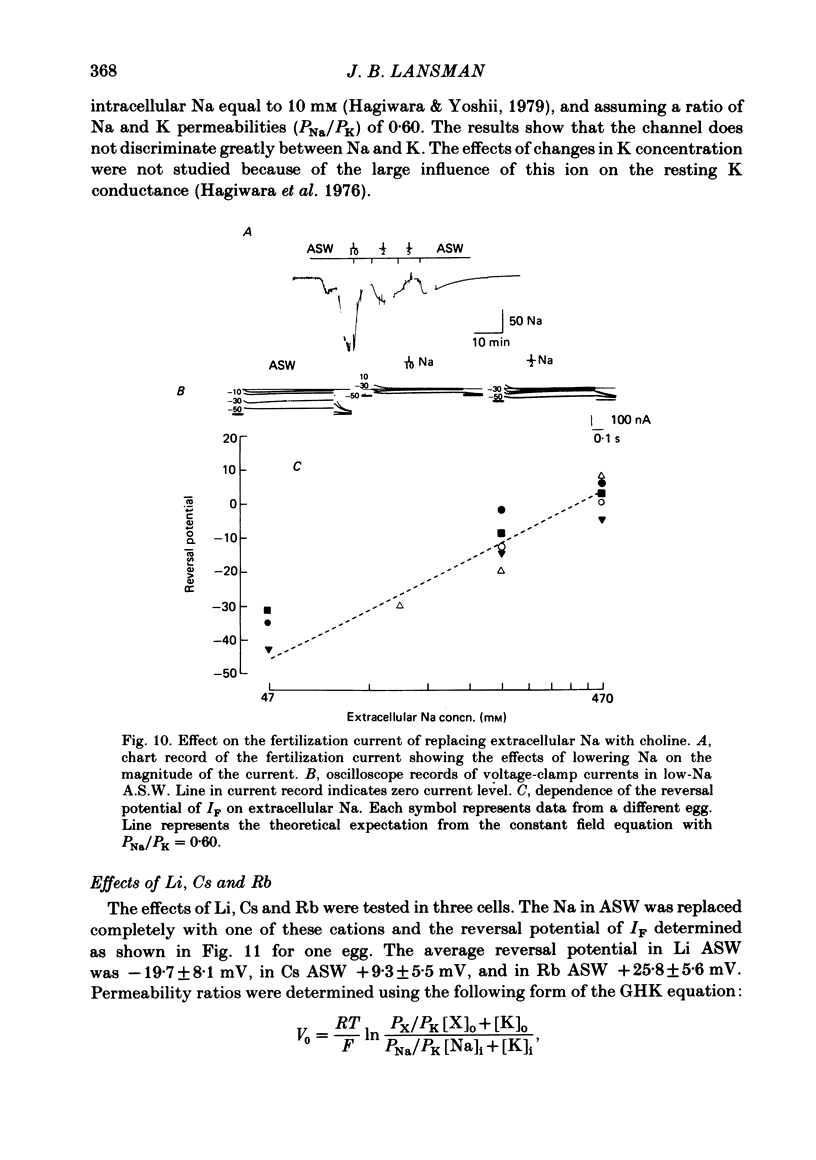
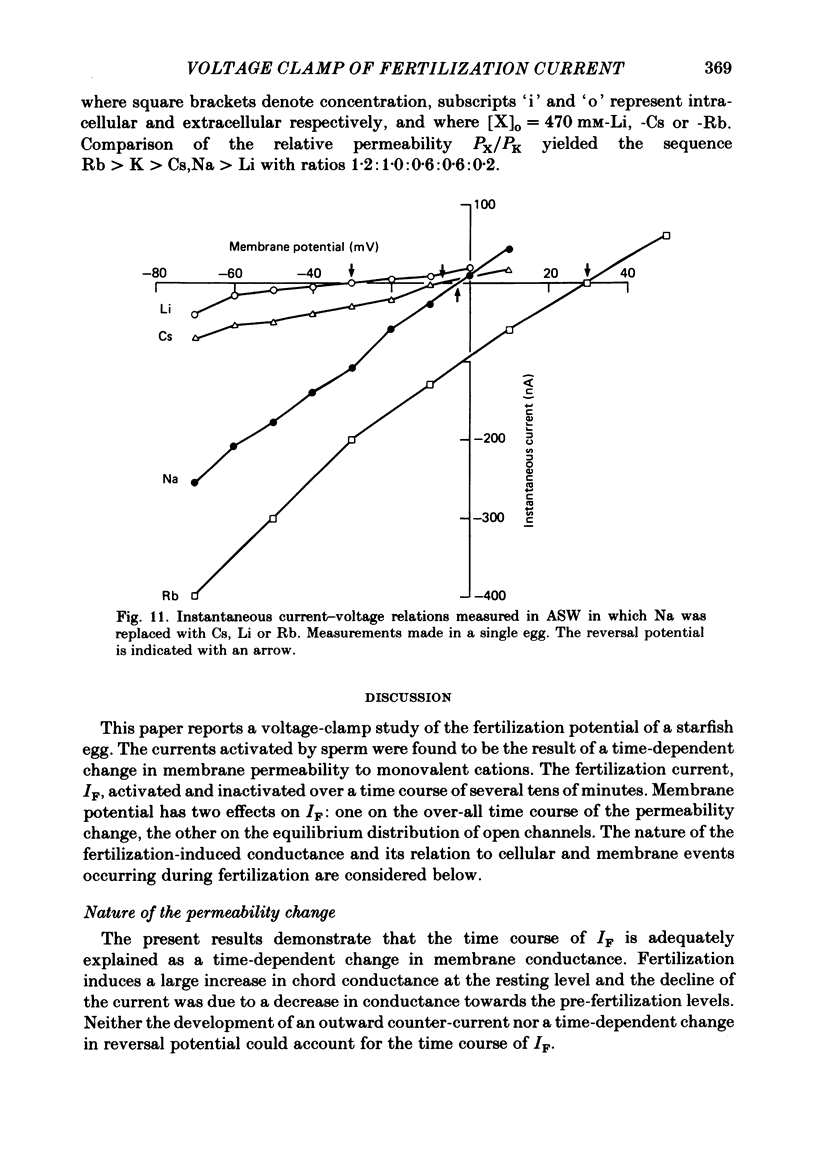
Abstract
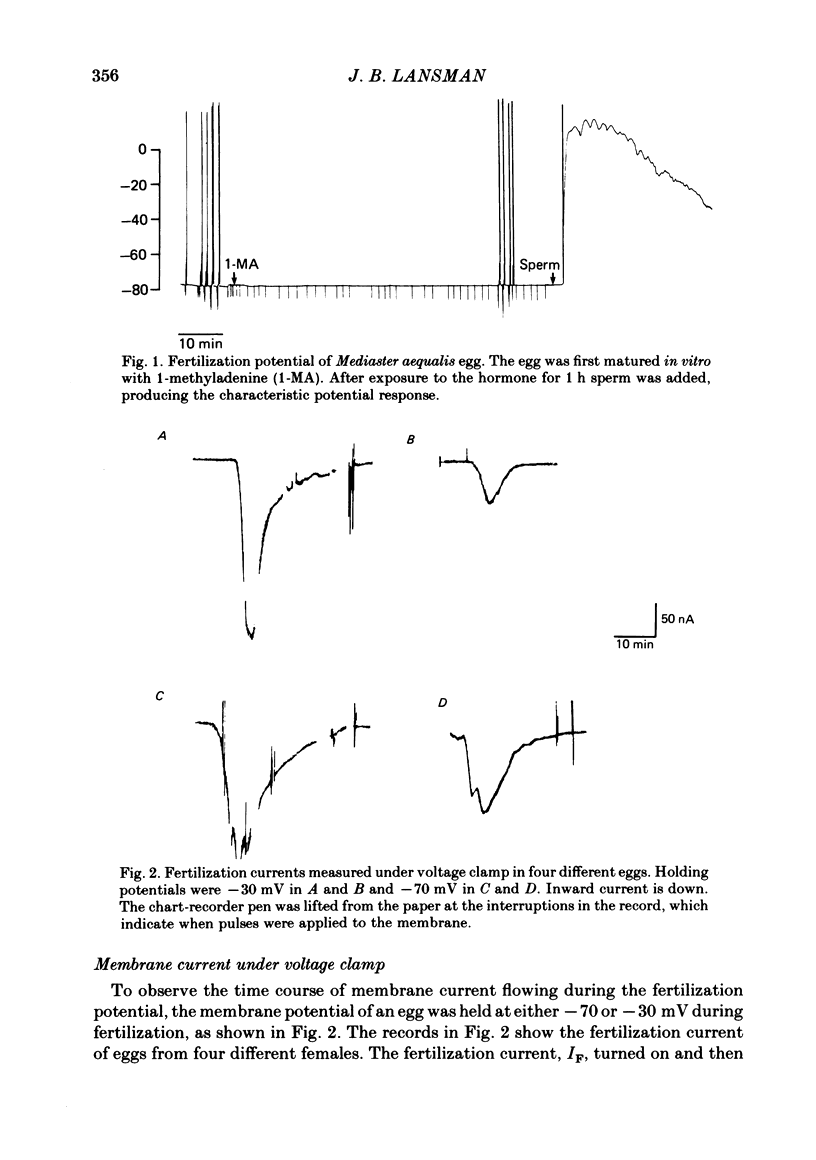
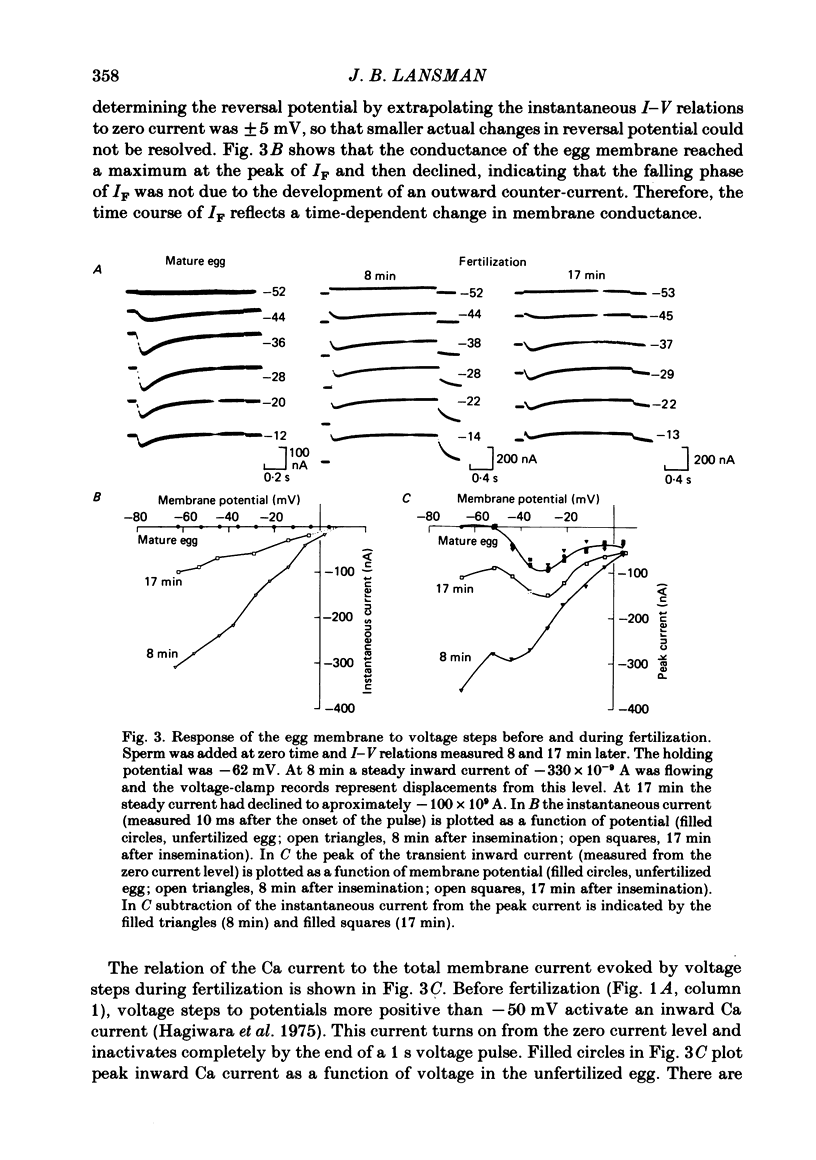
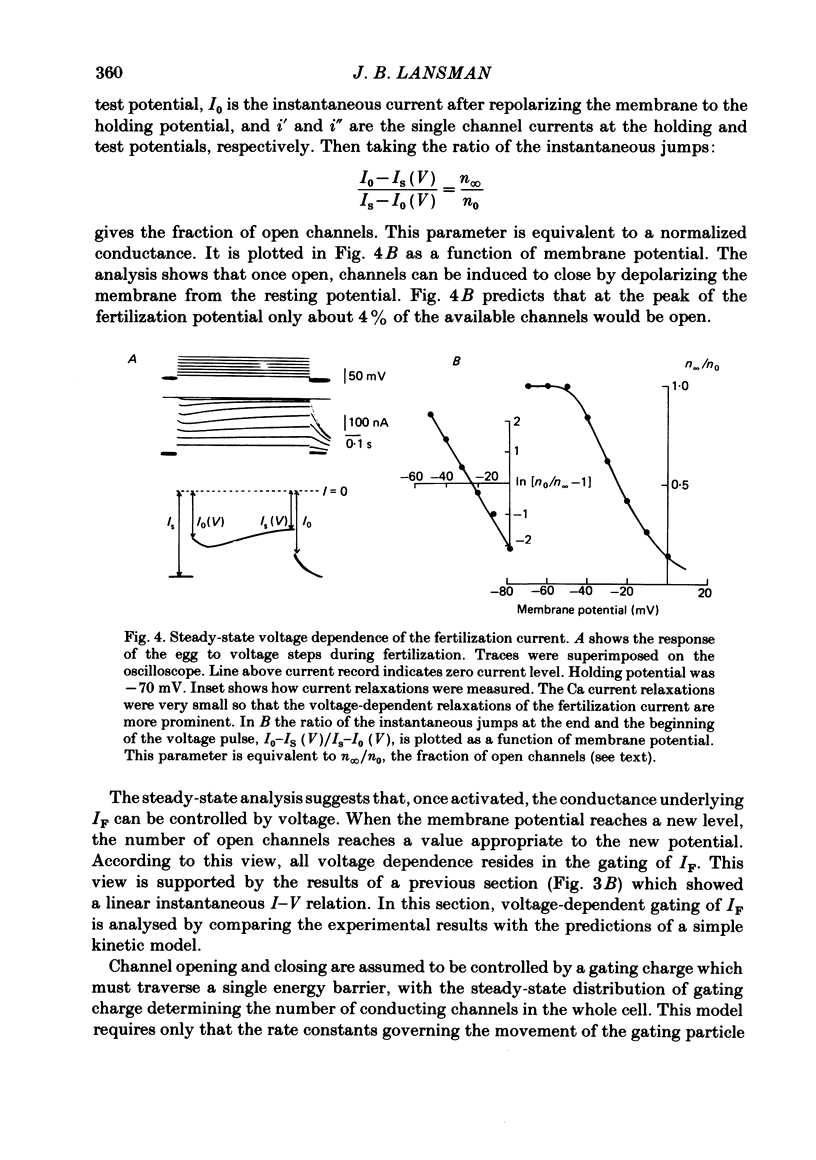
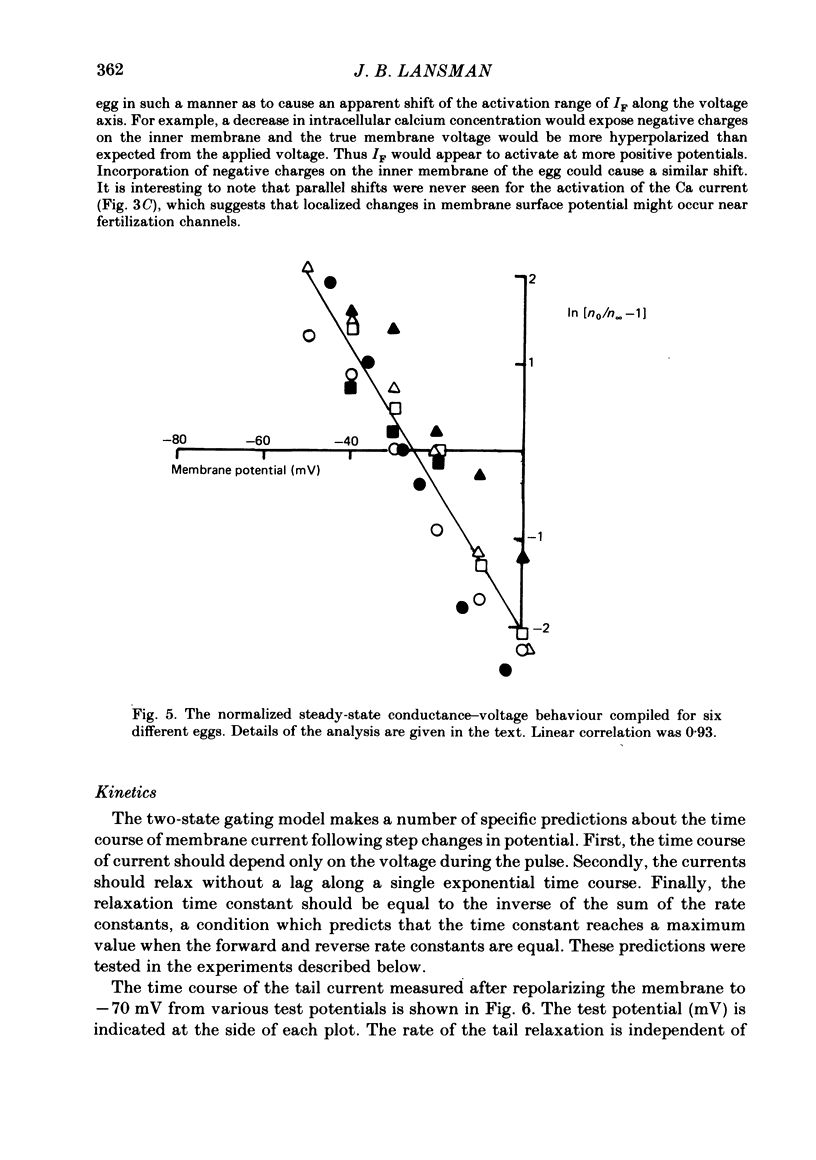
Ionic currents underlying the fertilization potential of the egg of the starfish Mediaster aequalis were studied using a two-micro-electrode voltage clamp. Mature eggs were fertilized in vitro under voltage-clamp conditions. The fertilization current, here termed IF, was induced by adding sperm to sea water bathing the egg. At a holding potential of -70 mV, IF was inward. It reached a peak within 2-4 min and then decayed over the next approximately 20 min with a rate which depended on the holding membrane potential. Instantaneous current-voltage relations measured at different times during IF were approximately linear and reversed at a potential of +6.0 +/- 5.8 mV (mean +/- S.D., n = 11). Membrane chord conductance was highest at the peak of inward current and the declining phase of IF was due to a decrease in conductance towards the pre-fertilization level. When the membrane potential was rapidly stepped to levels more positive than about -45 mV, the conductance underlying IF decreased in a manner which depended on both membrane potential and time. The fertilization-specific conductance showed a sigmoidal activation curve between -50 and +10 mV with a half-activation level of -25 mV. Analysis of the steady-state voltage dependence indicated that at the peak of the fertilization potential (+10 to +15 mV) only 4-5% of the total available channels would be open. Current relaxations followed first-order kinetics and the relaxation time constant depended upon the membrane potential during the voltage pulse. The relation between the time constant and voltage was bell-shaped, decreasing at potentials more negative than -40 and more positive than 0 mV. Both the steady-state conductance-voltage relation and the kinetics of the current relaxations were consistent with a simple two-state gating model in which the probability of a channel being open is determined by a single gating particle with an effective valency of -1.7 moving through the entire membrane field. The shifts in reversal potential with changes in external Na (at 10 mM-external K) were analysed using the constant field expression, which gave a relative permeability of Na to K of approximately 0.6.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chambers E. L., Pressman B. C., Rose B. The activation of sea urchin eggs by the divalent ionophores A23187 and X-537A. Biochem Biophys Res Commun. 1974 Sep 9;60(1):126–132. doi: 10.1016/0006-291x(74)90181-8. [DOI] [PubMed] [Google Scholar]
- Chambers E. L., de Armendi J. Membrane potential, action potential and activation potential of eggs of the sea urchin, Lytechinus variegatus. Exp Cell Res. 1979 Aug;122(1):203–218. doi: 10.1016/0014-4827(79)90575-5. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Cross N. L. Initiation of the activation potential by an increase in intracellular calcium in eggs of the frog, Rana pipiens. Dev Biol. 1981 Jul 30;85(2):380–384. doi: 10.1016/0012-1606(81)90269-4. [DOI] [PubMed] [Google Scholar]
- Gabel C. A., Eddy E. M., Shapiro B. M. After fertilization, sperm surface components remain as a patch in sea urchin and mouse embryos. Cell. 1979 Sep;18(1):207–215. doi: 10.1016/0092-8674(79)90369-6. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Jaffe L. A. Electrical properties of egg cell membranes. Annu Rev Biophys Bioeng. 1979;8:385–416. doi: 10.1146/annurev.bb.08.060179.002125. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ozawa S., Sand O. Voltage clamp analysis of two inward current mechanisms in the egg cell membrane of a starfish. J Gen Physiol. 1975 May;65(5):617–644. doi: 10.1085/jgp.65.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Yoshii M. Effects of internal potassium and sodium on the anomalous rectification of the starfish egg as examined by internal perfusion. J Physiol. 1979 Jul;292:251–265. doi: 10.1113/jphysiol.1979.sp012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Yoshioka K. Effect of various ionic compositions upon the membrane potentials during activation of sea urchin eggs. Exp Cell Res. 1973 Mar 30;78(1):191–200. doi: 10.1016/0014-4827(73)90054-2. [DOI] [PubMed] [Google Scholar]
- Jaffe L. A. Fast block to polyspermy in sea urchin eggs is electrically mediated. Nature. 1976 May 6;261(5555):68–71. doi: 10.1038/261068a0. [DOI] [PubMed] [Google Scholar]
- Jaffe L. A., Gould-Somero M., Holland L. Ionic mechanism of the fertilization potential of the marine worm, Urechis caupo (Echiura). J Gen Physiol. 1979 Apr;73(4):469–492. doi: 10.1085/jgp.73.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatani H. Maturation-inducing substance in starfishes. Int Rev Cytol. 1973;35:253–298. doi: 10.1016/s0074-7696(08)60356-3. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Lederer W. J., Tsien R. W., Weingart R. Role of calcium ions in transient inward currents and aftercontractions induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W., Weingart R. Ionic basis of transient inward current induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:209–226. doi: 10.1113/jphysiol.1978.sp012417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClendon J. F. ELECTROLYTIC EXPERIMENTS SHOWING INCREASE IN PERMEABILITY OF THE EGG TO IONS AT THE BEGINNING OF DEVELOPMENT. Science. 1910 Jul 22;32(812):122–124. doi: 10.1126/science.32.812.122. [DOI] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Miyazaki S. I., Ohmori H., Sasaki S. Action potential and non-linear current-voltage relation in starfish oocytes. J Physiol. 1975 Mar;246(1):37–54. doi: 10.1113/jphysiol.1975.sp010879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. I., Ohmori H., Sasaki S. Potassium rectifications of the starfish oocyte membrane and their changes during oocyte maturation. J Physiol. 1975 Mar;246(1):55–78. doi: 10.1113/jphysiol.1975.sp010880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. Fast polyspermy block and activation potential. Electrophysiological bases for their changes during oocyte maturation of a starfish. Dev Biol. 1979 Jun;70(2):341–354. doi: 10.1016/0012-1606(79)90032-0. [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Hirai S. Fast polyspermy block and activation potential. Correlated changes during oocyte maturation of a starfish. Dev Biol. 1979 Jun;70(2):327–340. doi: 10.1016/0012-1606(79)90031-9. [DOI] [PubMed] [Google Scholar]
- Moody W. J., Lansman J. B. Developmental regulation of Ca2+ and K+ currents during hormone-induced maturation of starfish oocytes. Proc Natl Acad Sci U S A. 1983 May;80(10):3096–3100. doi: 10.1073/pnas.80.10.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H., Yoshii M. Surface potential reflected in both gating and permeation mechanisms of sodium and calcium channels of the tunicate egg cell membrane. J Physiol. 1977 May;267(2):429–463. doi: 10.1113/jphysiol.1977.sp011821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichter L. C., Elinson R. P. Electrical responses of immature and mature Rana pipiens oocytes to sperm and other activating stimuli. Dev Biol. 1981 Apr 15;83(1):33–41. doi: 10.1016/s0012-1606(81)80005-x. [DOI] [PubMed] [Google Scholar]
- Shen S., Steinhardt R. A. An electrophysiological study of the membrane properties of the immature and mature oocyte of the batstar, Patiria miniata. Dev Biol. 1976 Jan;48(1):148–162. doi: 10.1016/0012-1606(76)90053-1. [DOI] [PubMed] [Google Scholar]
- Steinhardt R. A., Epel D. Activation of sea-urchin eggs by a calcium ionophore. Proc Natl Acad Sci U S A. 1974 May;71(5):1915–1919. doi: 10.1073/pnas.71.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt R. A., Lundin L., Mazia D. Bioelectric responses of the echinoderm egg to fertilization. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2426–2430. doi: 10.1073/pnas.68.10.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt R., Zucker R., Schatten G. Intracellular calcium release at fertilization in the sea urchin egg. Dev Biol. 1977 Jul 1;58(1):185–196. doi: 10.1016/0012-1606(77)90084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Yoshii M. Effects of internal free calcium upon the sodium and calcium channels in the tunicate egg analysed by the internal perfusion technique. J Physiol. 1978 Jun;279:519–549. doi: 10.1113/jphysiol.1978.sp012360. [DOI] [PMC free article] [PubMed] [Google Scholar]


