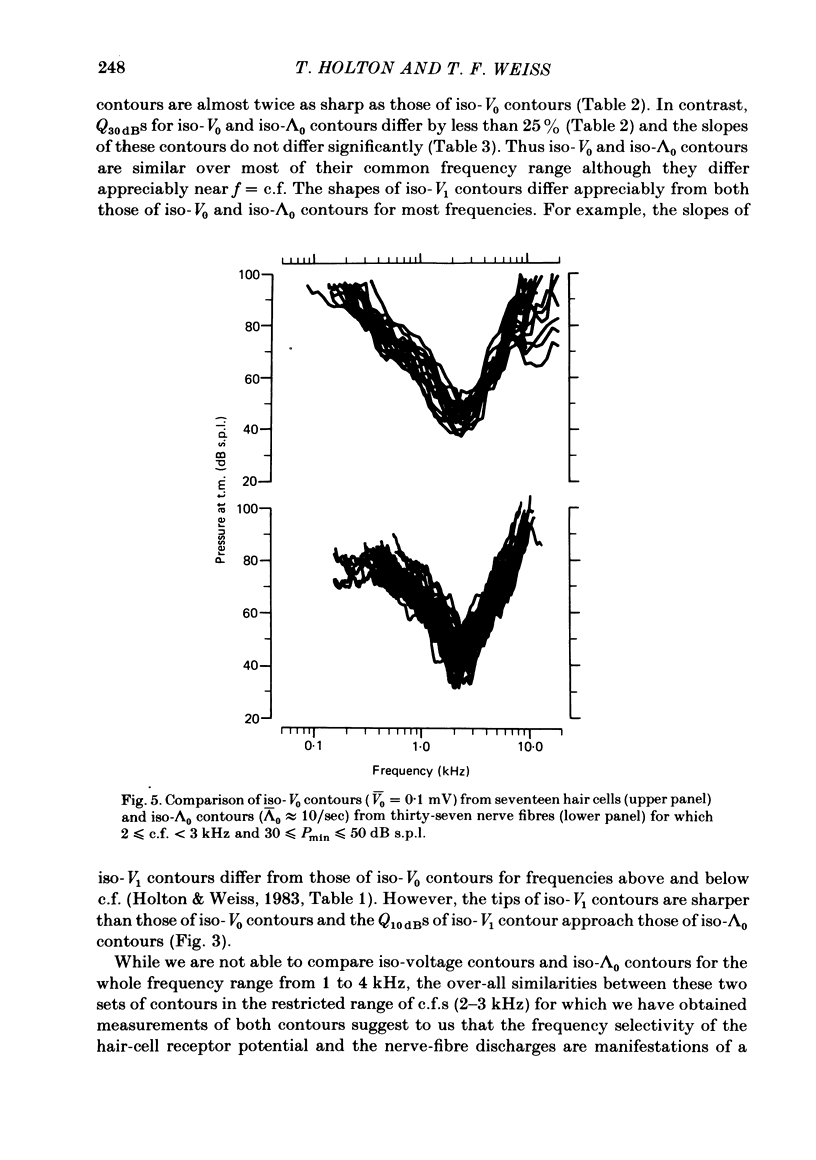
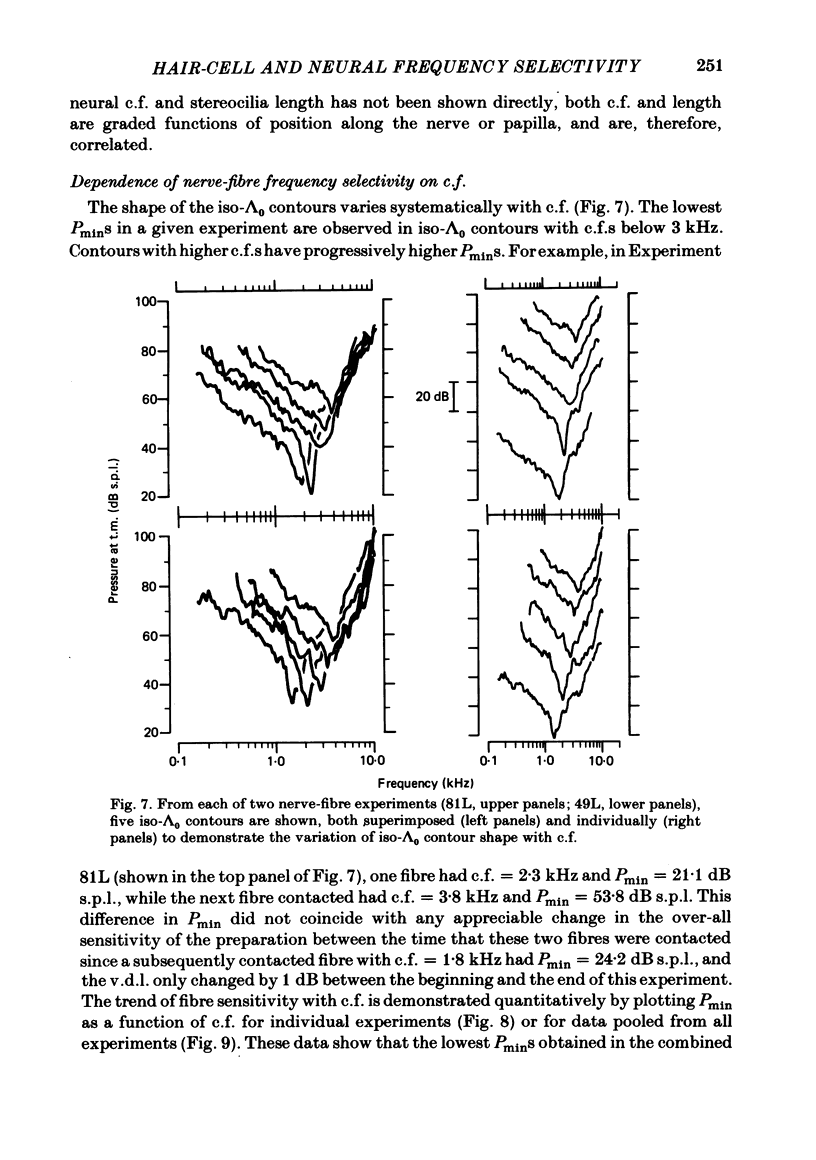
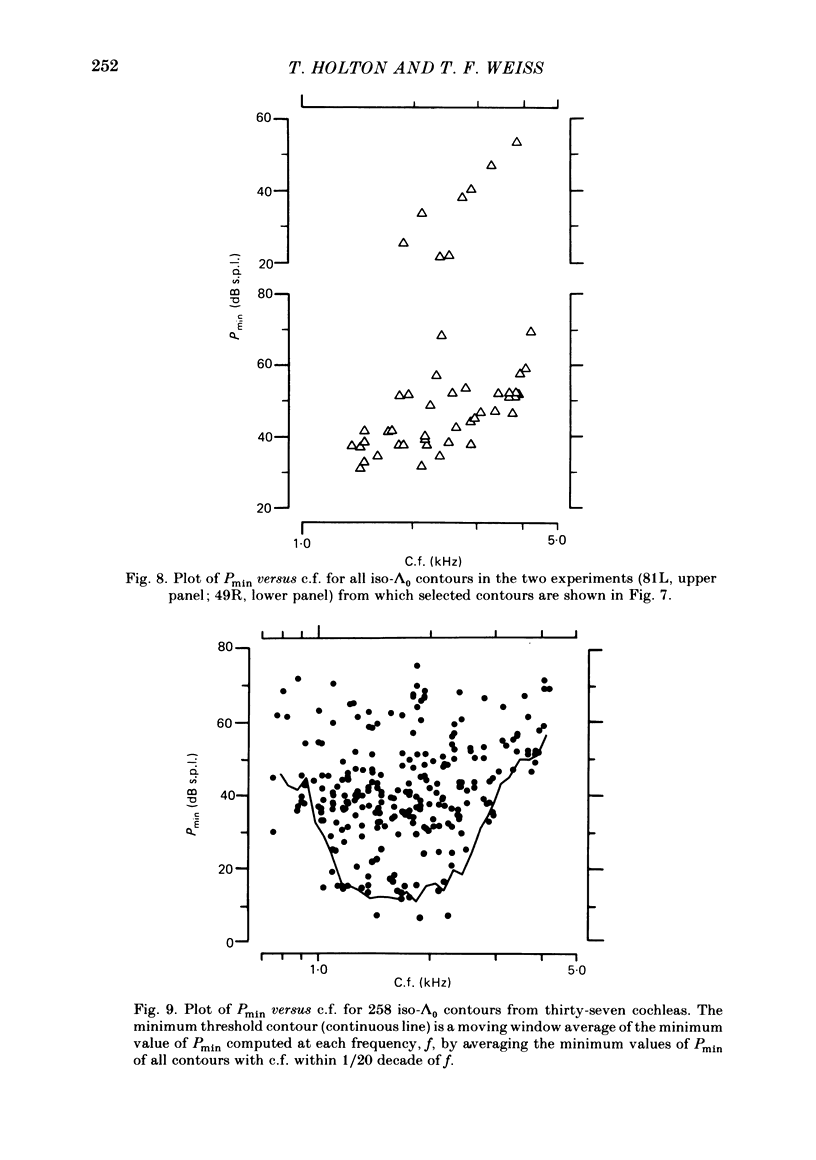
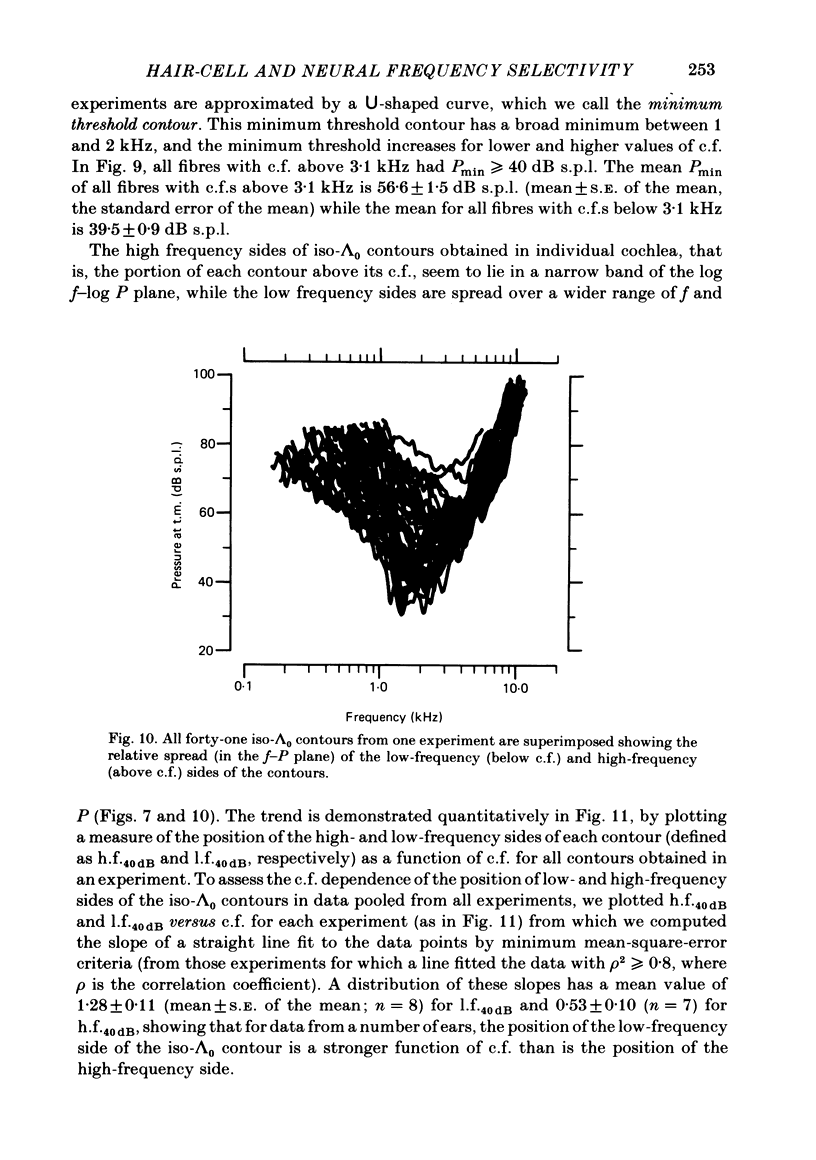
Abstract
Receptor potentials of hair cells and spike discharges of cochlear nerve fibres were recorded with micropipettes from the free-standing region of the basilar papilla of anaesthetized alligator lizards in response to tones. In this region the hair-cell stereocilia are free-standing, i.e. they protrude directly into endolymph and are not in contact with a tectorial membrane. The frequency selectivity of hair-cell responses was measured by means of isovoltage contours of the d.c. (V0) and fundamental-a.c. (V1) component of the receptor potential, i.e. iso-V0 and iso-V1 contours. The frequency selectivity of the nerve-fibre discharge was measured by iso-rate (iso-V0) contours. Iso-V0, iso-V1 and iso-V0 contours are basically V-shaped with a characteristic frequency (c.f.) defined as the frequency at which minimum sound pressure (Pmin) is required to evoke the criterion value of the response. Receptor potential iso-V0 contours and neural iso-V0 contours have similar slopes: the mean slopes of the low-frequency sides (dB/decade) are -43.0 and -44.3; the slopes of the high-frequency sides are 85.0 and 80.2. The band widths of iso-V0 and iso-V0 contours away from c.f. are similar (mean values of Q30dB are 0.40 and 0.53, respectively). The band widths of iso-V0 contours near c.f. are narrower than those of iso-V0 contours (mean values of Q10dB are 2.34 and 1.20, respectively). However, the shapes of the contours near c.f. depend on the iso-response criteria, and we have not determined whether or not iso-V0 and iso-V0 contours are similar near c.f. The shapes of iso-V1 contours differ from those of iso-V0 and iso-V0 contours. Nerve fibre c.f.s are tonotopically organized in the nerve, with lowest c.f.s recorded from fibres innervating the border of free-standing and tectorial regions, a region in which hair-cell stereocilia are longest, and the highest c.f.s recorded from fibres innervating the end of the free-standing region in which hair-cell stereocilia are shortest. The c.f. of nerve-fibre response (and by implication hair-cell response) is, therefore, correlated with the height of the stereociliary tuft. The shapes of iso-V0 contours vary systematically with c.f. and, therefore, tonotopically with nerve position.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baden-Kristensen K., Weiss T. F. Receptor potentials of lizard hair cells with free-standing stereocilia: responses to acoustic clicks. J Physiol. 1983 Feb;335:699–721. doi: 10.1113/jphysiol.1983.sp014559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns V., Goldbach M. Hair cells and tectorial membrane in the cochlea of the greater horseshoe bat. Anat Embryol (Berl) 1980;161(1):51–63. doi: 10.1007/BF00304668. [DOI] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. The frequency selectivity of auditory nerve fibres and hair cells in the cochlea of the turtle. J Physiol. 1980 Sep;306:79–125. doi: 10.1113/jphysiol.1980.sp013387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. F. The frequency response and other properties of single fibres in the guinea-pig cochlear nerve. J Physiol. 1972 Oct;226(1):263–287. doi: 10.1113/jphysiol.1972.sp009984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. F., Wilson J. P. Cochlear tuning properties: concurrent basilar membrane and single nerve fiber measurements. Science. 1975 Dec 19;190(4220):1218–1221. doi: 10.1126/science.1198110. [DOI] [PubMed] [Google Scholar]
- Geisler C. D., Rhode W. S., Kennedy D. T. Responses to tonal stimuli of single auditory nerve fibers and their relationship to basilar membrane motion in the squirrel monkey. J Neurophysiol. 1974 Nov;37(6):1156–1172. doi: 10.1152/jn.1974.37.6.1156. [DOI] [PubMed] [Google Scholar]
- Holton T. Relations between frequency selectivity and two-tone rate suppression in lizard cochlear-nerve fibers. Hear Res. 1980 Jan;2(1):21–38. doi: 10.1016/0378-5955(80)90014-3. [DOI] [PubMed] [Google Scholar]
- Holton T., Weiss T. F. Receptor potentials of lizard cochlear hair cells with free-standing stereocilia in response to tones. J Physiol. 1983 Dec;345:205–240. doi: 10.1113/jphysiol.1983.sp014975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton T., Weiss T. F. Two-tone rate suppression in lizard cochlear nerve fibers, relation to receptor organ morphology. Brain Res. 1978 Dec 22;159(1):219–222. doi: 10.1016/0006-8993(78)90123-3. [DOI] [PubMed] [Google Scholar]
- Johnstone B. M., Boyle A. J. Basilar membrane vibration examined with the Mössbauer technique. Science. 1967 Oct 20;158(3799):389–390. doi: 10.1126/science.158.3799.389. [DOI] [PubMed] [Google Scholar]
- Johnstone B. M., Taylor K. J., Boyle A. J. Mechanics of the guinea pig colea. J Acoust Soc Am. 1970 Feb;47(2):504–509. doi: 10.1121/1.1911921. [DOI] [PubMed] [Google Scholar]
- Johnstone B. M., Yates G. K. Basilar membrane tuning curves in the guinea pig. J Acoust Soc Am. 1974 Mar;55(3):584–587. doi: 10.1121/1.1914568. [DOI] [PubMed] [Google Scholar]
- Khanna S. M., Leonard D. G. Basilar membrane tuning in the cat cochlea. Science. 1982 Jan 15;215(4530):305–306. doi: 10.1126/science.7053580. [DOI] [PubMed] [Google Scholar]
- Kiang N. Y., Moxon E. C., Levine R. A. Auditory-nerve activity in cats with normal and abnormal cochleas. In: Sensorineural hearing loss. Ciba Found Symp. 1970:241–273. doi: 10.1002/9780470719756.ch15. [DOI] [PubMed] [Google Scholar]
- Le Page E. L., Johnstone B. M. Nonlinear mechanical behaviour of the basilar membrane in the basal turn of the guinea pig cochlea. Hear Res. 1980 Jun;2(3-4):183–189. doi: 10.1016/0378-5955(80)90056-8. [DOI] [PubMed] [Google Scholar]
- Lewis E. R. Suggested evolution of tonotopic organization in the frog amphibian papilla. Neurosci Lett. 1981 Jan 20;21(2):131–136. doi: 10.1016/0304-3940(81)90370-0. [DOI] [PubMed] [Google Scholar]
- Liberman M. C. Auditory-nerve response from cats raised in a low-noise chamber. J Acoust Soc Am. 1978 Feb;63(2):442–455. doi: 10.1121/1.381736. [DOI] [PubMed] [Google Scholar]
- Liberman M. C. The cochlear frequency map for the cat: labeling auditory-nerve fibers of known characteristic frequency. J Acoust Soc Am. 1982 Nov;72(5):1441–1449. doi: 10.1121/1.388677. [DOI] [PubMed] [Google Scholar]
- Lim D. J. Cochlear anatomy related to cochlear micromechanics. A review. J Acoust Soc Am. 1980 May;67(5):1686–1695. doi: 10.1121/1.384295. [DOI] [PubMed] [Google Scholar]
- Mulroy M. J. Cochlear anatomy of the alligator lizard. Brain Behav Evol. 1974;10(1-3):69–87. doi: 10.1159/000124303. [DOI] [PubMed] [Google Scholar]
- Peake W. T., Ling A., Jr Basilar-membrane motion in the alligator lizard: its relation to tonotopic organization and frequency selectivity. J Acoust Soc Am. 1980 May;67(5):1736–1745. doi: 10.1121/1.384300. [DOI] [PubMed] [Google Scholar]
- Rhode W. S. Cochlear partition vibration--recent views. J Acoust Soc Am. 1980 May;67(5):1696–1703. doi: 10.1121/1.384296. [DOI] [PubMed] [Google Scholar]
- Rhode W. S. Observations of the vibration of the basilar membrane in squirrel monkeys using the Mössbauer technique. J Acoust Soc Am. 1971 Apr;49(4 Suppl):1218+–1218+. doi: 10.1121/1.1912485. [DOI] [PubMed] [Google Scholar]
- Rhode W. S. Some observations on cochlear mechanics. J Acoust Soc Am. 1978 Jul;64(1):158–176. doi: 10.1121/1.381981. [DOI] [PubMed] [Google Scholar]
- Russell I. J., Sellick P. M. Intracellular studies of hair cells in the mammalian cochlea. J Physiol. 1978 Nov;284:261–290. doi: 10.1113/jphysiol.1978.sp012540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellick P. M., Patuzzi R., Johnstone B. M. Measurement of basilar membrane motion in the guinea pig using the Mössbauer technique. J Acoust Soc Am. 1982 Jul;72(1):131–141. doi: 10.1121/1.387996. [DOI] [PubMed] [Google Scholar]
- Turner R. G., Muraski A. A., Nielsen D. W. Cilium length: influence on neural tonotopic organization. Science. 1981 Sep 25;213(4515):1519–1521. doi: 10.1126/science.7280673. [DOI] [PubMed] [Google Scholar]
- Weiss T. F., Mulroy M. J., Turner R. G., Pike C. L. Tuning of single fibers in the cochlear nerve of the alligator lizard: relation to receptor morphology. Brain Res. 1976 Oct 8;115(1):71–90. doi: 10.1016/0006-8993(76)90823-4. [DOI] [PubMed] [Google Scholar]
- Wilson J. P., Johnstone J. R. Basilar membrane and middle-ear vibration in guinea pig measured by capacitive probe. J Acoust Soc Am. 1975 Mar;57(3):705–723. doi: 10.1121/1.380472. [DOI] [PubMed] [Google Scholar]


