Abstract
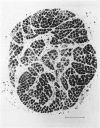
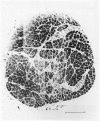
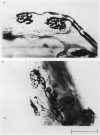
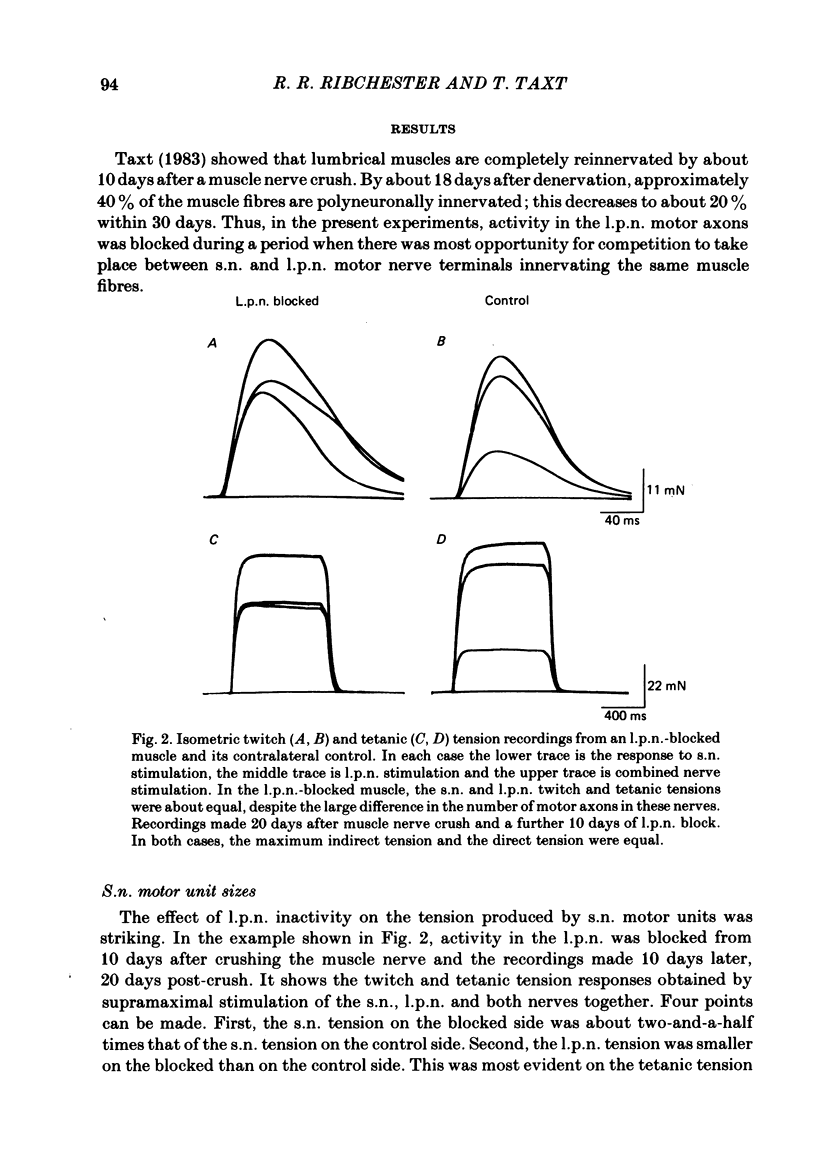
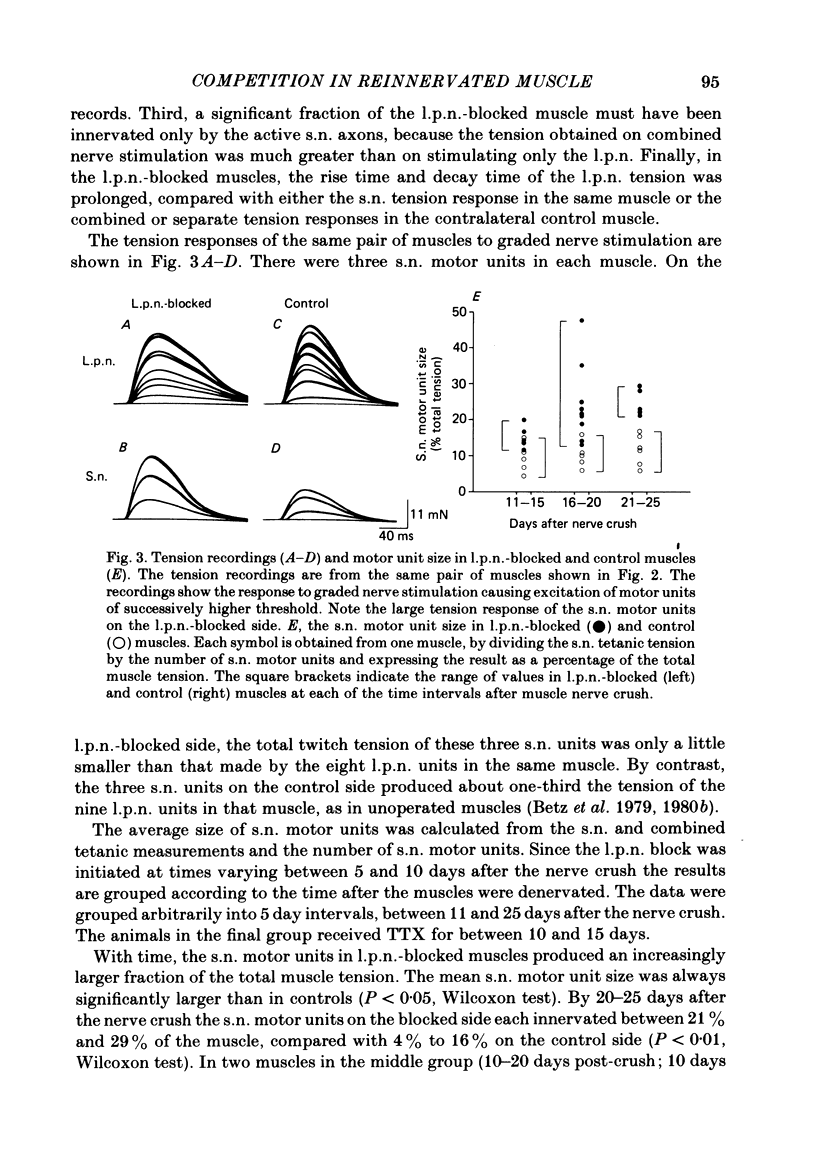
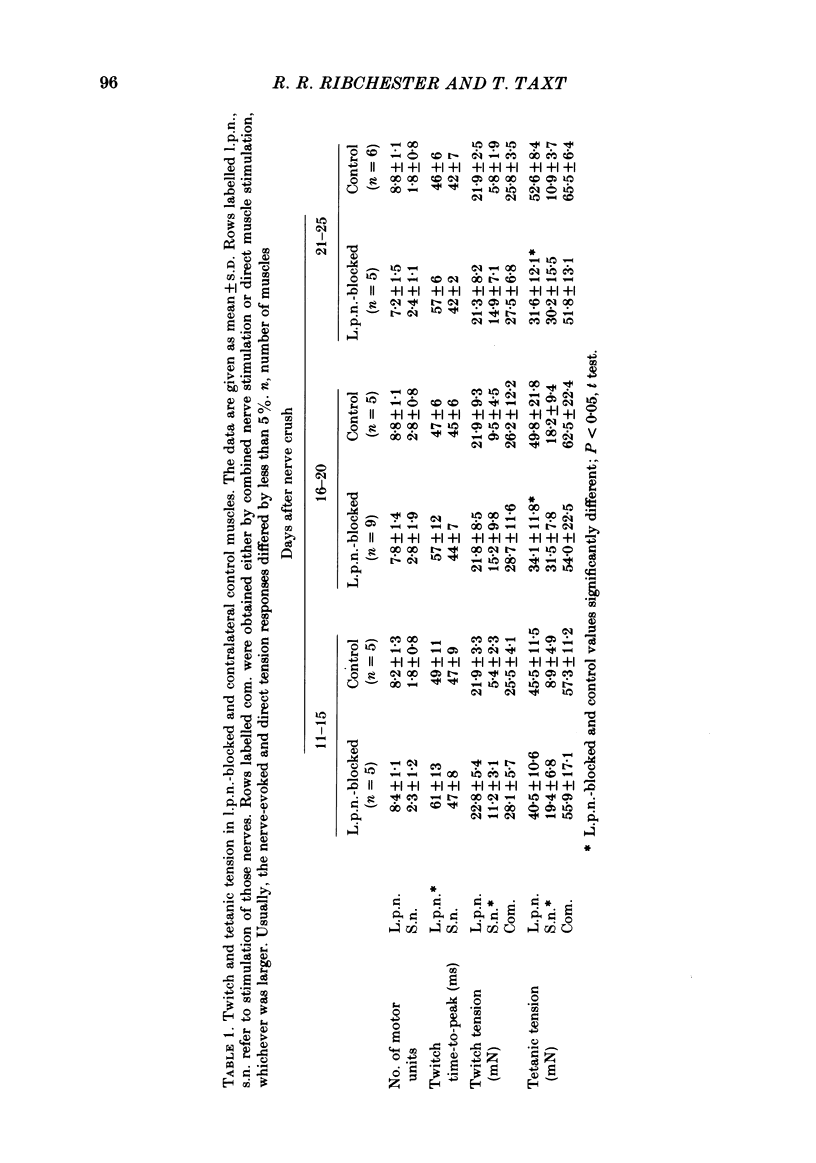
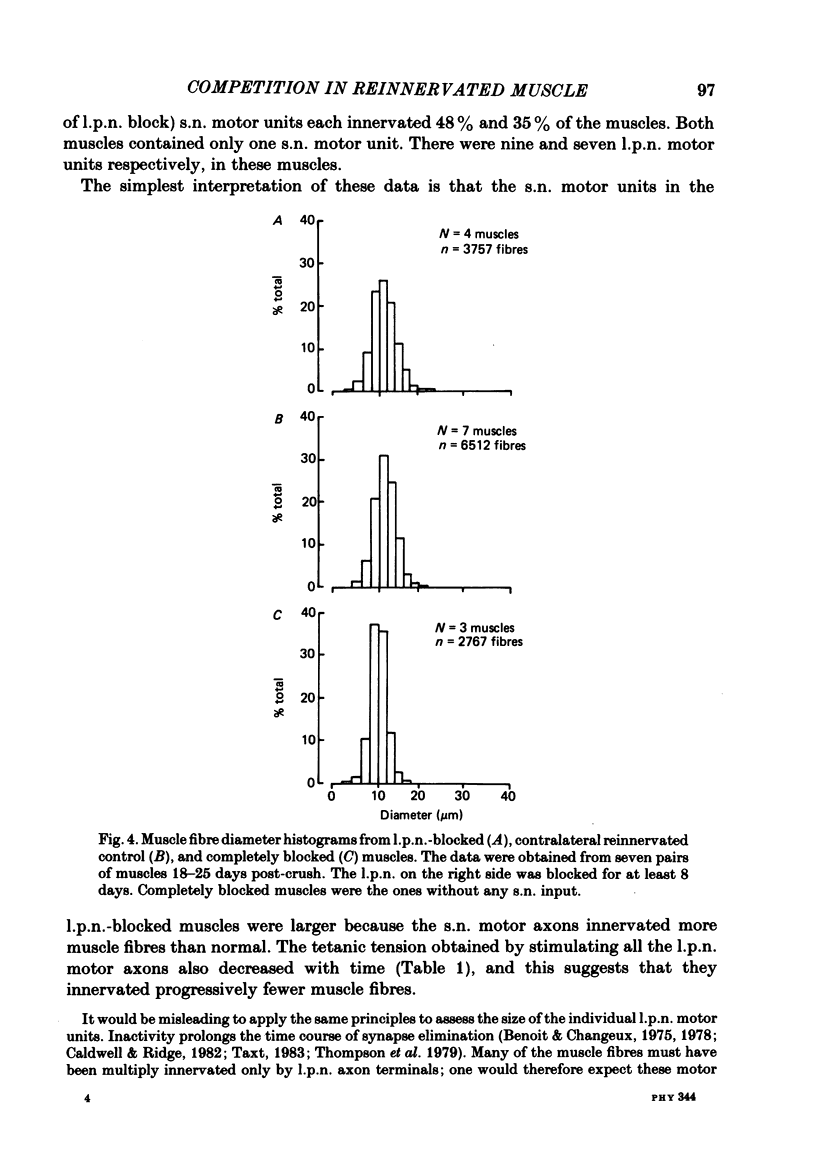
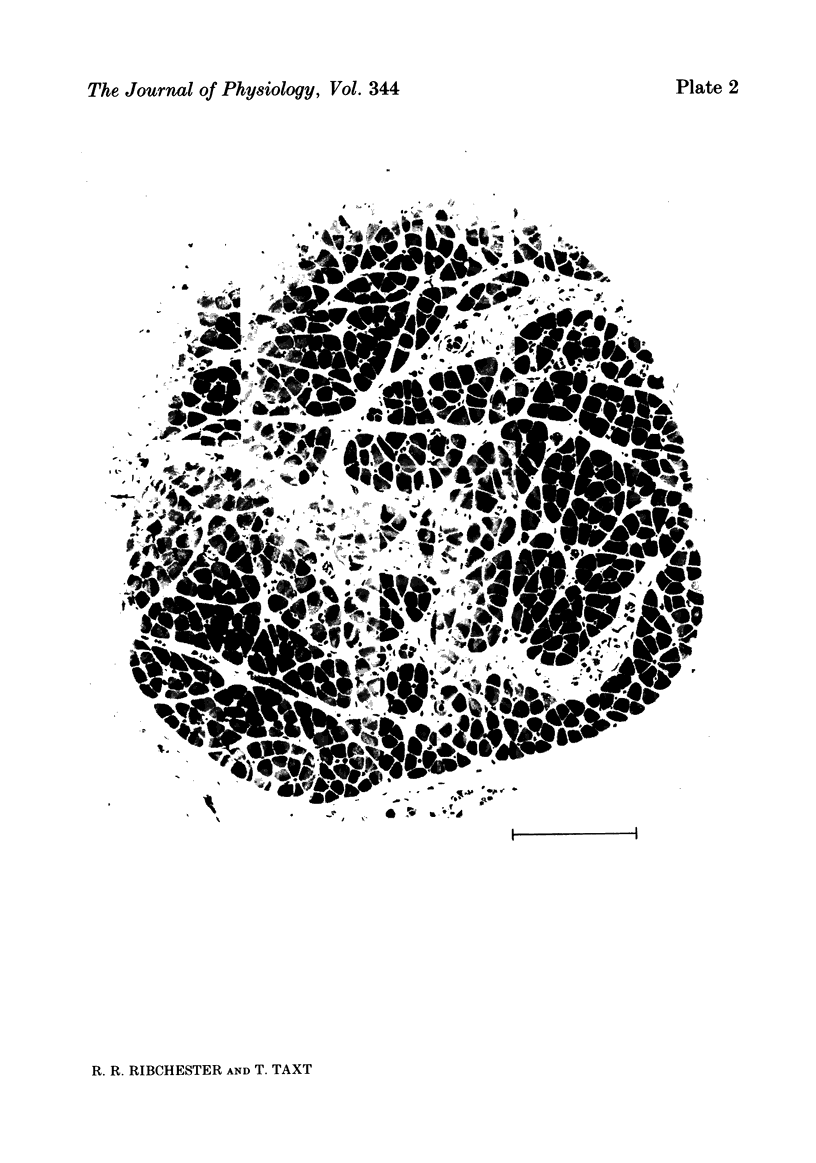
The size of motor units has been measured in adult rat muscles reinnervated by active and inactive motor axons. The results suggest that active nerve terminals have a competitive advantage over inactive terminals during neuromuscular synapse elimination. The experiments were done using the fourth deep lumbrical muscle in the rat hind foot, which receives its motor innervation from the lateral plantar nerve (l.p.n.) and the sural nerve (s.n.). The muscles were denervated by a nerve crush close to the muscle. Five to ten days later, nerve impulse conduction in the l.p.n. was blocked for 1-2 weeks by chronic superfusion of the nerve with tetrodotoxin (Betz, Caldwell & Ribchester, 1980 b). After 2 weeks of l.p.n. block, the isometric tetanic tension of s.n. motor units increased about two-fold, compared with contralateral control muscles. This was due to an increase in the number of muscle fibres innervated by s.n. motor axons. Intracellular recordings showed that more fibres were innervated by the s.n. than in normal muscles. In some animals, the blocked l.p.n. was cut 1-2 weeks later. The l.p.n. terminals were allowed to degenerate for 1-2 days. There were more s.n. terminals in zinc iodide-osmium stained preparations of these muscles than in normal muscles. Calculation of tetanic tension overlap between l.p.n. and s.n. motor units, and the amount of mono-neuronal innervation seen in intracellular recordings suggested that a larger fraction of the muscles was innervated only by s.n. motor nerve terminals than in controls. This fraction increased with time, ultimately reaching about 14% of the muscle per s.n. motor unit. The expansion of the s.n. motor units appeared to take place by terminal and preterminal sprouting of motor axons. The l.p.n.-evoked tetanic tension decreased in parallel with the increase in the s.n. tetanic tension. The decrease in the l.p.n. twitch tension did not parallel the increase in the s.n. twitch tension. At least part of this discrepancy was due to repetitive firing of the regenerated, inactive l.p.n. terminals when the nerve was stimulated electrically. The results support the notion that modifications in connectivity between pre- and post-synaptic cells can come about by growth or withdrawal of terminals, as a result of differences in the level of activity in the presynaptic cells (Hebb, 1949).
Full text
PDF





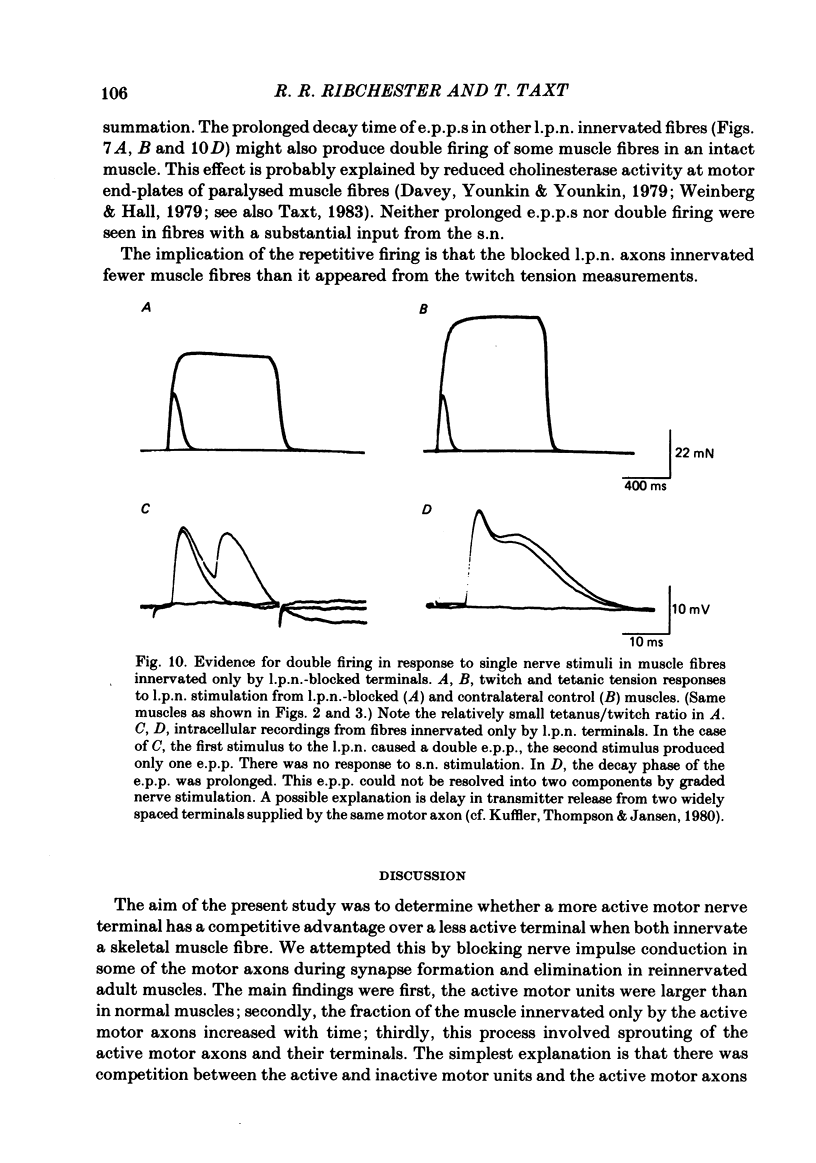






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akert K., Sandri C. An electron-microscopic study of zinc iodide-osmium impregnation of neurons. I. Staining of synaptic vesicles at cholinergic junctions. Brain Res. 1968 Feb;7(2):286–295. doi: 10.1016/0006-8993(68)90104-2. [DOI] [PubMed] [Google Scholar]
- BARSTAD J. A. Presynaptic effect of the neuro-muscular transmitter. Experientia. 1962 Dec 15;18:579–580. doi: 10.1007/BF02172193. [DOI] [PubMed] [Google Scholar]
- BROWN M. C., MATTHEWS P. B. An investigation into the possible existence of polyneuronal innervation of individual skeletal muscle fibres in certain hind-limb muscles of the cat. J Physiol. 1960 Jun;151:436–457. doi: 10.1113/jphysiol.1960.sp006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagust J., Lewis D. M., Luck J. C., Westerman R. A. Development of motor units in a fast twitch muscle of the cat hind limb. J Physiol. 1972 Jul;224(1):35P–37P. [PubMed] [Google Scholar]
- Benoit P., Changeux J. P. Consequences of blocking the nerve with a local anaesthetic on the evolution of multiinnervation at the regenerating neuromuscular junction of the rat. Brain Res. 1978 Jun 23;149(1):89–96. doi: 10.1016/0006-8993(78)90589-9. [DOI] [PubMed] [Google Scholar]
- Benoit P., Changeux J. P. Consequences of tenotomy on the evolution of multiinnervation in developing rat soleus muscle. Brain Res. 1975 Dec 5;99(2):354–358. doi: 10.1016/0006-8993(75)90036-0. [DOI] [PubMed] [Google Scholar]
- Betz W. J., Caldwell J. H., Ribchester R. R. Sprouting of active nerve terminals in partially inactive muscles of the rat. J Physiol. 1980 Jun;303:281–297. doi: 10.1113/jphysiol.1980.sp013285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J., Caldwell J. H., Ribchester R. R. The effects of partial denervation at birth on the development of muscle fibres and motor units in rat lumbrical muscle. J Physiol. 1980 Jun;303:265–279. doi: 10.1113/jphysiol.1980.sp013284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixby J. L. Ultrastructural observations on synapse elimination in neonatal rabbit skeletal muscle. J Neurocytol. 1981 Feb;10(1):81–100. doi: 10.1007/BF01181746. [DOI] [PubMed] [Google Scholar]
- Bray J. J., Hubbard J. I., Mills R. G. The trophic influence of tetrodotoxin-inactive nerves on normal and reinnervated rat skeletal muscles. J Physiol. 1979 Dec;297(0):479–491. doi: 10.1113/jphysiol.1979.sp013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Goodwin G. M., Ironton R. Prevention of motor nerve sprouting in botulinum toxin poisoned mouse soleus muscles by direct stimulation of the muscle [proceedings]. J Physiol. 1977 May;267(1):42P–43P. [PubMed] [Google Scholar]
- Brown M. C., Holland R. L., Hopkins W. G. Motor nerve sprouting. Annu Rev Neurosci. 1981;4:17–42. doi: 10.1146/annurev.ne.04.030181.000313. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Ironton R. Sprouting and regression of neuromuscular synapses in partially denervated mammalian muscles. J Physiol. 1978 May;278:325–348. doi: 10.1113/jphysiol.1978.sp012307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Mikoshiba K. Genetic and 'epigenetic' factors regulating synapse formation in vertebrate cerebellum and neuromuscular junction. Prog Brain Res. 1978;48:43–66. doi: 10.1016/S0079-6123(08)61015-8. [DOI] [PubMed] [Google Scholar]
- Davey B., Younkin L. H., Younkin S. G. Neural control of skeletal muscle cholinesterase: a study using organ-cultured rat muscle. J Physiol. 1979 Apr;289:501–515. doi: 10.1113/jphysiol.1979.sp012749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen L. W., Tonge D. A. The effects of implantation of an extra nerve on axonal sprouting usually induced by botulinum toxin in skeletal muscle of the mouse. J Anat. 1977 Sep;124(Pt 1):205–215. [PMC free article] [PubMed] [Google Scholar]
- Edström L., Kugelberg E. Histochemical composition, distribution of fibres and fatiguability of single motor units. Anterior tibial muscle of the rat. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):424–433. doi: 10.1136/jnnp.31.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J. K., Thompson W., Kuffler D. P. The formation and maintenance of synaptic connections as illustrated by studies of the neuromuscular junction. Prog Brain Res. 1978;48:3–19. doi: 10.1016/S0079-6123(08)61012-2. [DOI] [PubMed] [Google Scholar]
- Kuffler D. P., Thompson W., Jansen J. K. The fate of foreign endplates in cross-innervated rat soleus muscle. Proc R Soc Lond B Biol Sci. 1980 Jun 24;208(1171):189–222. doi: 10.1098/rspb.1980.0049. [DOI] [PubMed] [Google Scholar]
- Lavoie P. A., Collier B., Tenenhouse A. Role of skeletal muscle activity in the control of muscle acetylcholine sensitivity. Exp Neurol. 1977 Jan;54(1):148–171. doi: 10.1016/0014-4886(77)90242-4. [DOI] [PubMed] [Google Scholar]
- Lewis D. M. The effect of denervation on the mechanical and electrical responses of fast and slow mammalian twitch muscle. J Physiol. 1972 Apr;222(1):51–75. doi: 10.1113/jphysiol.1972.sp009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCPHEDRAN A. M., WUERKER R. B., HENNEMAN E. PROPERTIES OF MOTOR UNITS IN A HETEROGENEOUS PALE MUSCLE (M. GASTROCNEMIUS) OF THE CAT. J Neurophysiol. 1965 Jan;28:85–99. doi: 10.1152/jn.1965.28.1.85. [DOI] [PubMed] [Google Scholar]
- MCPHEDRAN A. M., WUERKER R. B., HENNEMAN E. PROPERTIES OF MOTOR UNITS IN A HOMOGENEOUS RED MUSCLE (SOLEUS) OF THE CAT. J Neurophysiol. 1965 Jan;28:71–84. doi: 10.1152/jn.1965.28.1.71. [DOI] [PubMed] [Google Scholar]
- McArdle J. J. Complex end-plate potentials at the regenerating neuromuscular junction of the rat. Exp Neurol. 1975 Dec;49(3):629–638. doi: 10.1016/0014-4886(75)90048-5. [DOI] [PubMed] [Google Scholar]
- O'Brien R. A., Ostberg A. J., Vrbová G. Observations on the elimination of polyneuronal innervation in developing mammalian skeletal muscle. J Physiol. 1978 Sep;282:571–582. doi: 10.1113/jphysiol.1978.sp012482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs S., Hollingsworth D. Dependence of fast axoplasmic transport in nerve on oxidative metabolism. J Neurochem. 1971 Jan;18(1):107–114. doi: 10.1111/j.1471-4159.1971.tb00172.x. [DOI] [PubMed] [Google Scholar]
- Rauschecker J. P., Singer W. The effects of early visual experience on the cat's visual cortex and their possible explanation by Hebb synapses. J Physiol. 1981 Jan;310:215–239. doi: 10.1113/jphysiol.1981.sp013545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley D. A. Ultrastructural evidence for axon retraction during the spontaneous elimination of polyneuronal innervation of the rat soleus muscle. J Neurocytol. 1981 Jun;10(3):425–440. doi: 10.1007/BF01262414. [DOI] [PubMed] [Google Scholar]
- Roper S., Ko C. P. Impulse blockade in frog cardiac ganglion does not resemble partial denervation in changing synaptic organization. Science. 1978 Oct 6;202(4363):66–68. doi: 10.1126/science.308697. [DOI] [PubMed] [Google Scholar]
- Salmons S., Sréter F. A. Significance of impulse activity in the transformation of skeletal muscle type. Nature. 1976 Sep 2;263(5572):30–34. doi: 10.1038/263030a0. [DOI] [PubMed] [Google Scholar]
- Taxt T. Local and systemic effects of tetrodotoxin on the formation and elimination of synapses in reinnervated adult rat muscle. J Physiol. 1983 Jul;340:175–194. doi: 10.1113/jphysiol.1983.sp014757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W., Jansen J. K. The extent of sprouting of remaining motor units in partly denervated immature and adult rat soleus muscle. Neuroscience. 1977;2(4):523–535. doi: 10.1016/0306-4522(77)90049-5. [DOI] [PubMed] [Google Scholar]
- Thompson W., Kuffler D. P., Jansen J. K. The effect of prolonged, reversible block of nerve impulses on the elimination of polyneuronal innervation of new-born rat skeletal muscle fibers. Neuroscience. 1979;4(2):271–281. doi: 10.1016/0306-4522(79)90088-5. [DOI] [PubMed] [Google Scholar]
- Thompson W. Reinnervation of partially denervated rat soleus muscle. Acta Physiol Scand. 1978 May;103(1):81–91. doi: 10.1111/j.1748-1716.1978.tb06193.x. [DOI] [PubMed] [Google Scholar]
- Thompson W. Synapse elimination in neonatal rat muscle is sensitive to pattern of muscle use. Nature. 1983 Apr 14;302(5909):614–616. doi: 10.1038/302614a0. [DOI] [PubMed] [Google Scholar]
- Walsh J. V., Jr, Burke R. E., Rymer W. Z., Tsairis P. Effect of compensatory hypertrophy studied in individual motor units in medial gastrocnemius muscle of the cat. J Neurophysiol. 1978 Mar;41(2):496–508. doi: 10.1152/jn.1978.41.2.496. [DOI] [PubMed] [Google Scholar]
- Weinberg C. B., Hall Z. W. Junctional form of acetylcholinesterase restored at nerve-free endplates. Dev Biol. 1979 Feb;68(2):631–635. doi: 10.1016/0012-1606(79)90233-1. [DOI] [PubMed] [Google Scholar]
- Willshaw D. J. The establishment and the subsequent elimination of polyneuronal innervation of developing muscle: theoretical considerations. Proc R Soc Lond B Biol Sci. 1981 May 22;212(1187):233–252. doi: 10.1098/rspb.1981.0036. [DOI] [PubMed] [Google Scholar]