responding in rats. Journal of the Experimental Analysis of Behavior. 1993;59:543–549. doi: 10.1901/jeab.1993.59-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado A. Learning the temporal dynamics of behavior. Psychological Review. 1997;104:241–265. doi: 10.1037/0033-295x.104.2.241. [DOI] [PubMed] [Google Scholar]
- Machado A, Cevik M. Acquisition and extinction under periodic reinforcement. Behavioural Processes. 1998;44:237–262. doi: 10.1016/s0376-6357(98)00052-7. [DOI] [PubMed] [Google Scholar]
- Morgan M.J. Fixed-interval schedules and delay of reinforcement. Quarterly Journal of Experimental Psychology. 1970;22:663–673. [Google Scholar]
- Neuringer A.J, Schneider B.A. Separating the effects of interreinforcement time and number of interreinforcement responses. Journal of the Experimental Analysis of Behavior. 1968;11:661–667. doi: 10.1901/jeab.1968.11-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palya W.L. Bipolar control in fixed interfood intervals. Journal of the Experimental Analysis of Behavior. 1993;60:345–359. doi: 10.1901/jeab.1993.60-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider B. A two-state analysis of fixed-interval responding in the pigeon. Journal of the Experimental Analysis of Behavior. 1969;12:667–687. doi: 10.1901/jeab.1969.12-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull R.L. The response-reinforcement dependency in fixed-interval schedules of reinforcement. Journal of the Experimental Analysis of Behavior. 1970;14:55–60. doi: 10.1901/jeab.1970.14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull R.L, Guilkey M, Witty W. Changing the response unit from a single peck to a fixed number of pecks in fixed-interval schedules. Journal of the Experimental Analysis of Behavior. 1972;17:193–200. doi: 10.1901/jeab.1972.17-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbain C, Poling A, Millam J, Thompson T. d-amphetamine and fixed-interval performance: Effects of operant history. Journal of the Experimental Analysis of Behavior. 1978;29:385–392. doi: 10.1901/jeab.1978.29-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanchisen B.A, Tatham T.A, Mooney S.E. Variable-ratio conditioning history produces high-and low-rate fixed-interval performance in rats. Journal of the Experimental Analysis of Behavior. 1989;52:167–179. doi: 10.1901/jeab.1989.52-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. Conditioning history and human fixed-interval performance. Journal of the Experimental Analysis of Behavior. 1964;7:383–385. doi: 10.1901/jeab.1964.7-383. [DOI] [PMC free article] [PubMed] [Google Scholar]