Abstract
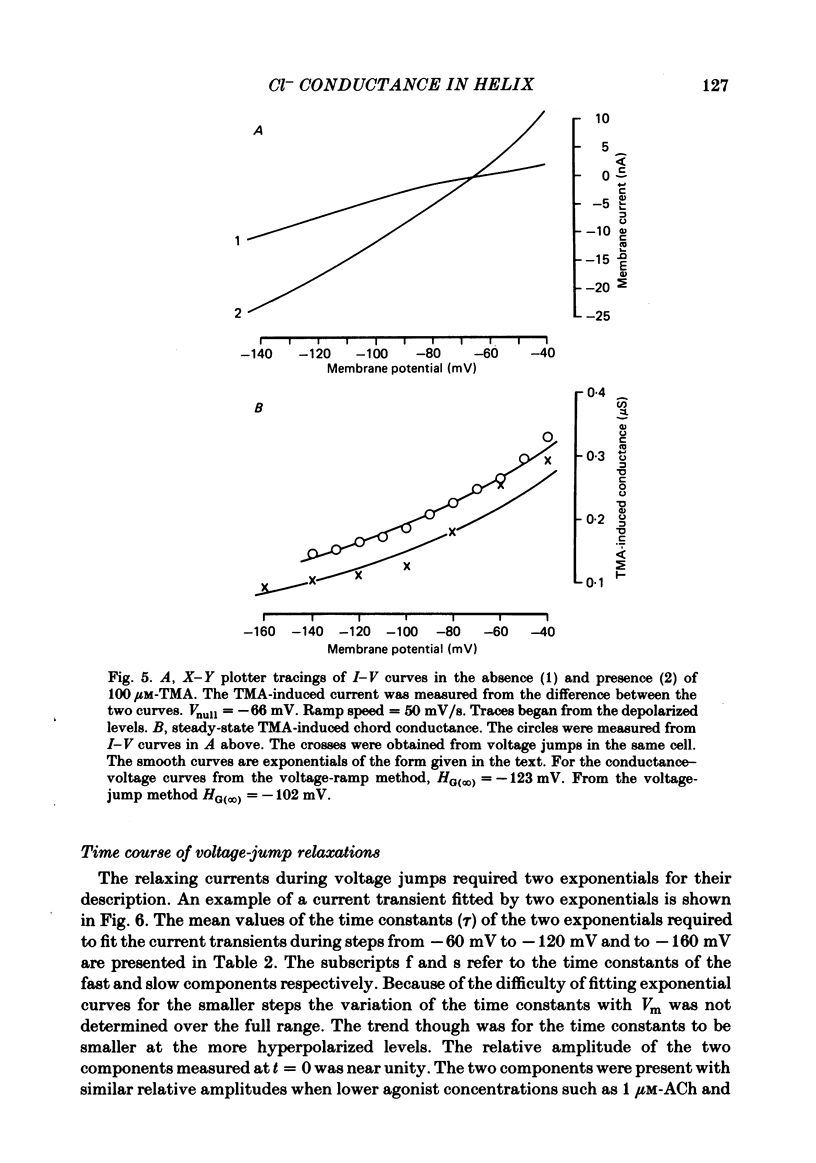
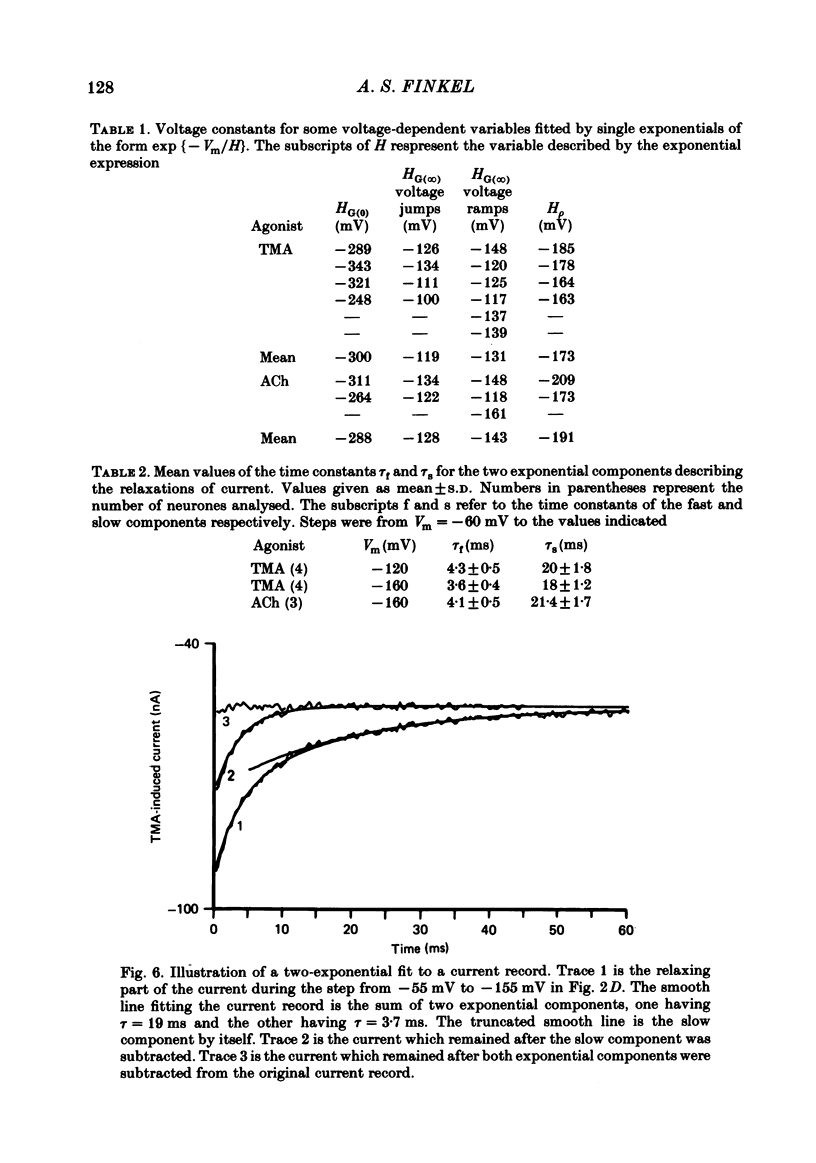
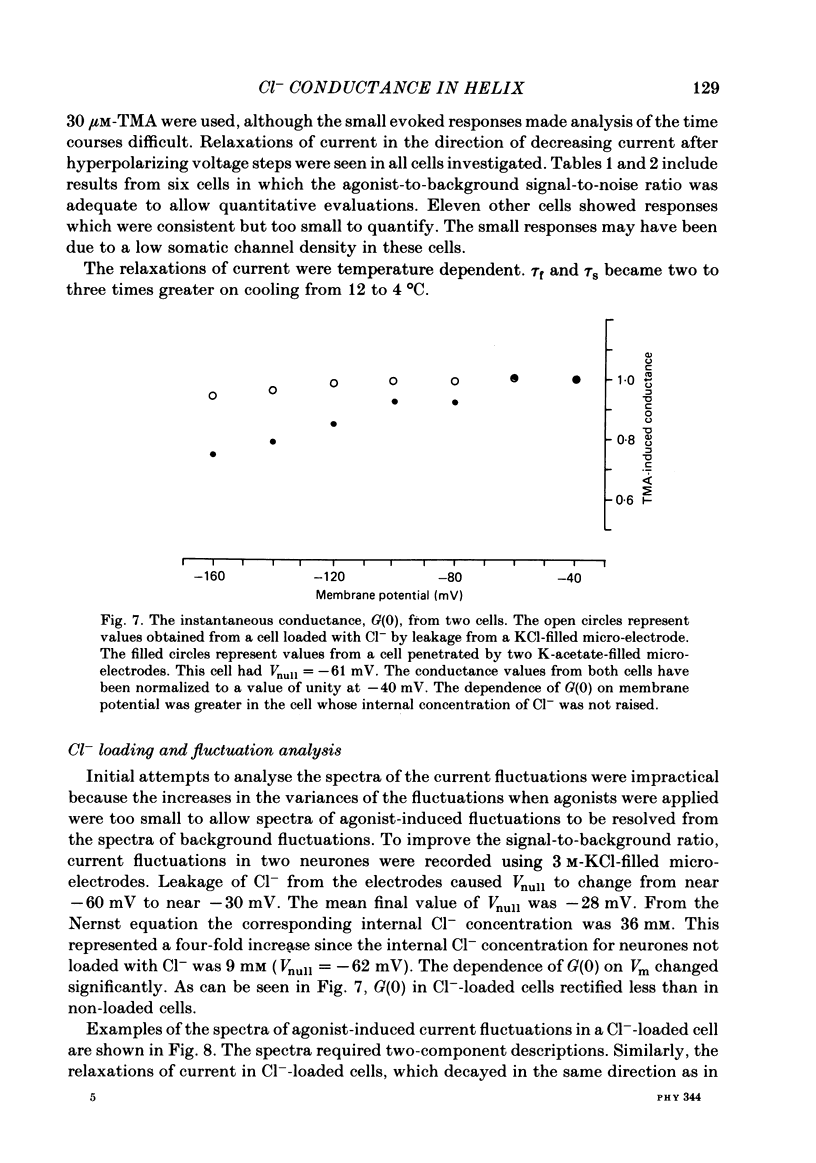
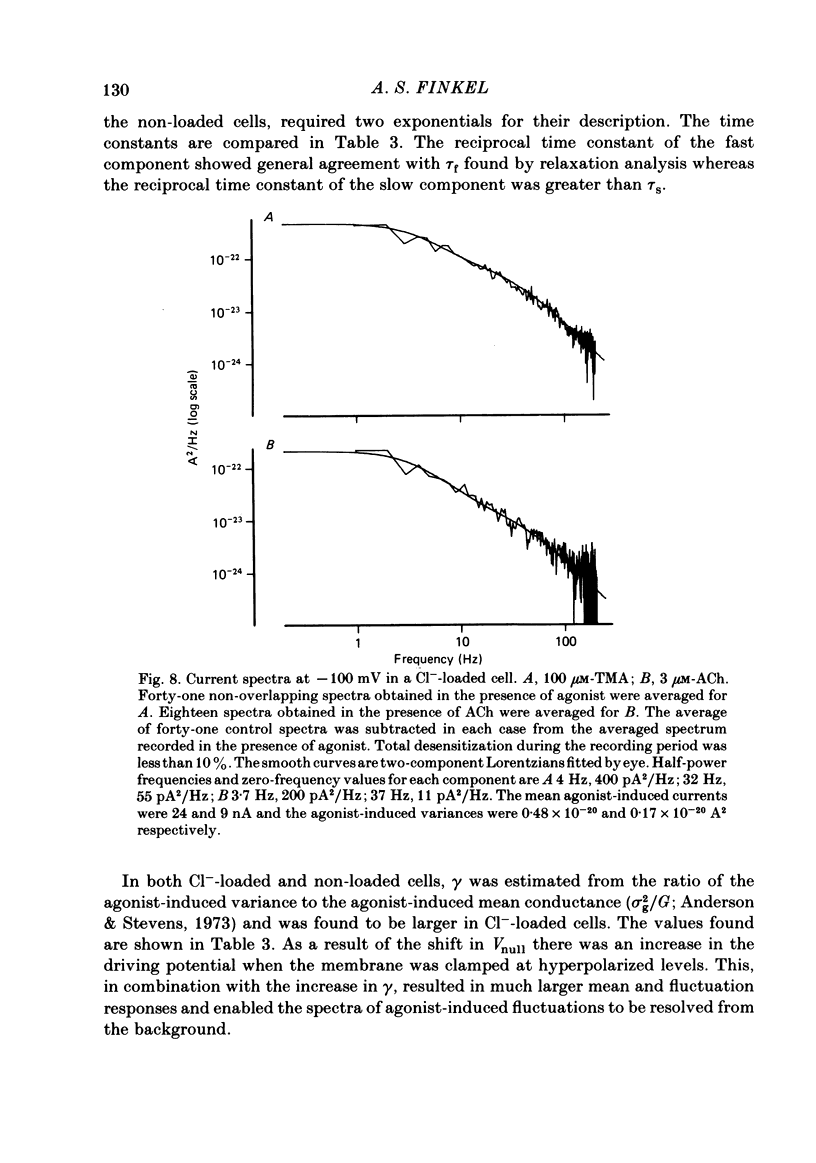
Inhibitory Cl- -mediated currents through cholinergic channels on the soma of identified neurones from the right parietal ganglion of Helix aspersa were studied under voltage clamp. Voltage-jump relaxation analysis showed that these currents decreased with hyperpolarization. In 3 microM-acetylcholine (ACh), the normalized fraction of channels in the open configuration (rho) decreased e-fold with each 191 mV of membrane hyperpolarization. The steady-state membrane conductance, G(infinity), decreased e-fold with each 128 mV of membrane hyperpolarization. The difference in the voltage sensitivities of rho and G(infinity) arose because of the voltage sensitivity of the instantaneous membrane conductance, G(0). G(0) rectified in the direction predicted by the Goldman-Hodgkin-Katz conductance model. The degree of rectification decreased when the internal Cl- concentration was raised. The relaxing currents were composed of two exponential components. At membrane potential (Vm) = -160 mV, 12 degrees C, the time constants of the two components were 4.1 ms and 21 ms in 3 microM-ACh, and 3.6 ms and 18 ms in 100 microM-tetramethylammonium (TMA). Fluctuation analysis in neurones loaded with Cl- yielded spectra which were composed of two Lorentzian components. In 3 microM-ACh the mean single-channel conductance (gamma) appeared to rise from a low value observed in cells with normal intracellular Cl- to 2.7 pS in cells whose internal Cl- concentration was raised four-fold. The voltage sensitivity of rho was attributed to the conformational change step of a gating mechanism having three kinetically distinguishable states.
Full text
PDF


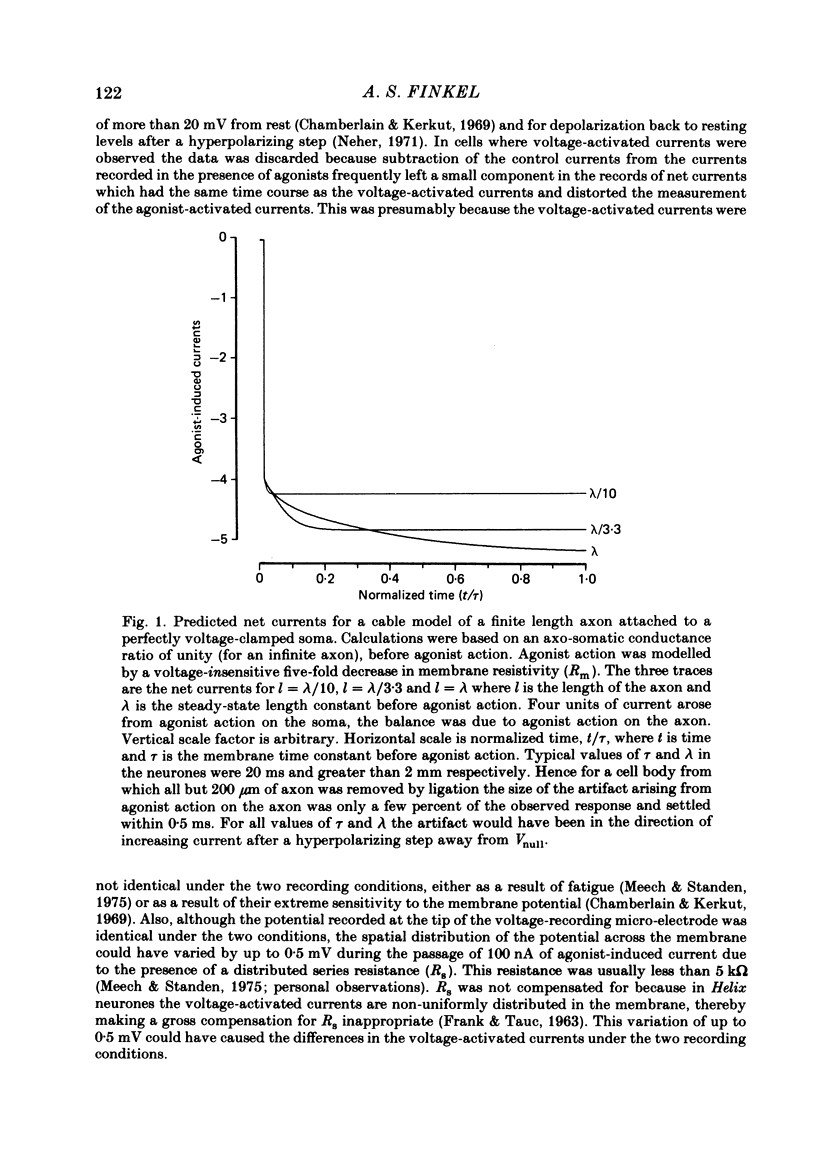

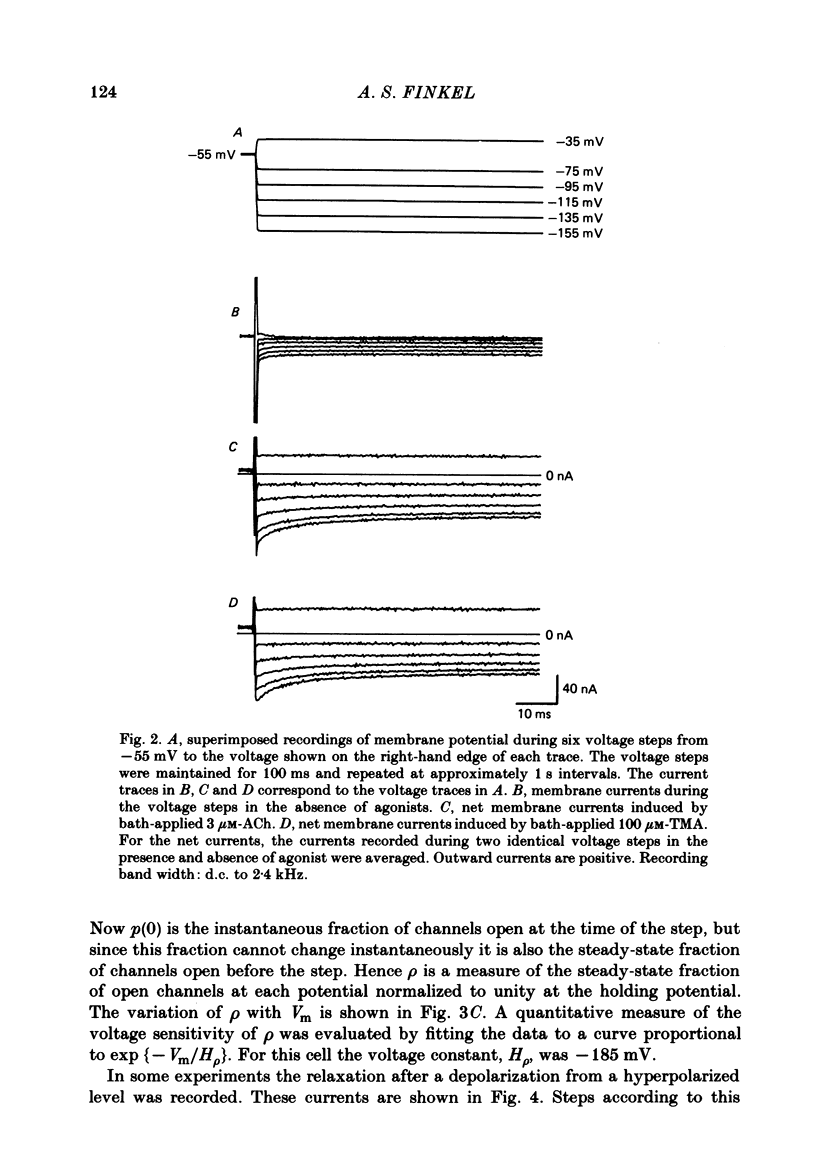
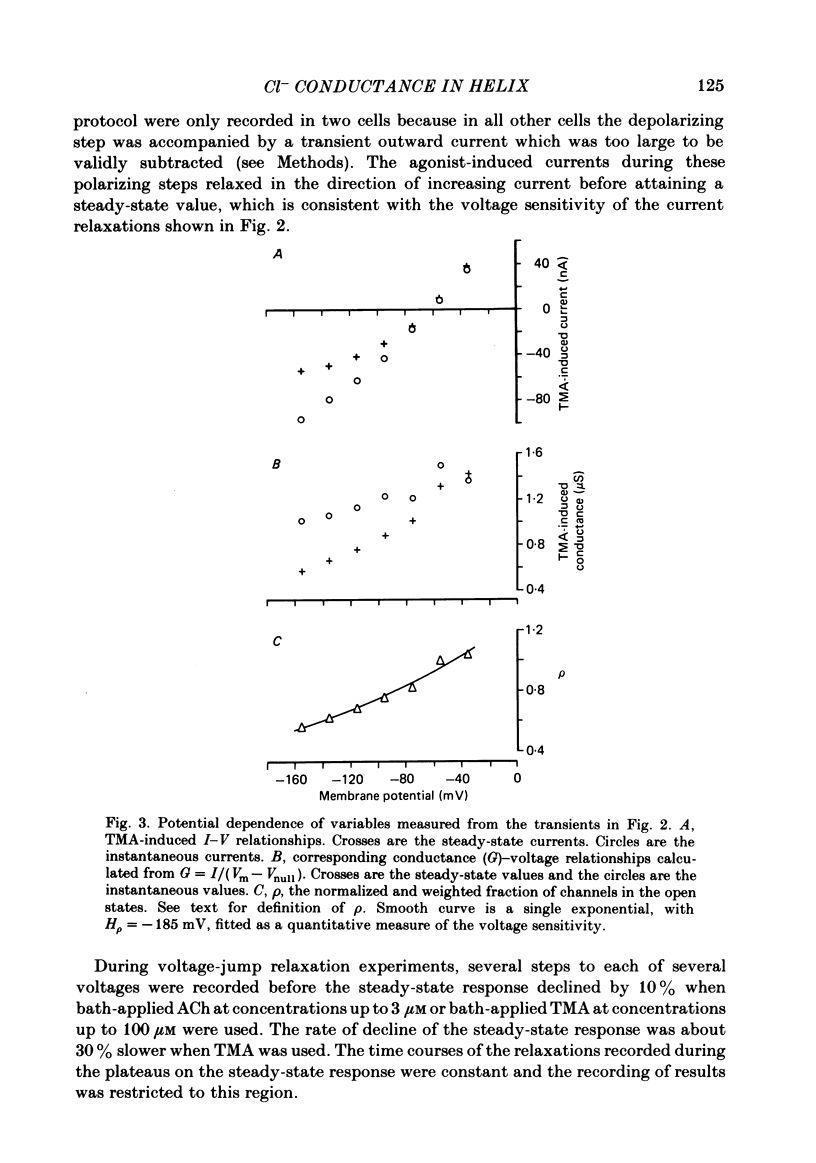
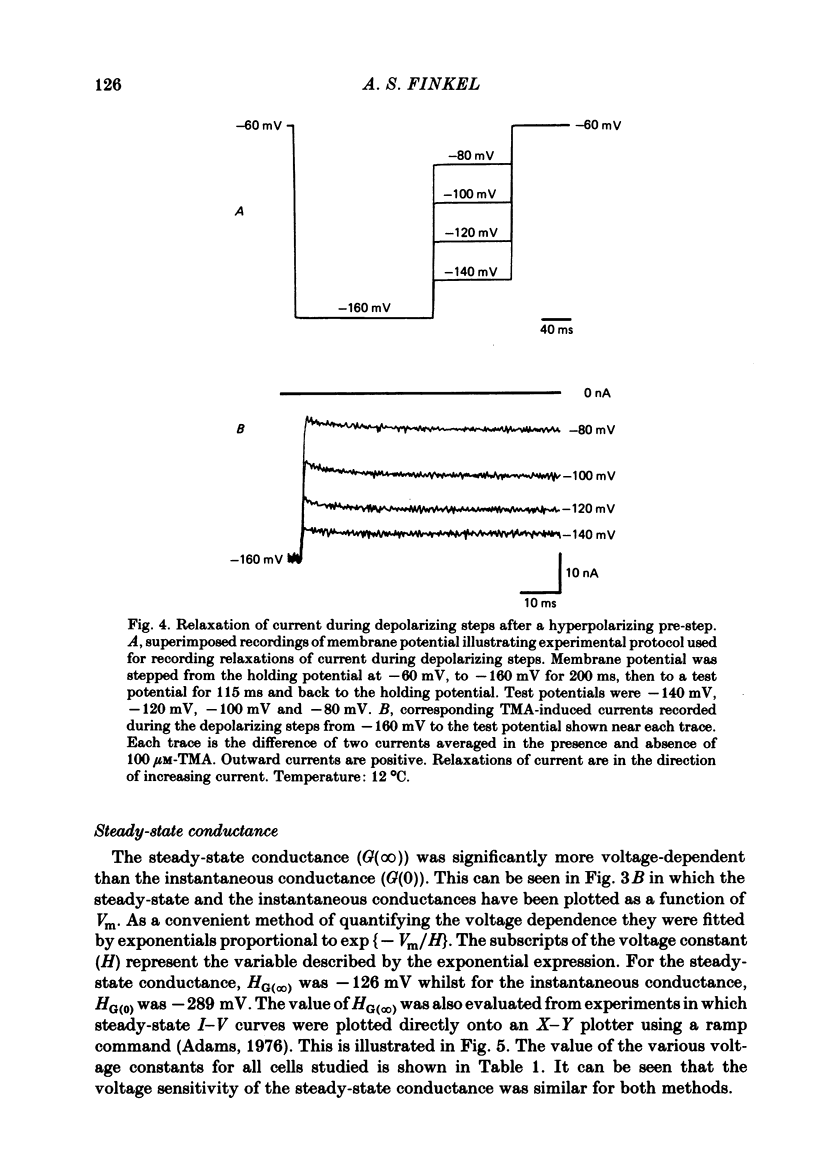




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Gage P. W., Hamill O. P. Voltage sensitivity of inhibitory postsynaptic current in Aplysia buccal ganglia. Brain Res. 1976 Oct 22;115(3):506–511. doi: 10.1016/0006-8993(76)90368-1. [DOI] [PubMed] [Google Scholar]
- Adams P. R. Kinetics of agonist conductance changes during hyperolarization at frog endplates. Br J Pharmacol. 1975 Feb;53(2):308–310. doi: 10.1111/j.1476-5381.1975.tb07364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Voltage dependence of agonist responses at voltage-clamped frog endplates. Pflugers Arch. 1976 Jan 30;361(2):145–151. doi: 10.1007/BF00583458. [DOI] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Marty A., Neild T. O. Life time and elementary conductance of the channels mediating the excitatory effects of acetylcholine in Aplysia neurones. J Physiol. 1978 May;278:177–206. doi: 10.1113/jphysiol.1978.sp012299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregestovski P. D., Iljin V. I. Effect of "calcium antagonist" D-600 on the postsynaptic membrane. J Physiol (Paris) 1980 Sep;76(5):515–522. [PubMed] [Google Scholar]
- Chapman J. B. On current-voltage relations of ionic channels. J Theor Biol. 1980 Jul 7;85(1):165–169. doi: 10.1016/0022-5193(80)90287-8. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Large W. A., Rang H. P. An analysis of the action of a false transmitter at the neuromuscular junction. J Physiol. 1977 Apr;266(2):361–395. doi: 10.1113/jphysiol.1977.sp011772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Inward and delayed outward membrane currents in isolated neural somata under voltage clamp. J Physiol. 1971 Feb;213(1):1–19. doi: 10.1113/jphysiol.1971.sp009364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Van Helden D. Effects of permeant monovalent cations on end-plate channels. J Physiol. 1979 Mar;288:509–528. [PMC free article] [PubMed] [Google Scholar]
- Gardner D. Membrane-potential effects on an inhibitory post-synaptic conductance in Aplysia buccal ganglia. J Physiol. 1980 Jul;304:165–180. doi: 10.1113/jphysiol.1980.sp013317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner D., Stevens C. F. Rate-limiting step of inhibitory post-synaptic current decay in Aplysia buccal ganglia. J Physiol. 1980 Jul;304:145–164. doi: 10.1113/jphysiol.1980.sp013316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol Rev. 1973 Jan;53(1):1–119. doi: 10.1152/physrev.1973.53.1.1. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L., Kado R. T. Voltage-current relationship of a carbachol-induced potassium-ion pathway in Aplysia neurones. J Physiol. 1975 Mar;245(3):713–725. doi: 10.1113/jphysiol.1975.sp010870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. R., Martin A. R. Intracellular Cl- accumulation reduces Cl- conductance in inhibitory synaptic channels. Nature. 1982 Oct 28;299(5886):828–830. doi: 10.1038/299828a0. [DOI] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graubard K. Voltage attenuation within Aplysia neurons: the effect of branching pattern. Brain Res. 1975 May 2;88(2):325–332. doi: 10.1016/0006-8993(75)90394-7. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Three acetylcholine receptors in Aplysia neurones. J Physiol. 1972 Aug;225(1):115–146. doi: 10.1113/jphysiol.1972.sp009931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C. A. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979 Jan;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., Ascher P. Slow relaxations of acetylcholine-induced potassium currents in Aplysia neurones. Nature. 1978 Aug 3;274(5670):494–497. doi: 10.1038/274494a0. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974 Mar;237(2):259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Voltage-dependence of drug-induced conductance in frog neuromuscular junction. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2140–2144. doi: 10.1073/pnas.72.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff R. L. A quantitative analysis of local anaesthetic alteration of miniature end-plate currents and end-plate current fluctuations. J Physiol. 1977 Jan;264(1):89–124. doi: 10.1113/jphysiol.1977.sp011659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan R. E., Lester H. A. Rates and equilibria at the acetylcholine receptor of Electrophorus electroplaques: a study of neurally evoked postsynaptic currents and of voltage-jump relaxations. J Gen Physiol. 1977 Aug;70(2):187–219. [PMC free article] [PubMed] [Google Scholar]
- Sheridan R. E., Lester H. A. Relaxation measurements on the acetylcholine receptor. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3496–3500. doi: 10.1073/pnas.72.9.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonneau M., Tauc L., Baux G. Quantal release of acetylcholine examined by current fluctuation analysis at an identified neuro-neuronal synapse of Aplysia. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1661–1665. doi: 10.1073/pnas.77.3.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavari P., Walker R. J., Kerkut G. A. The pA2 values of cholinergic antagonists on identified neurons of the snail Helix aspersa. Comp Biochem Physiol C. 1979;63C(1):39–52. doi: 10.1016/0306-4492(79)90128-x. [DOI] [PubMed] [Google Scholar]


