Abstract
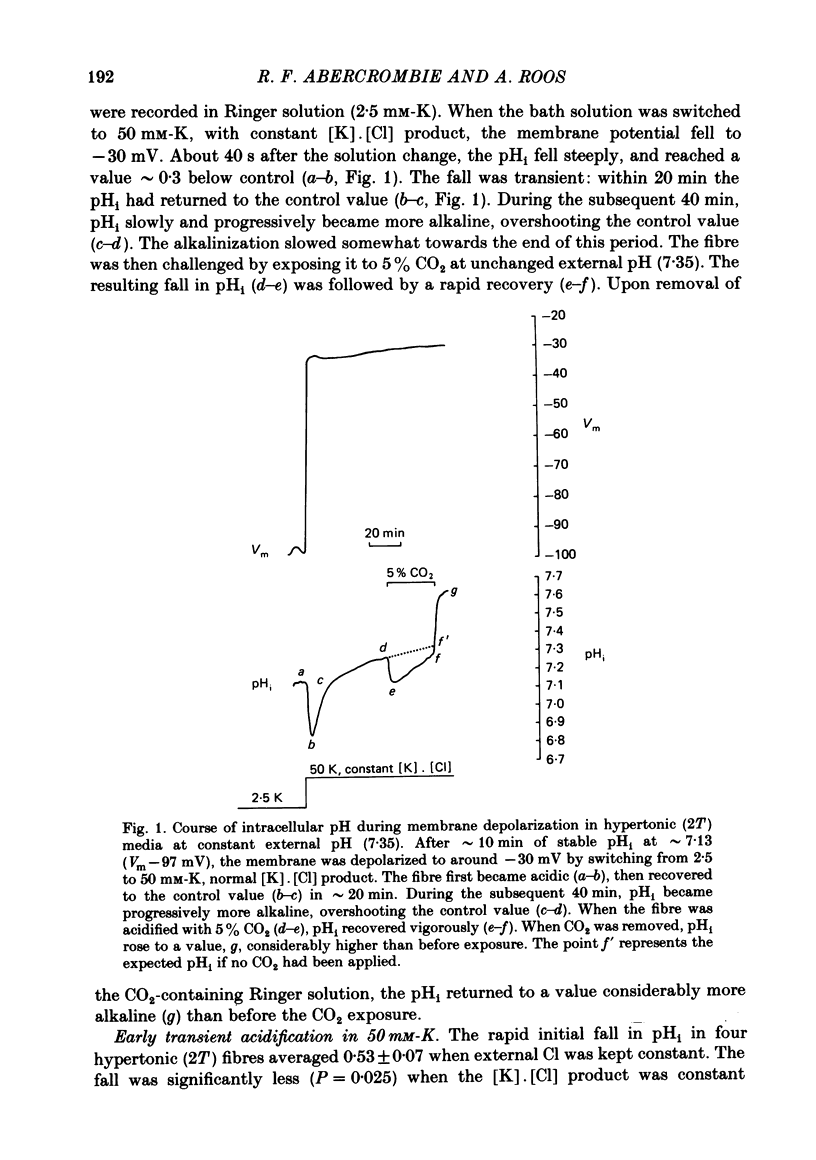
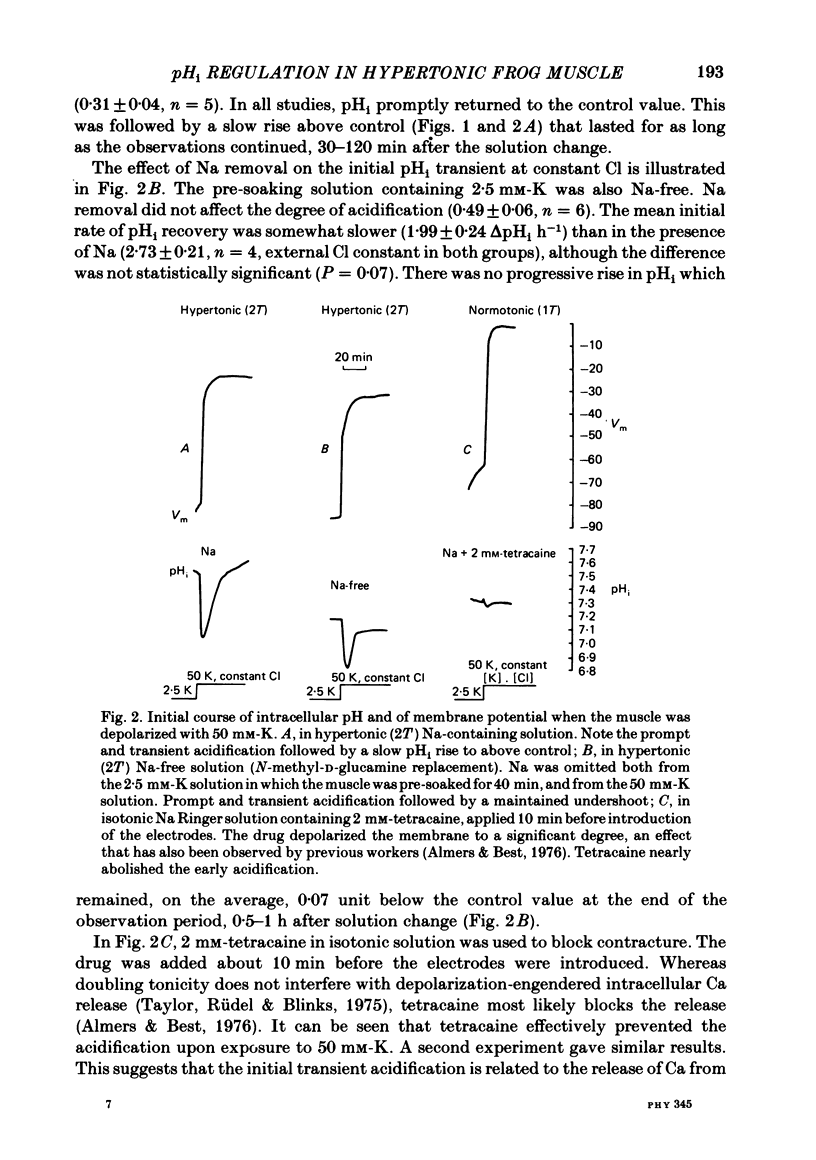
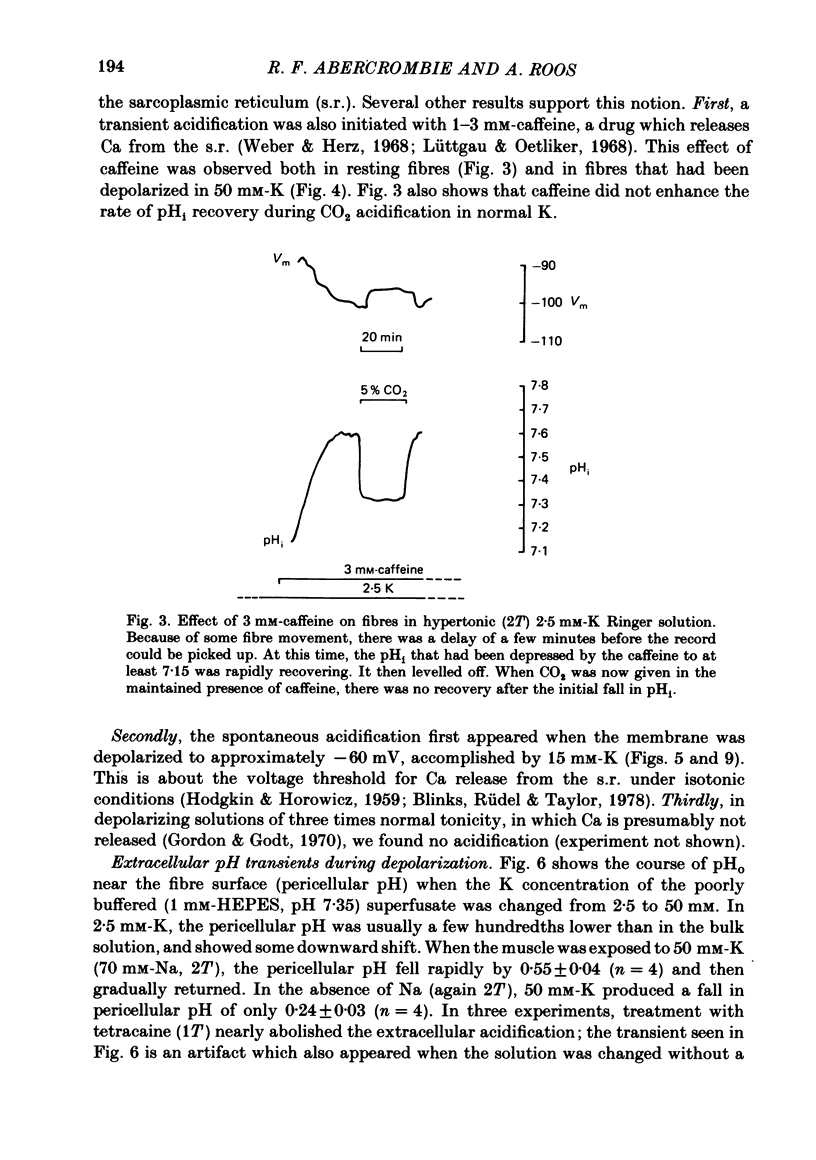
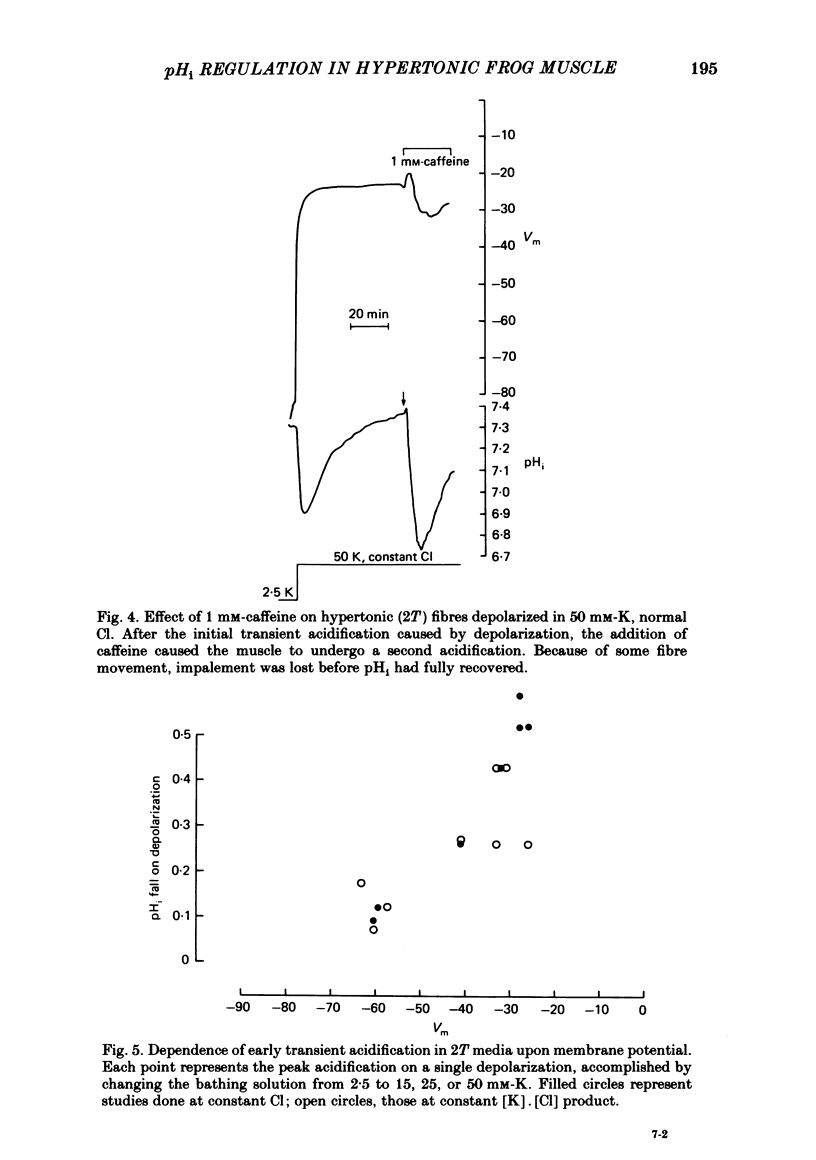
Intracellular pH (pHi) was followed with micro-electrodes in frog semitendinosus muscle, superfused at 22 degrees C with hypertonic solutions (external pH, pHo, 7.35) containing 2.5, 15 or 50 mM-K. Tonicity was doubled by addition of 250 mM-mannitol or, in a few cases, 125 mM-extra NaCl. Tripling of tonicity was accomplished by adding 500 mM-mannitol. Because of the ability of hypertonicity to minimize contracture, the course of pHi could be followed from the start of depolarization. The pHi of fibres after about 40 min in Ringer solution (2.5 mM-K, HEPES buffer) of twice normal tonicity was 7.40 +/- 0.04 (S.E. of mean) (n = 17), about 0.2 higher than at normal tonicity. The membrane potential, Vm, was -87.7 +/- 1.3 mV. When the muscle was depolarized in 50 mM-K to about -30 mV, the pHi rapidly fell by 0.3-0.5 unit (n = 9), and then promptly returned. This recovery was followed by a much slower and progressive rise to above control. Removing Na from the medium did not affect the degree of acidification, but the pHi recovered at a slightly slower rate, did not reach control value and showed no progressive rise. A less pronounced transient acidification was also observed when the muscle was depolarized in 15 mM-K to about -60 mV. When contracture was prevented either by 1-2 mM-tetracaine under isotonic conditions or by raising tonicity 3-fold, 50 mM-K produced no transient acidification. When the pHi of resting fibres in Ringer solution (2.5 mM-K) of twice normal tonicity was reduced by 5% CO2 from 7.40 to 7.12 +/- 0.07 (n = 3), it recovered at a slow rate (0.06 +/- 0.03 delta pHi h-1). Depolarization by 15 or 50 mM-K enhanced recovery rate 4-6-fold. These solutions of twice normal tonicity, as compared to those of normal tonicity, shifted the curve relating pHi recovery rate and membrane potential along the potential axis in the direction of hyperpolarization. This shift may be due to increased ionic shielding of fixed negative charges at the inner membrane surface. At twice normal tonicity, the very slow pHi recovery of resting fibres from CO2-induced acidification, as well as the more rapid recovery in depolarized fibres, could be abolished by 1 mM-amiloride or by removing Na. The application of amiloride during pHi recovery in 50 mM-K was not associated with an observable change in Vm. SITS had no significant effect on recovery.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie R. F., Putnam R. W., Roos A. The intracellular pH of frog skeletal muscle: its regulation in isotonic solutions. J Physiol. 1983 Dec;345:175–187. doi: 10.1113/jphysiol.1983.sp014973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman J. J., Lowry M., Radda G. K., Ross B. D., Wong G. G. The role of intrarenal pH in regulation of ammoniagenesis: [31P]NMR studies of the isolated perfused rat kidney. J Physiol. 1981;319:65–79. doi: 10.1113/jphysiol.1981.sp013892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Rakowski R. F. Charge movement and mechanical repriming in skeletal muscle. J Physiol. 1976 Jan;254(2):361–388. doi: 10.1113/jphysiol.1976.sp011236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Best P. M. Effects of tetracaine on displacement currents and contraction of frog skeletal muscle. J Physiol. 1976 Nov;262(3):583–611. doi: 10.1113/jphysiol.1976.sp011611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Dichroic components of Arsenazo III and dichlorophosphonazo III signals in skeletal muscle fibres. J Physiol. 1982 Oct;331:179–210. doi: 10.1113/jphysiol.1982.sp014369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Optical measurements of intracellular pH and magnesium in frog skeletal muscle fibres. J Physiol. 1982 Oct;331:105–137. doi: 10.1113/jphysiol.1982.sp014367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONWAY E. J. Nature and significance of concentration relations of potassium and sodium ions in skeletal muscle. Physiol Rev. 1957 Jan;37(1):84–132. doi: 10.1152/physrev.1957.37.1.84. [DOI] [PubMed] [Google Scholar]
- Chandler W. K., Hodgkin A. L., Meves H. The effect of changing the internal solution on sodium inactivation and related phenomena in giant axons. J Physiol. 1965 Oct;180(4):821–836. doi: 10.1113/jphysiol.1965.sp007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Klee C. B., Picton C., Shenolikar S. Calcium control of muscle phosphorylase kinase through the combined action of calmodulin and troponin. Ann N Y Acad Sci. 1980;356:151–161. doi: 10.1111/j.1749-6632.1980.tb29608.x. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- Curtin N. A., Woledge R. C. Energy changes and muscular contraction. Physiol Rev. 1978 Jul;58(3):690–761. doi: 10.1152/physrev.1978.58.3.690. [DOI] [PubMed] [Google Scholar]
- DYDYNSKA M., WILKIE D. R. THE OSMOTIC PROPERTIES OF STRIATED MUSCLE FIBERS IN HYPERTONIC SOLUTIONS. J Physiol. 1963 Nov;169:312–329. doi: 10.1113/jphysiol.1963.sp007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M., Blinks J. R. Inconstant association of aequorin luminescence with tension during calcium release in skinned muscle fibres. Nat New Biol. 1973 Dec 19;246(155):218–221. doi: 10.1038/newbio246218a0. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAHAME D. C. The electrical double layer and the theory of electrocapillarity. Chem Rev. 1947 Dec;41(3):441–501. doi: 10.1021/cr60130a002. [DOI] [PubMed] [Google Scholar]
- Gordon A. M., Godt R. E. Some effects of hypertonic solutions on contraction and excitation-contraction coupling in frog skeletal muscles. J Gen Physiol. 1970 Feb;55(2):254–275. doi: 10.1085/jgp.55.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttgau H. C., Oetliker H. The action of caffeine on the activation of the contractile mechanism in straited muscle fibres. J Physiol. 1968 Jan;194(1):51–74. doi: 10.1113/jphysiol.1968.sp008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa E., Hosoi K., Ebashi S. Reversible stimulation of muscle phosphorylase b kinase by low concentrations of calcium ions. J Biochem. 1967 Apr;61(4):531–533. doi: 10.1093/oxfordjournals.jbchem.a128582. [DOI] [PubMed] [Google Scholar]
- Robertson S. P., Johnson J. D., Potter J. D. The time-course of Ca2+ exchange with calmodulin, troponin, parvalbumin, and myosin in response to transient increases in Ca2+. Biophys J. 1981 Jun;34(3):559–569. doi: 10.1016/S0006-3495(81)84868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. M., Boron W. F. Role of choloride transport in regulation of intracellular pH. Nature. 1976 Nov 4;264(5581):73–74. doi: 10.1038/264073a0. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Gonzalez-Serratos H. G., Shuman H., McClellan G., Somlyo A. P. Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: an electron-probe study. J Cell Biol. 1981 Sep;90(3):577–594. doi: 10.1083/jcb.90.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. R., Rüdel R., Blinks J. R. Calcium transients in amphibian muscle. Fed Proc. 1975 Apr;34(5):1379–1381. [PubMed] [Google Scholar]
- Thomas R. C. Recovery of pHi in snail neurones exposed to high external potassium [proceedings]. J Physiol. 1979 Nov;296:77P–77P. [PubMed] [Google Scholar]
- Thomas R. C. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol. 1977 Dec;273(1):317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A., Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968 Nov;52(5):750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]


