Abstract
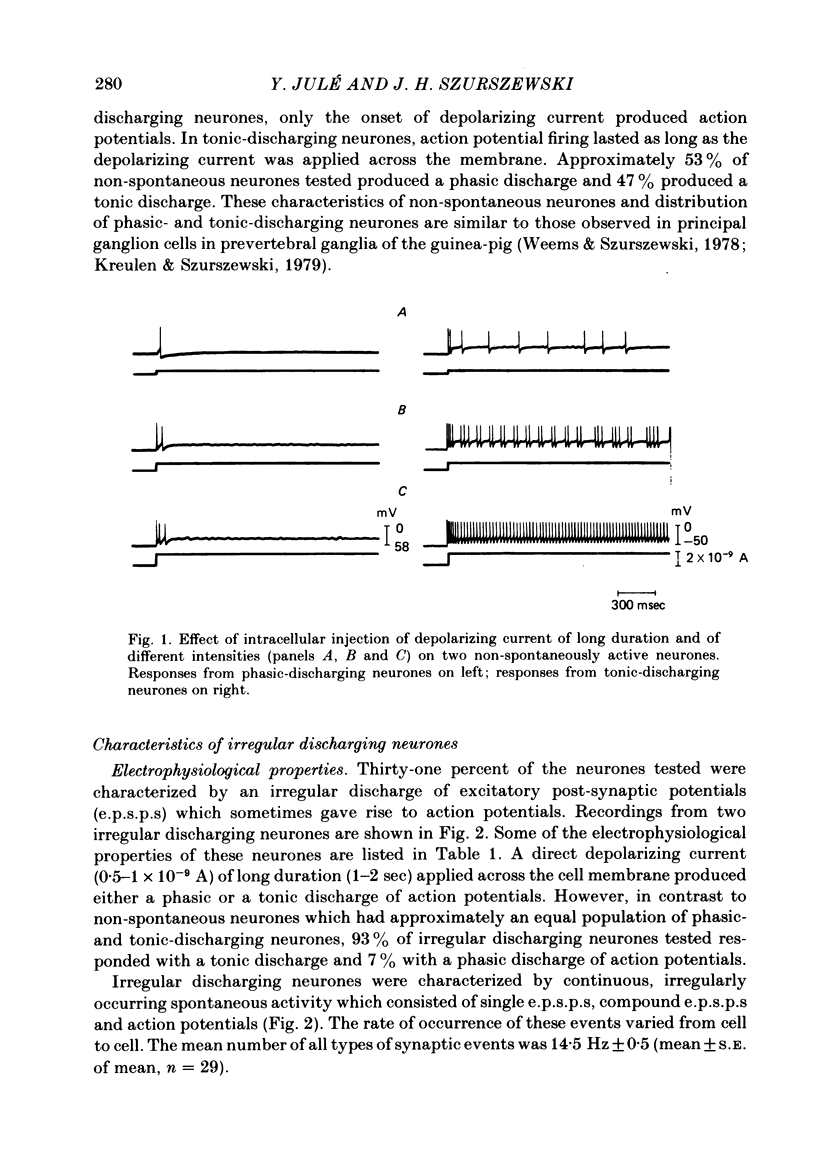
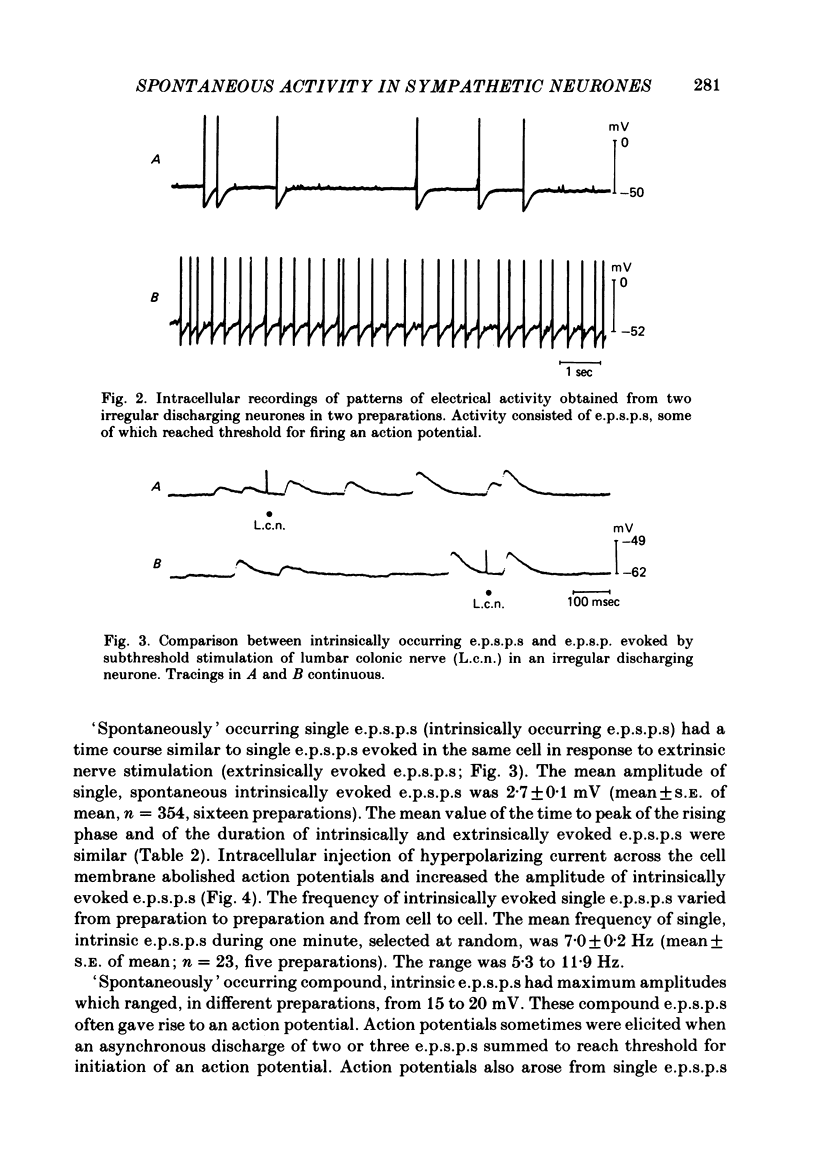
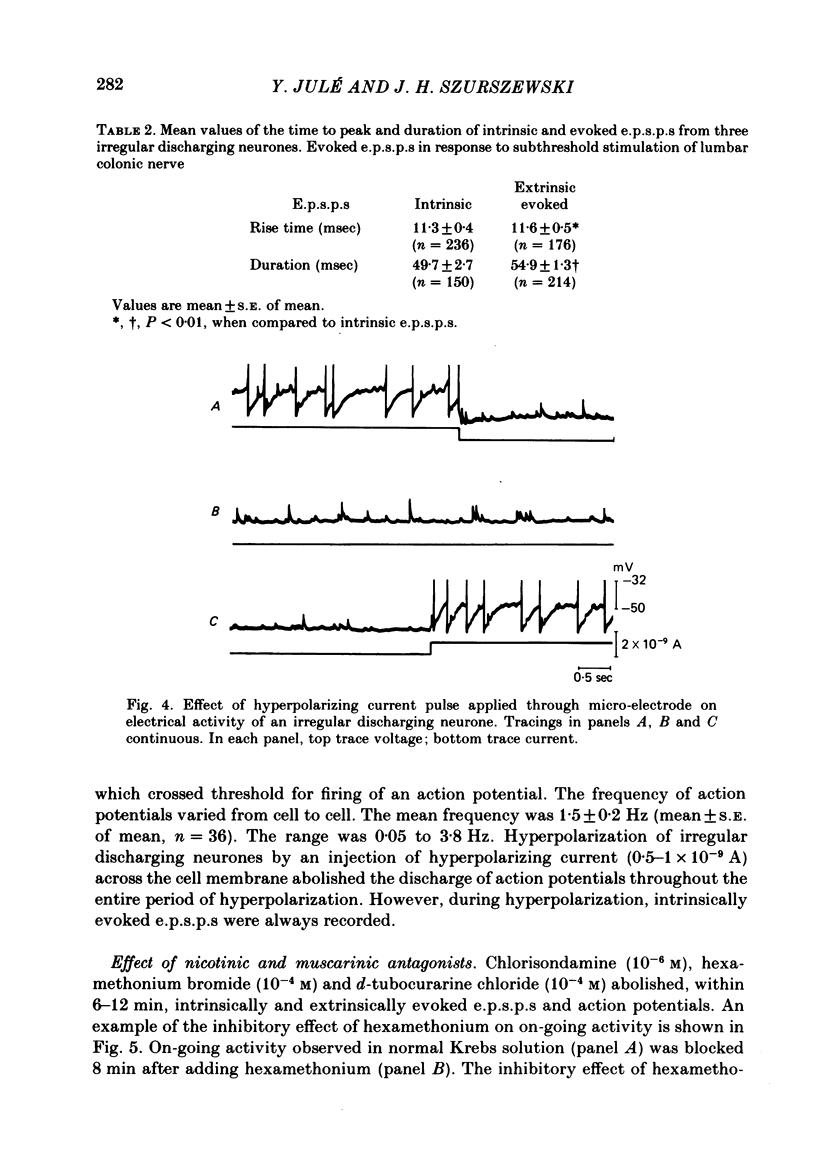
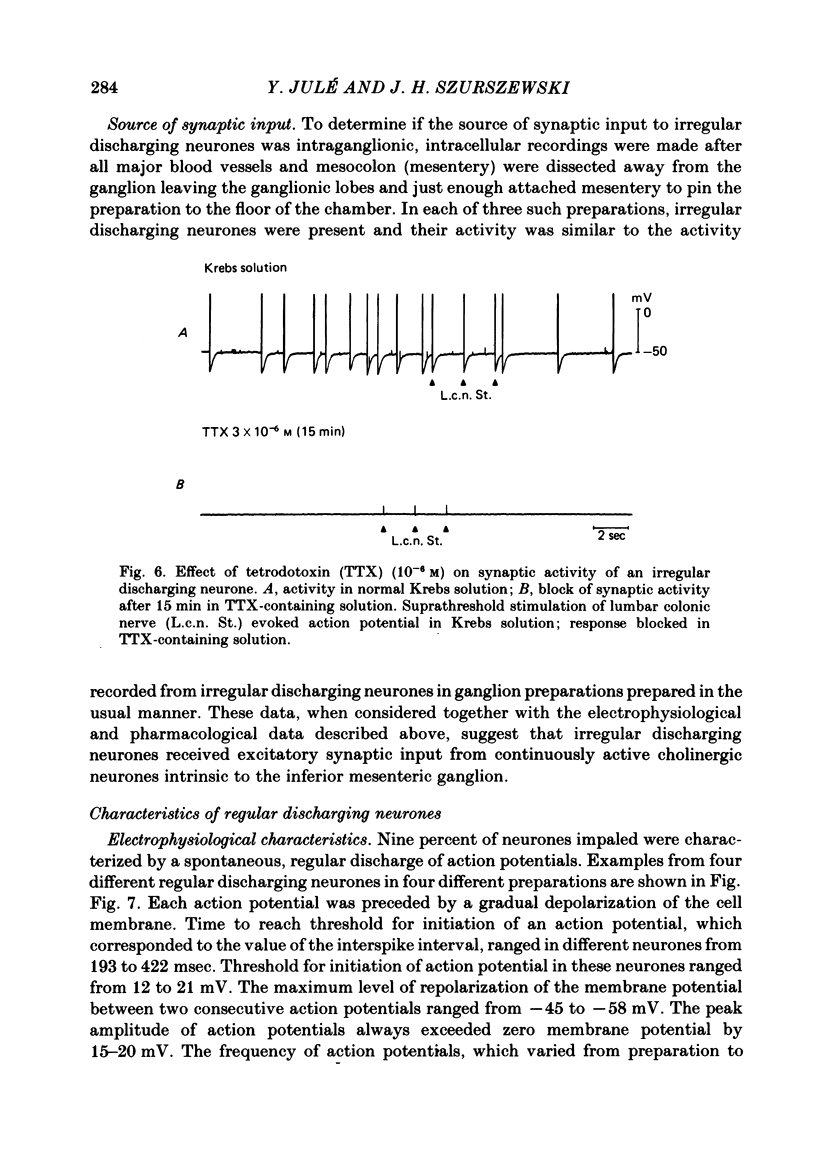
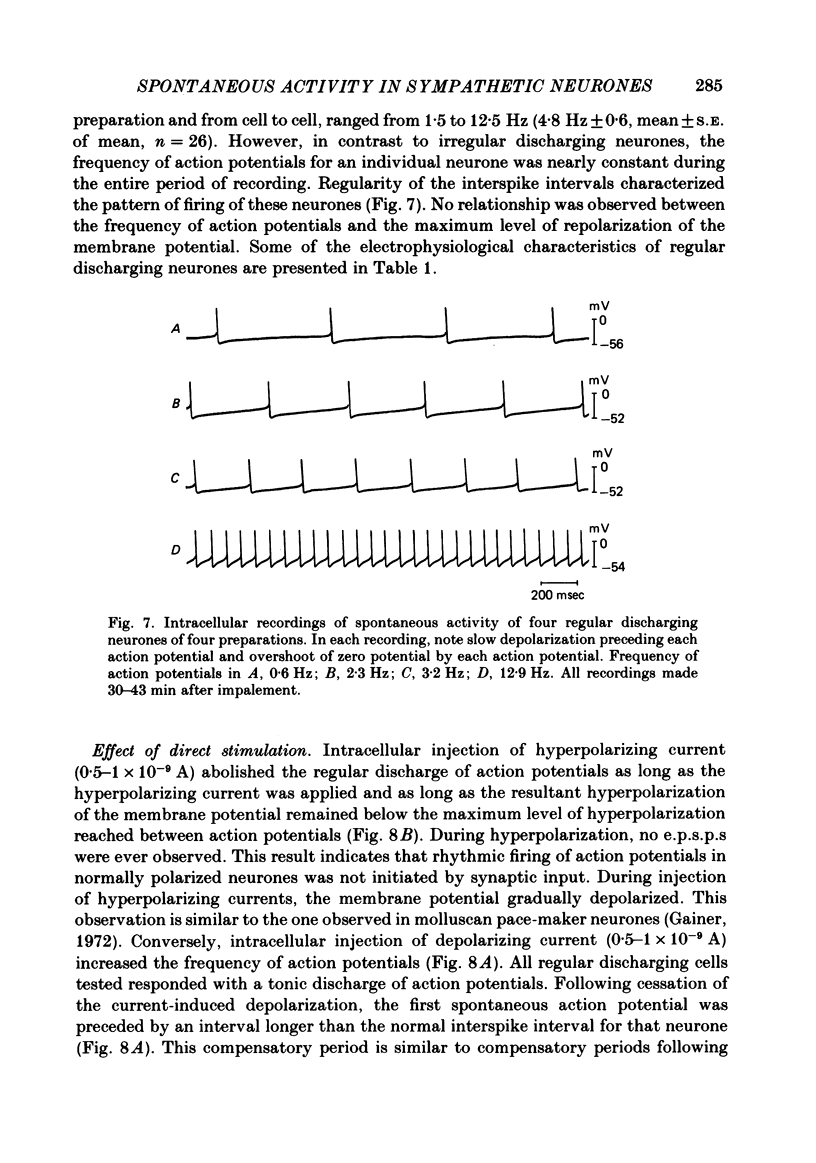
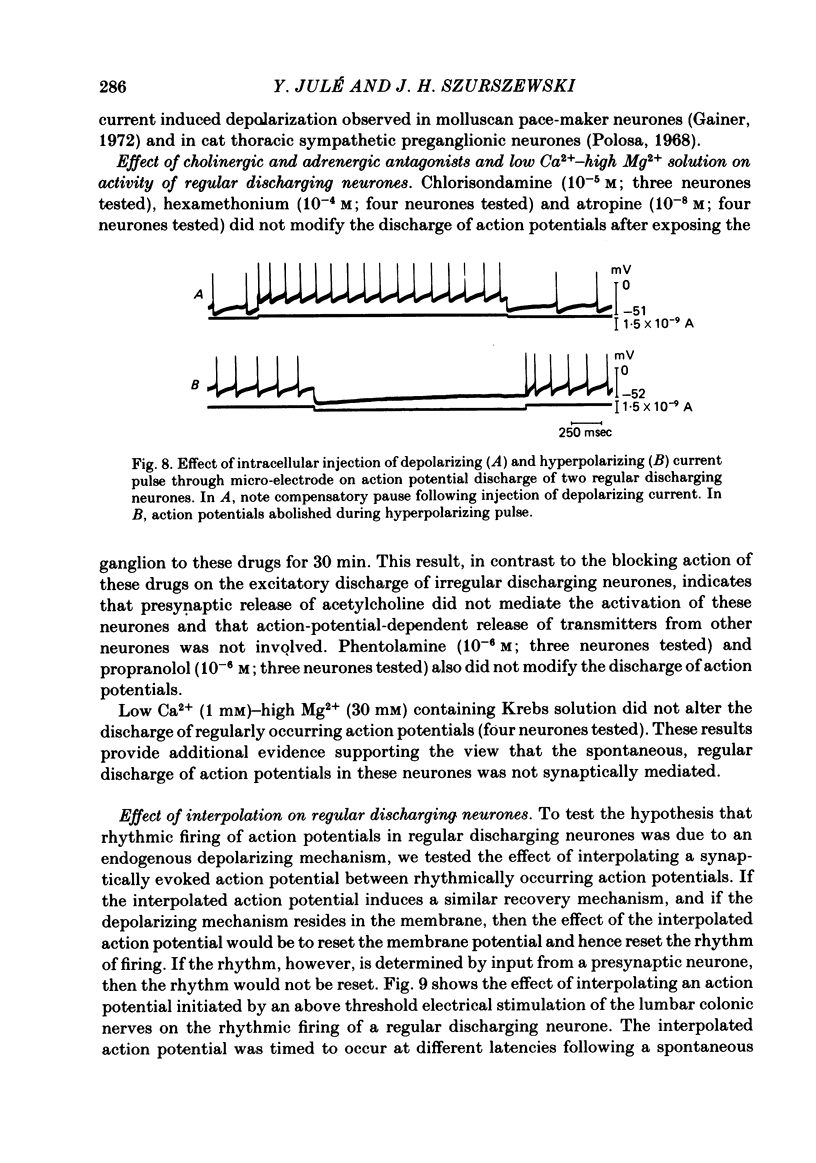
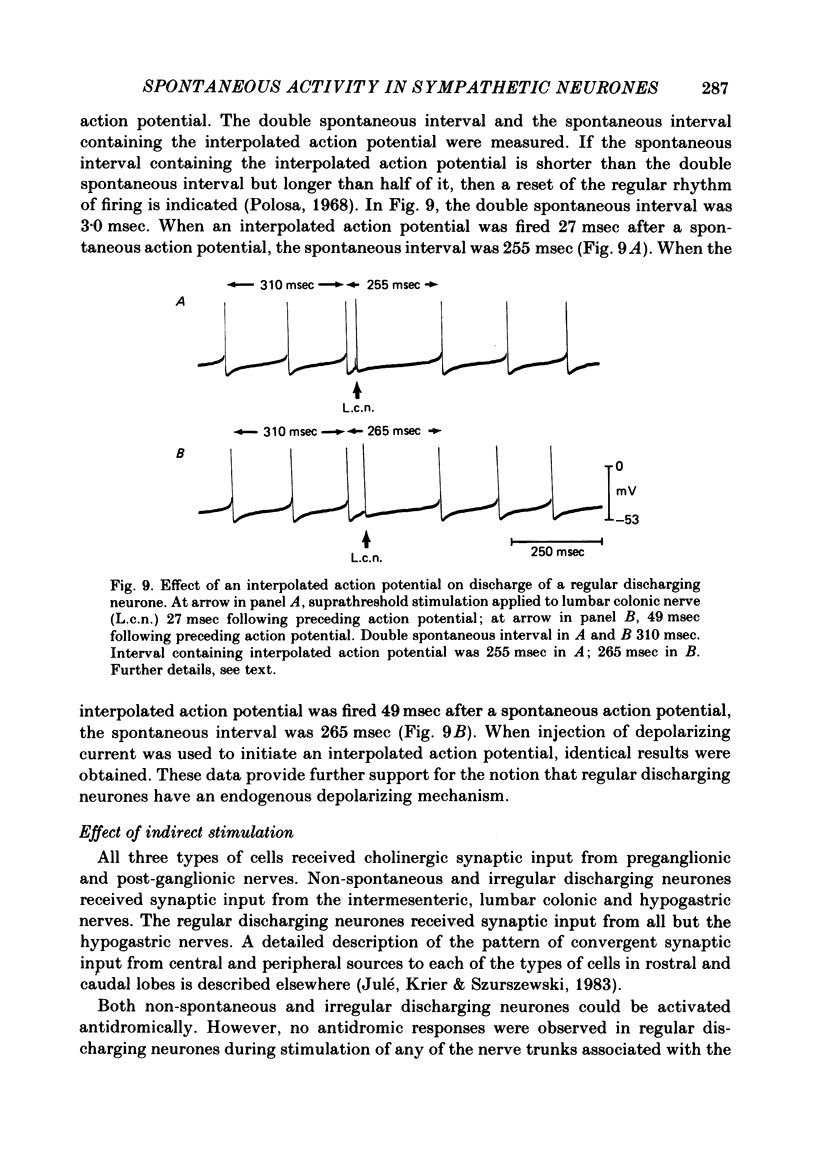
Intracellular recordings were obtained from cells in vitro in the inferior mesenteric ganglia of the cat. Neurones could be classified into three types: non-spontaneous, irregular discharging and regular discharging neurones. Non-spontaneous neurones had a stable resting membrane potential and responded with action potentials to indirect preganglionic nerve stimulation and to intracellular injection of depolarizing current. Irregular discharging neurones were characterized by a discharge of excitatory post-synaptic potentials (e.p.s.p.s.) which sometimes gave rise to action potentials. This activity was abolished by hexamethonium bromide, chlorisondamine and d-tubocurarine chloride. Tetrodotoxin and a low Ca2+ -high Mg2+ solution also blocked on-going activity in irregular discharging neurones. Regular discharging neurones were characterized by a rhythmic discharge of action potentials. Each action potential was preceded by a gradual depolarization of the intracellularly recorded membrane potential. Intracellular injection of hyperpolarizing current abolished the regular discharge of action potential. No synaptic potentials were observed during hyperpolarization of the membrane potential. Nicotinic, muscarinic and adrenergic receptor blocking drugs did not modify the discharge of action potentials in regular discharging neurones. A low Ca2+ -high Mg2+ solution also had no effect on the regular discharge of action potentials. Interpolation of an action potential between spontaneous action potentials in regular discharging neurones reset the rhythm of discharge. It is suggested that regular discharging neurones were endogenously active and that these neurones provided synaptic input to irregular discharging neurones.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian E. D., Bronk D. W., Phillips G. Discharges in mammalian sympathetic nerves. J Physiol. 1932 Feb 8;74(2):115–133. doi: 10.1113/jphysiol.1932.sp002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alving B. O. Spontaneous activity in isolated somata of Aplysia pacemaker naurons. J Gen Physiol. 1968 Jan;51(1):29–45. doi: 10.1085/jgp.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEACHAM W. S., PERL E. R. BACKGROUND AND REFLEX DISCHARGE OF SYMPATHETIC PREGANGLIONIC NEURONES IN THE SPINAL CAT. J Physiol. 1964 Aug;172:400–416. doi: 10.1113/jphysiol.1964.sp007428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman J. G., Crowcroft P. J., Devine C. E., Holman M. E., Yonemura K. Transmission from pregnanglionic fibres in the hypogastric nerve to peripheral ganglia of male guinea-pigs. J Physiol. 1969 May;201(3):723–743. doi: 10.1113/jphysiol.1969.sp008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman J. G., Purves R. D. Intracellular recordings from ganglia of the thoracic sympathetic chain of the guinea-pig. J Physiol. 1969 Jul;203(1):173–198. doi: 10.1113/jphysiol.1969.sp008858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein J. C. Spontaneous multiquantal release at synapses in guinea-pig hypogastric ganglia: evidence that release can occur in bursts. J Physiol. 1978 Sep;282:375–398. doi: 10.1113/jphysiol.1978.sp012470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosnjak Z. J., Seagard J. L., Kampine J. P. Peripheral neural input to neurons of the stellate ganglion in dog. Am J Physiol. 1982 Mar;242(3):R237–R243. doi: 10.1152/ajpregu.1982.242.3.R237. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Holman M. E. Junction potentials at adrenergic synapses. Pharmacol Rev. 1966 Mar;18(1):481–493. [PubMed] [Google Scholar]
- Crowcroft P. J., Szurszewski J. H. A study of the inferior mesenteric and pelvic ganglia of guinea-pigs with intracellular electrodes. J Physiol. 1971 Dec;219(2):421–441. doi: 10.1113/jphysiol.1971.sp009670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat W. C., Krier J. The central control of the lumbar sympathetic pathway to the large intestine of the cat. J Physiol. 1979 Apr;289:449–468. doi: 10.1113/jphysiol.1979.sp012746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erulkar S. D., Woodward J. K. Intracellular recording from mammalian superior cervical ganglion in situ. J Physiol. 1968 Nov;199(1):189–203. doi: 10.1113/jphysiol.1968.sp008648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainer H. Electrophysiological behavior of an endogenously active neurosecretory cell. Brain Res. 1972 Apr 28;39(2):403–418. doi: 10.1016/0006-8993(72)90444-1. [DOI] [PubMed] [Google Scholar]
- Garry R. C. The nervous control of the caudal region of the large bowel in the cat. J Physiol. 1933 Mar 15;77(4):422–431. doi: 10.1113/jphysiol.1933.sp002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith W. H., 3rd, Gallagher J. P., Shinnick-Gallagher P. An intracellular investigation of cat vesical pelvic ganglia. J Neurophysiol. 1980 Feb;43(2):343–354. doi: 10.1152/jn.1980.43.2.343. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the release of transmitter by nerve impulses. J Physiol. 1968 May;196(1):75–86. doi: 10.1113/jphysiol.1968.sp008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the spontaneous release of transmitter from mammalian motor nerve terminals. J Physiol. 1968 Feb;194(2):355–380. doi: 10.1113/jphysiol.1968.sp008413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGGO A., VOGT M. Preganglionic sympathetic activity in normal and in reserpine-treated cats. J Physiol. 1960 Jan;150:114–133. doi: 10.1113/jphysiol.1960.sp006377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julé Y., Krier J., Szurszewski J. H. Patterns of innervation of neurones in the inferior mesenteric ganglion of the cat. J Physiol. 1983 Nov;344:293–304. doi: 10.1113/jphysiol.1983.sp014940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Further study of the role of calcium in synaptic transmission. J Physiol. 1970 May;207(3):789–801. doi: 10.1113/jphysiol.1970.sp009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreulen D. L., Szurszewski J. H. Nerve pathways in celiac plexus of the guinea pig. Am J Physiol. 1979 Jul;237(1):E90–E97. doi: 10.1152/ajpendo.1979.237.1.E90. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Nicholls J. G. The physiology of neuroglial cells. Ergeb Physiol. 1966;57:1–90. [PubMed] [Google Scholar]
- Langley J. N., Anderson H. K. On the Innervation of the Pelvic and Adjoining Viscera: Part I. The Lower Portion of the Intestine. J Physiol. 1895 May 20;18(1-2):67–105. doi: 10.1113/jphysiol.1895.sp000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa C. Spontaneous activity of sympathetic preganglionic neurons. Can J Physiol Pharmacol. 1968 Nov;46(6):887–896. doi: 10.1139/y68-138. [DOI] [PubMed] [Google Scholar]
- Tomita T. Current spread in the smooth muscle of the guinea-pig vas deferens. J Physiol. 1967 Mar;189(1):163–176. doi: 10.1113/jphysiol.1967.sp008161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALL P. D. Repetitive discharge of neurons. J Neurophysiol. 1959 May;22(3):305–320. doi: 10.1152/jn.1959.22.3.305. [DOI] [PubMed] [Google Scholar]
- Weems W. A., Szurszewski J. H. An intracellular analysis of some intrinsic factors controlling neural output from inferior mesenteric ganglion of guinea pigs. J Neurophysiol. 1978 Mar;41(2):305–321. doi: 10.1152/jn.1978.41.2.305. [DOI] [PubMed] [Google Scholar]
- Wood J. D., Mayer C. J. Intracellular study of electrical activity of Auerbach's plexus in guinea-pig small intestine. Pflugers Arch. 1978 May 31;374(3):265–275. doi: 10.1007/BF00585604. [DOI] [PubMed] [Google Scholar]


