Abstract
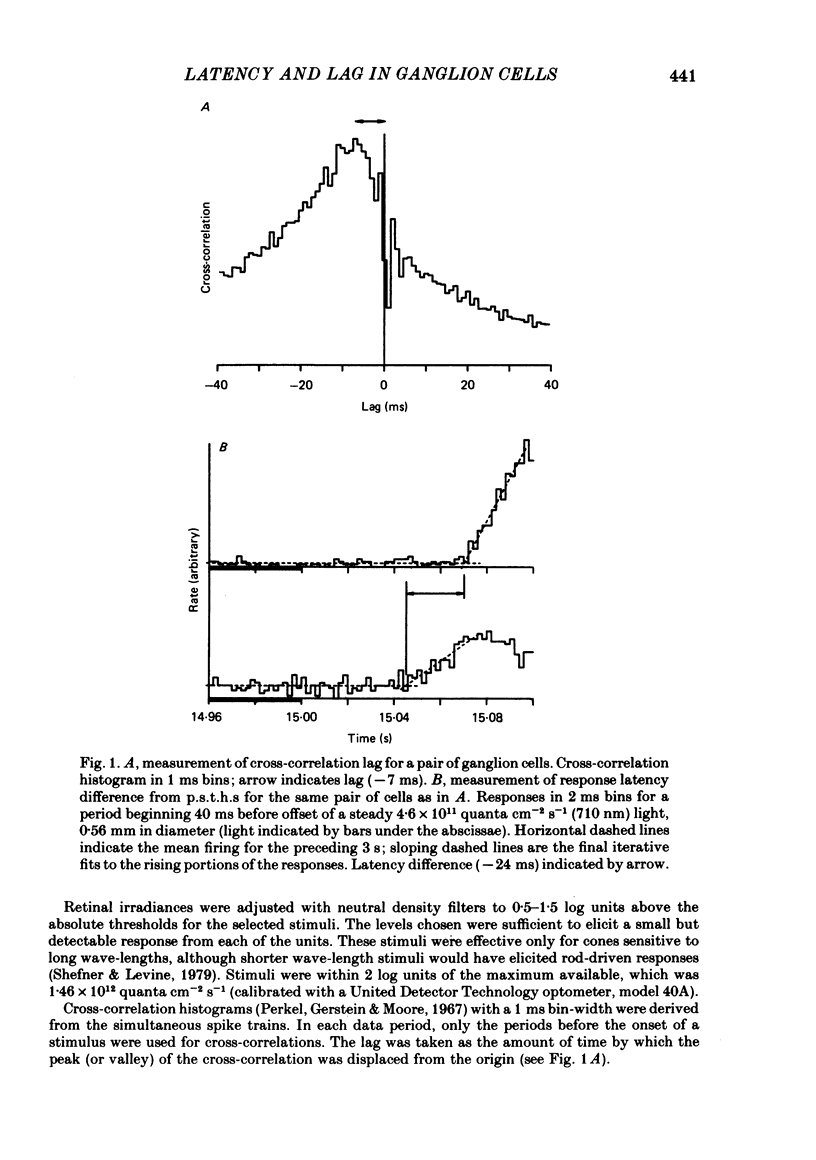
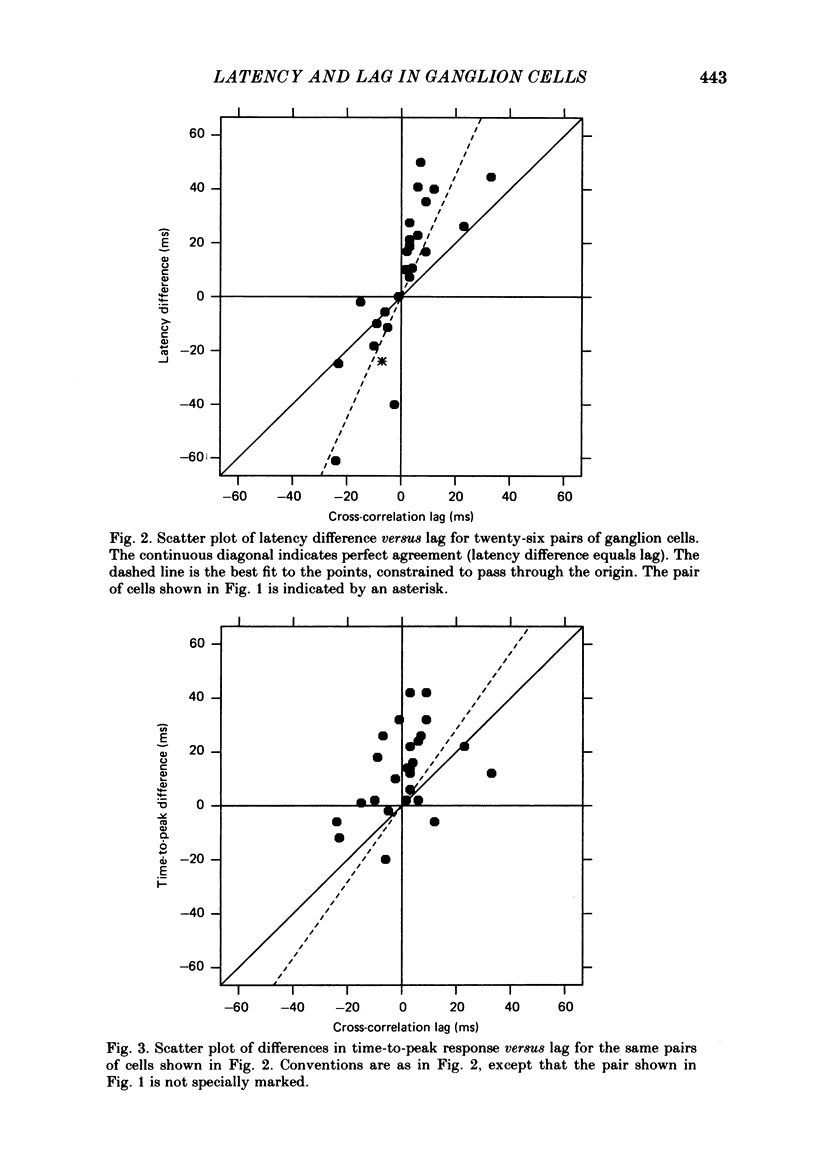
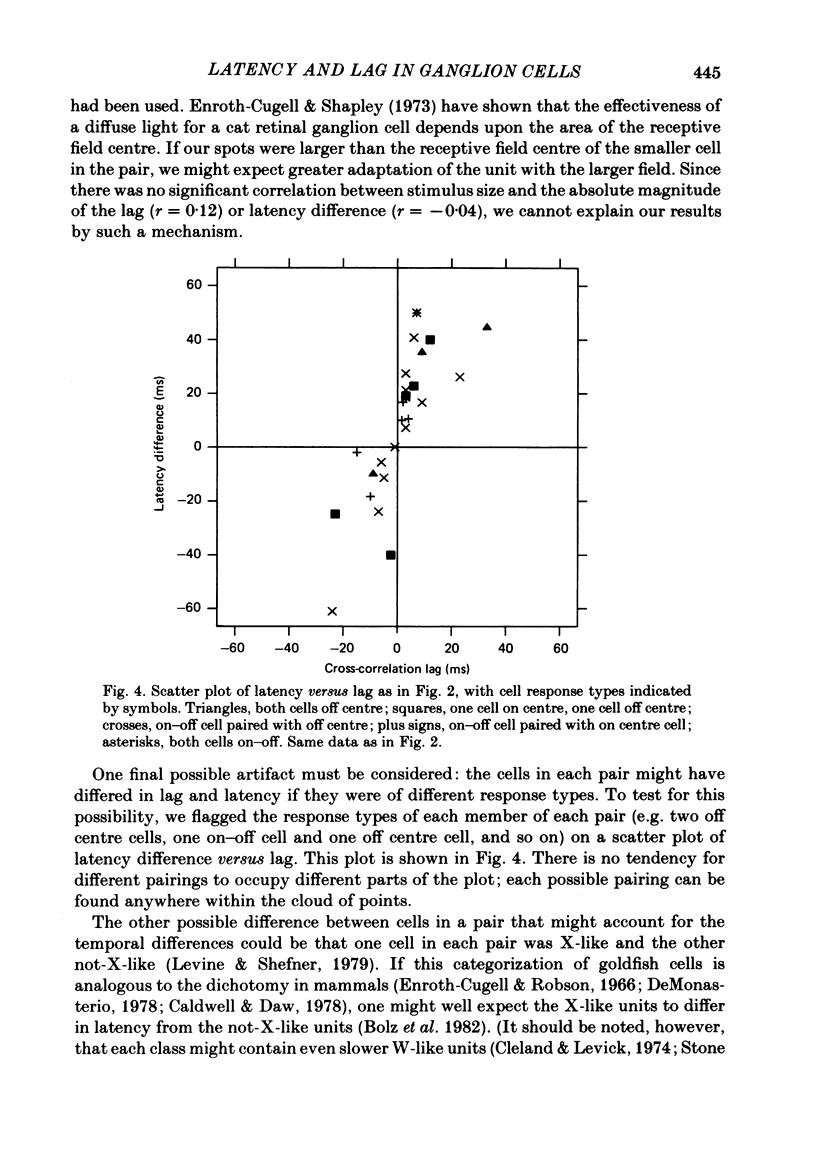
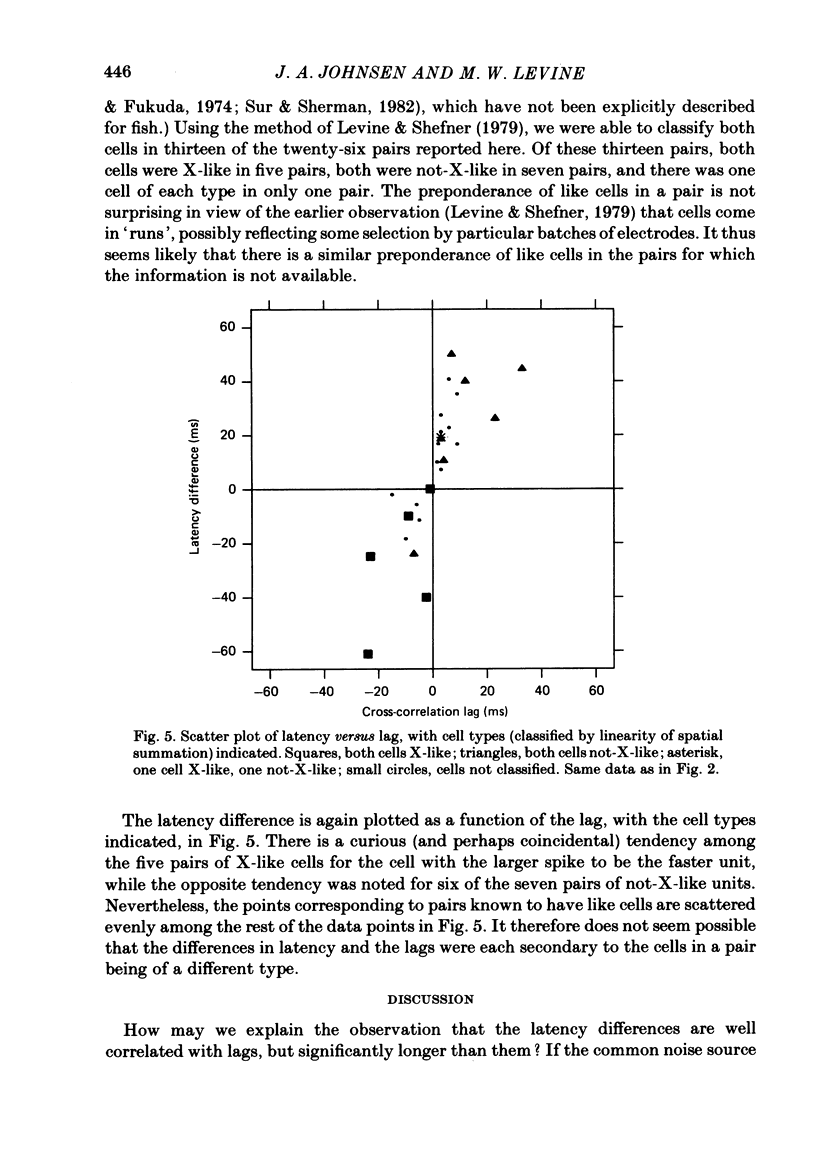
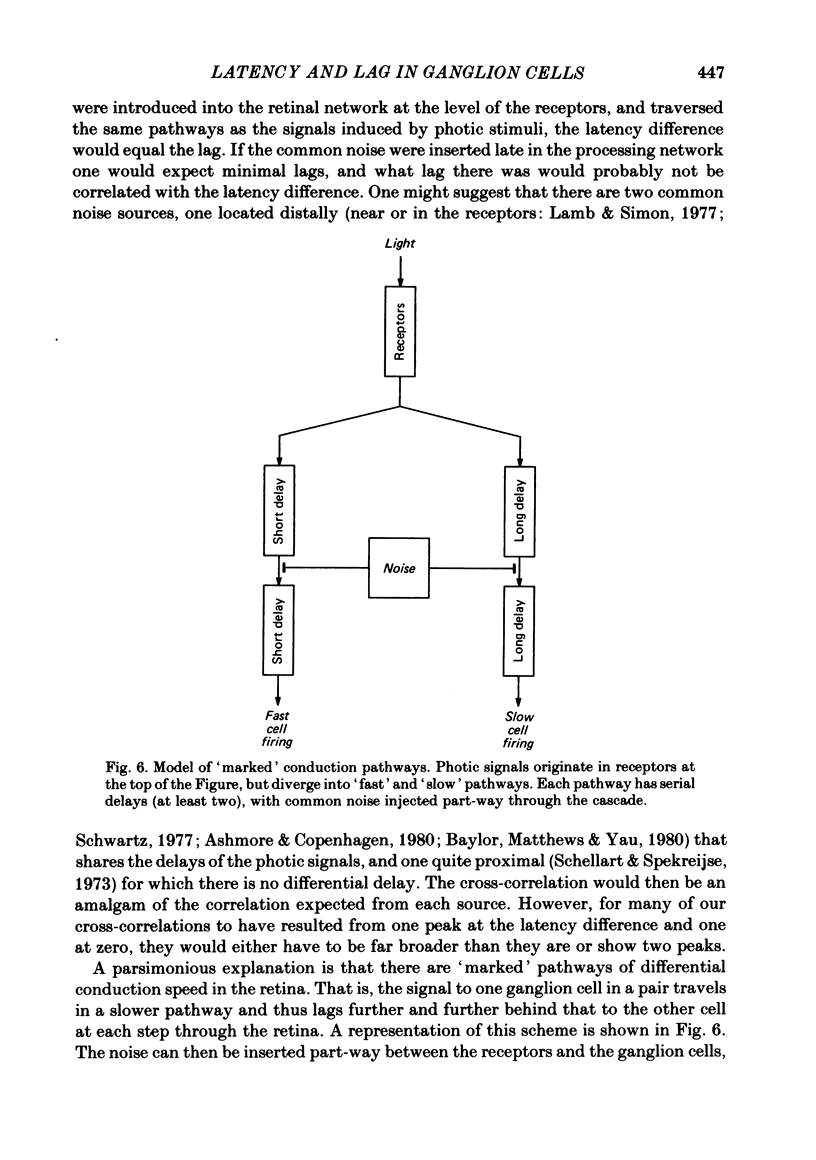
Pairs of retinal ganglion cells in the isolated goldfish retina were recorded simultaneously with a single electrode. Repeated flashes of light were delivered to evaluate the response latency of each of the units. The cross-correlation histogram for the maintained discharge of each pair of cells was examined, and its temporal relationships (lags) were compared with the differences in response latencies of the two units. There was a strong correlation between these measures; however, the differences between latencies were often at least twice as great as the lags. The differences between the times to the peaks of the responses of the two units were less reliably related to the lags of the pairs, although the correlation was positive and the differences in time-to-peak generally greater than the lags. The weaker relationship between the difference in time-to-peak and lag than between latency difference and lag is apparently a manifestation of a negative correlation between latency and rise time (from first response to peak). This indicates that cells with a longer latency compensate with a faster rise time. There was a negative correlation between the mean maintained rate of a neurone and its response latency. That is, cells with faster maintained discharge rates respond sooner than those with slower maintained rates. There was virtually no relationship between the lags or the differences in latency and the differences between the magnitudes of the responses to light. Thus, it is unlikely that differences in latency (or lags) could be attributed to unequal effectiveness of the stimuli for the two units. The relationship between differences in latency and lags did not depend on the response categorizations of the two units. Specifically, it did not matter whether the members of the pair were on centre, off centre or on-off centre; neither did it matter whether they were X-like or not-X-like neurones. Consideration of these data leads to the conclusion that there must be 'marked' pathways of differential conduction velocity through the retina.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramov I., Levine M. W. The effects of carbon dioxide on the excised goldfish retina. Vision Res. 1972 Nov;12(11):1881–1895. doi: 10.1016/0042-6989(72)90077-6. [DOI] [PubMed] [Google Scholar]
- Arnett D. W. Statistical dependence between neighboring retinal ganglion cells in goldfish. Exp Brain Res. 1978 May 12;32(1):49–53. doi: 10.1007/BF00237389. [DOI] [PubMed] [Google Scholar]
- Arnett D., Spraker T. E. Cross-correlation analysis of the maintained discharge of rabbit retinal ganglion cells. J Physiol. 1981 Aug;317:29–47. doi: 10.1113/jphysiol.1981.sp013812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J. F., Copenhagen D. R. Different postsynaptic events in two types of retinal bipolar cell. Nature. 1980 Nov 6;288(5786):84–86. doi: 10.1038/288084a0. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Yau K. W. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980 Dec;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz J., Rosner G., Wässle H. Response latency of brisk-sustained (X) and brisk-transient (Y) cells in the cat retina. J Physiol. 1982 Jul;328:171–190. doi: 10.1113/jphysiol.1982.sp014258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. H., Daw N. W. New properties of rabbit retinal ganglion cells. J Physiol. 1978 Mar;276:257–276. doi: 10.1113/jphysiol.1978.sp012232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-Cugell C. Quantitative aspects of gain and latency in the cat retina. J Physiol. 1970 Jan;206(1):73–91. doi: 10.1113/jphysiol.1970.sp008998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Properties of rarely encountered types of ganglion cells in the cat's retina and an overall classification. J Physiol. 1974 Jul;240(2):457–492. doi: 10.1113/jphysiol.1974.sp010618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W. Colour-coded ganglion cells in the goldfish retina: extension of their receptive fields by means of new stimuli. J Physiol. 1968 Aug;197(3):567–592. doi: 10.1113/jphysiol.1968.sp008575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Hertz G., Lennie P. Cone signals in the cat's retina. J Physiol. 1977 Jul;269(2):273–296. doi: 10.1113/jphysiol.1977.sp011902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Shapley R. M. Flux, not retinal illumination, is what cat retinal ganglion cells really care about. J Physiol. 1973 Sep;233(2):311–326. doi: 10.1113/jphysiol.1973.sp010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Simon E. J. Analysis of electrical noise in turtle cones. J Physiol. 1977 Nov;272(2):435–468. doi: 10.1113/jphysiol.1977.sp012053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennie P. The physiological basis of variations in visual latency. Vision Res. 1981;21(6):815–824. doi: 10.1016/0042-6989(81)90180-2. [DOI] [PubMed] [Google Scholar]
- Levine M. W., Shefner J. M. X-like and not X-like cells in goldfish retina. Vision Res. 1979;19(1):95–97. doi: 10.1016/0042-6989(79)90127-5. [DOI] [PubMed] [Google Scholar]
- Perkel D. H., Gerstein G. L., Moore G. P. Neuronal spike trains and stochastic point processes. II. Simultaneous spike trains. Biophys J. 1967 Jul;7(4):419–440. doi: 10.1016/S0006-3495(67)86597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck R. W. Maintained activity of cat retinal ganglion cells. J Neurophysiol. 1967 Sep;30(5):1043–1071. doi: 10.1152/jn.1967.30.5.1043. [DOI] [PubMed] [Google Scholar]
- Schellart N. A., Spekreijse H. Origin of the stochastic nature of ganglion cell activity in isolated goldfish retina. Vision Res. 1973 Feb;13(2):337–345. doi: 10.1016/0042-6989(73)90111-9. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Voltage noise observed in rods of the turtle retina. J Physiol. 1977 Nov;272(2):217–246. doi: 10.1113/jphysiol.1977.sp012042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefner J. M., Levine M. W. A comparison of properties of goldfish retinal ganglion cells as a function of lighting conditions during dissection. Vision Res. 1979;19(1):83–89. doi: 10.1016/0042-6989(79)90125-1. [DOI] [PubMed] [Google Scholar]
- Spekreijse H., Wagner H. G., Wolbarsht M. L. Spectral and spatial coding of ganglion cell responses in goldfish retina. J Neurophysiol. 1972 Jan;35(1):73–86. doi: 10.1152/jn.1972.35.1.73. [DOI] [PubMed] [Google Scholar]
- Stone J., Fukuda Y. Properties of cat retinal ganglion cells: a comparison of W-cells with X- and Y-cells. J Neurophysiol. 1974 Jul;37(4):722–748. doi: 10.1152/jn.1974.37.4.722. [DOI] [PubMed] [Google Scholar]
- Sur M., Sherman S. M. Linear and nonlinear W-cells in C-laminae of the cat's lateral geniculate nucleus. J Neurophysiol. 1982 May;47(5):869–884. doi: 10.1152/jn.1982.47.5.869. [DOI] [PubMed] [Google Scholar]
- de Monasterio F. M. Properties of concentrically organized X and Y ganglion cells of macaque retina. J Neurophysiol. 1978 Nov;41(6):1394–1417. doi: 10.1152/jn.1978.41.6.1394. [DOI] [PubMed] [Google Scholar]


