Abstract
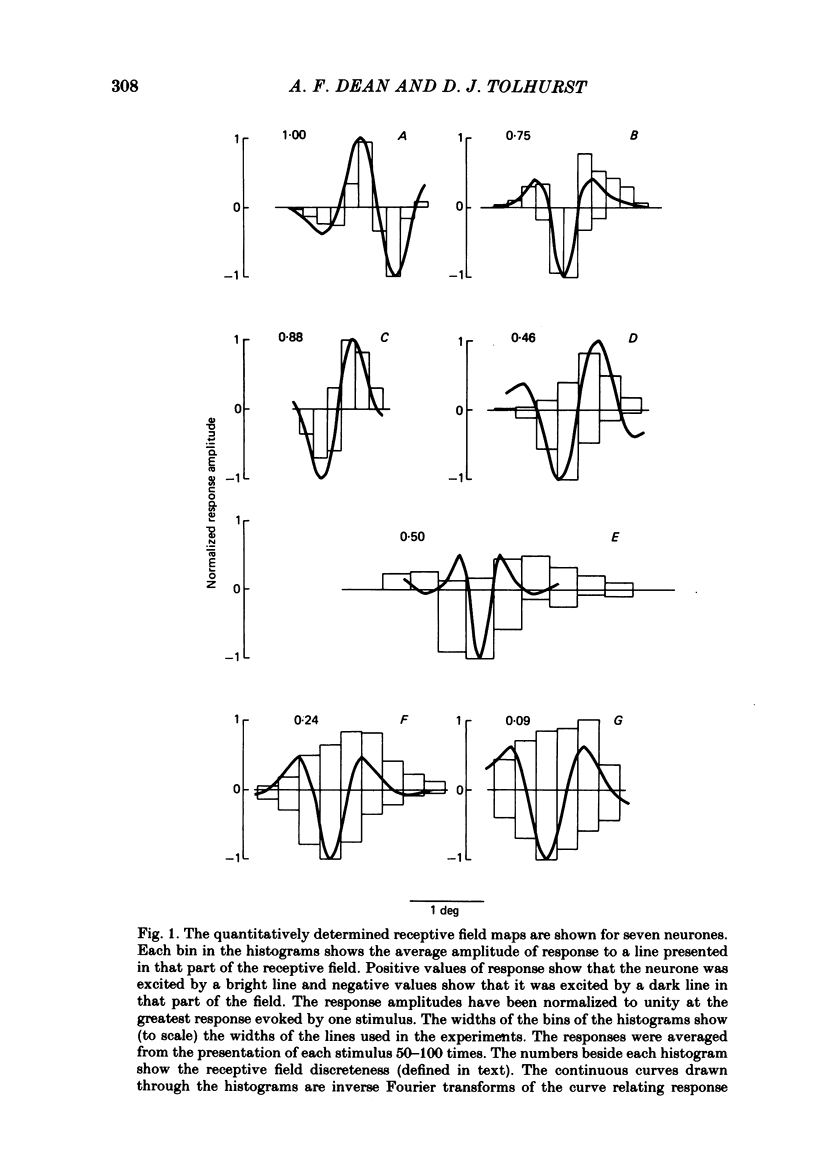
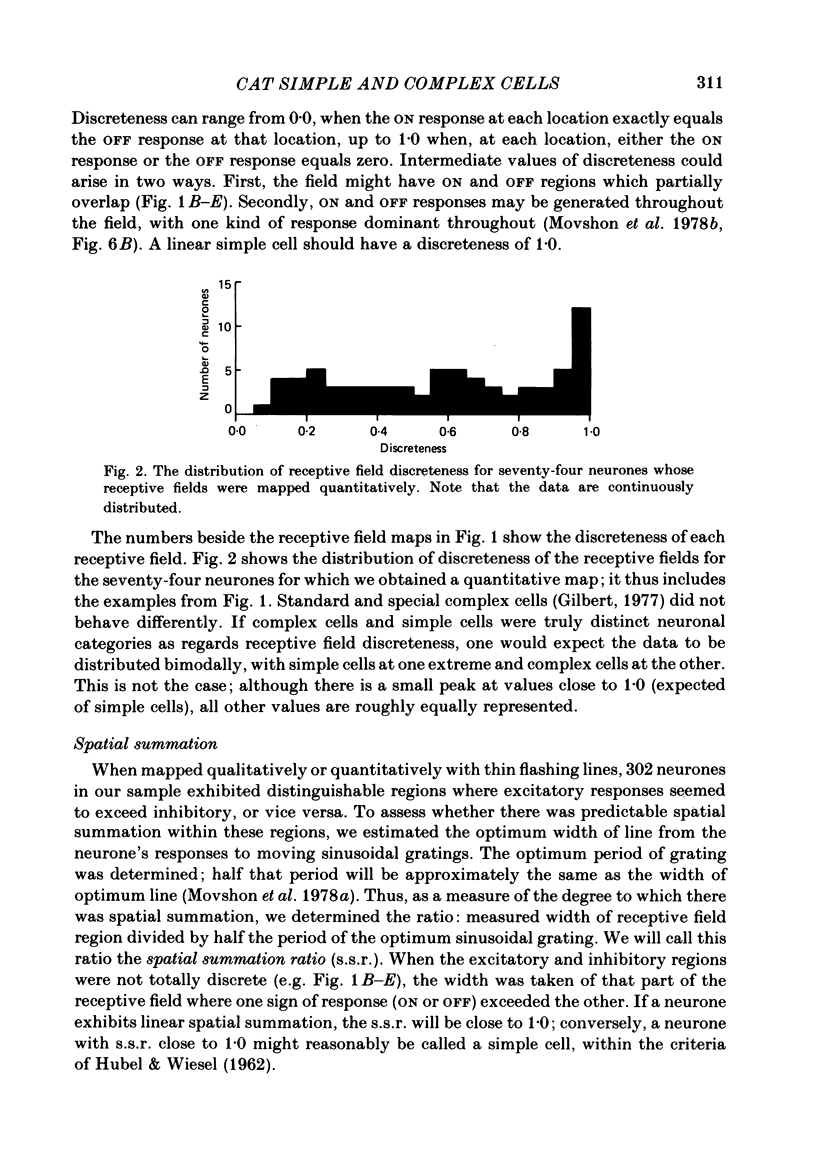
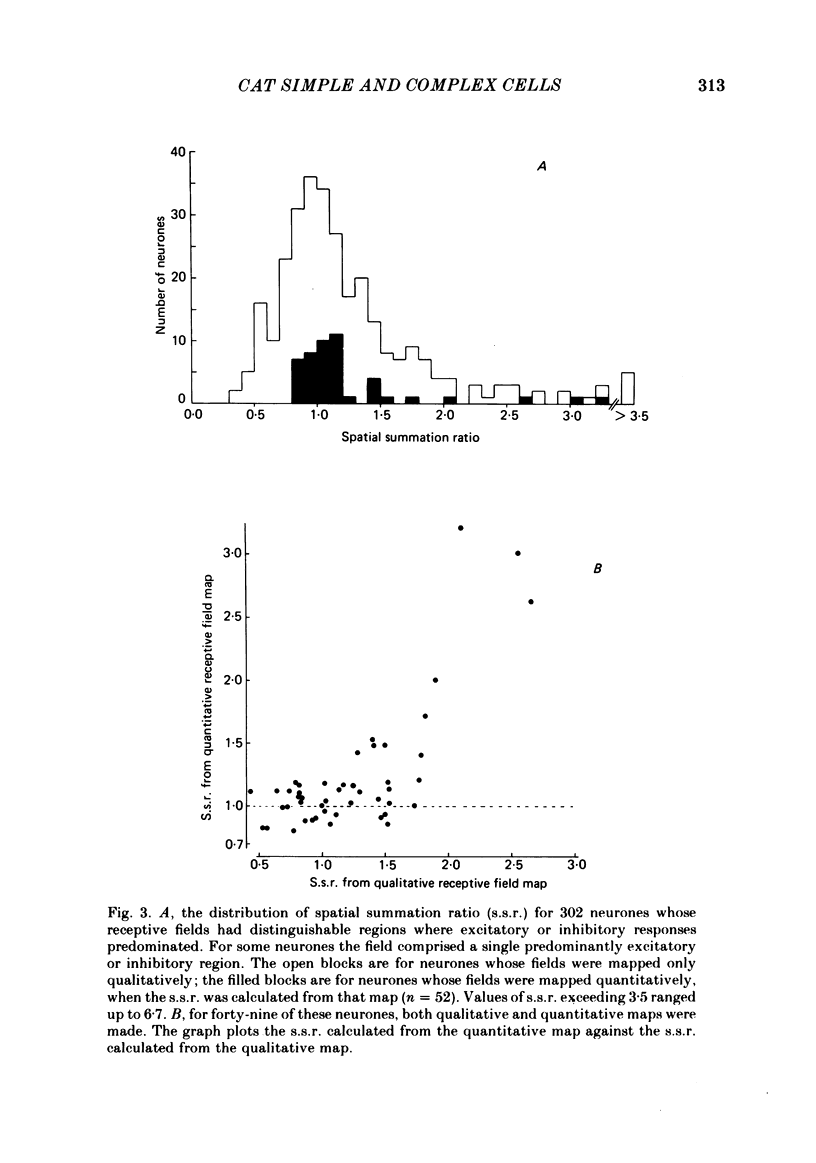
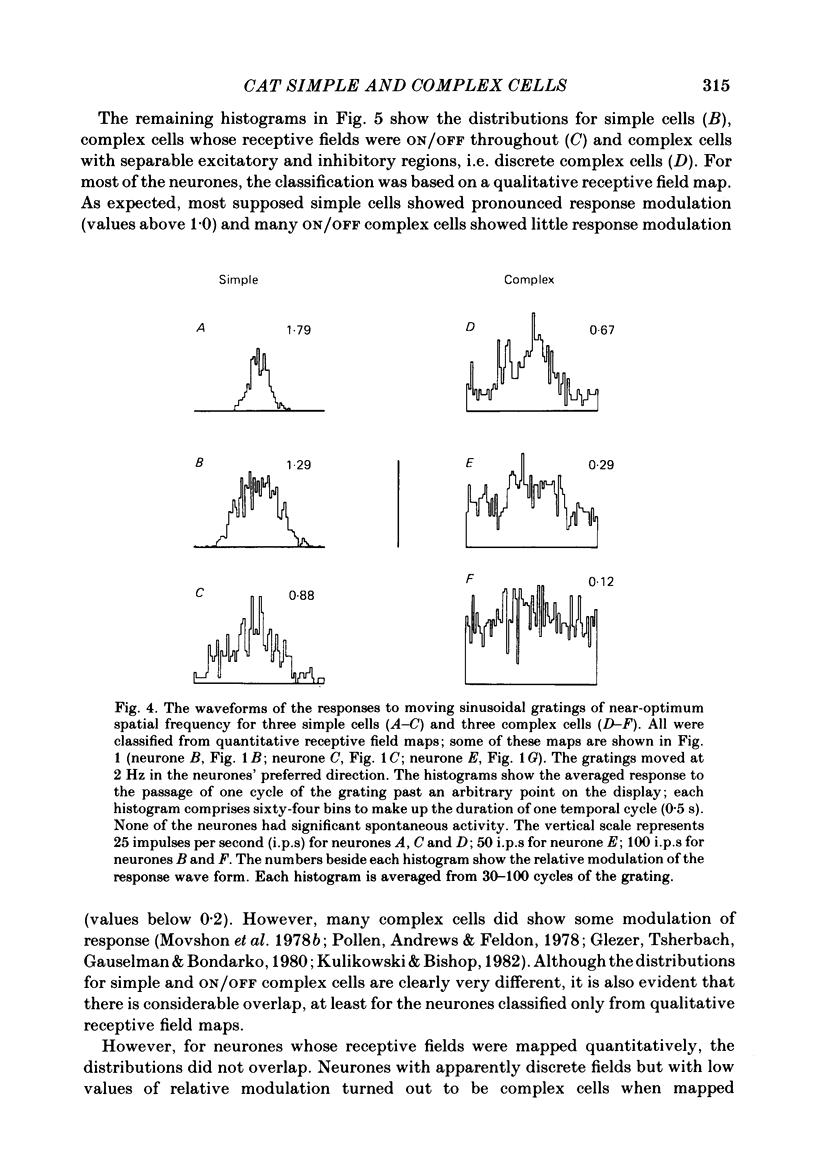
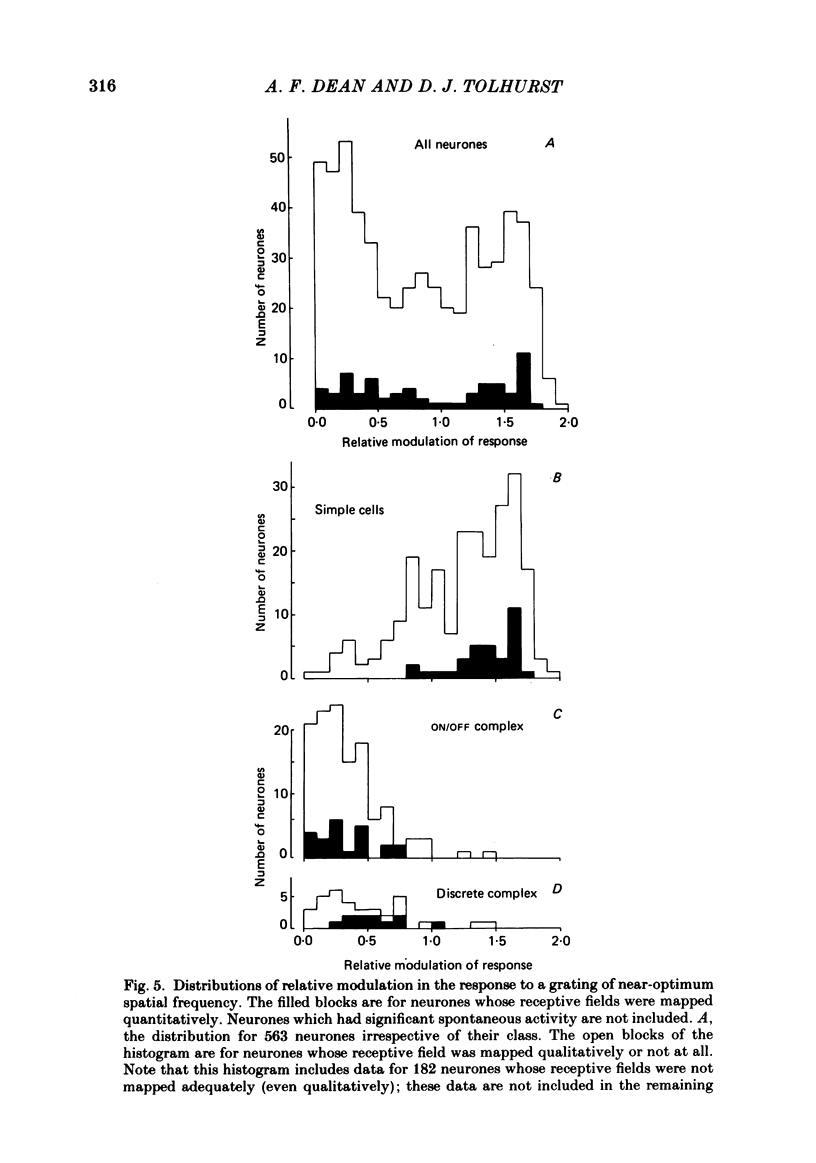
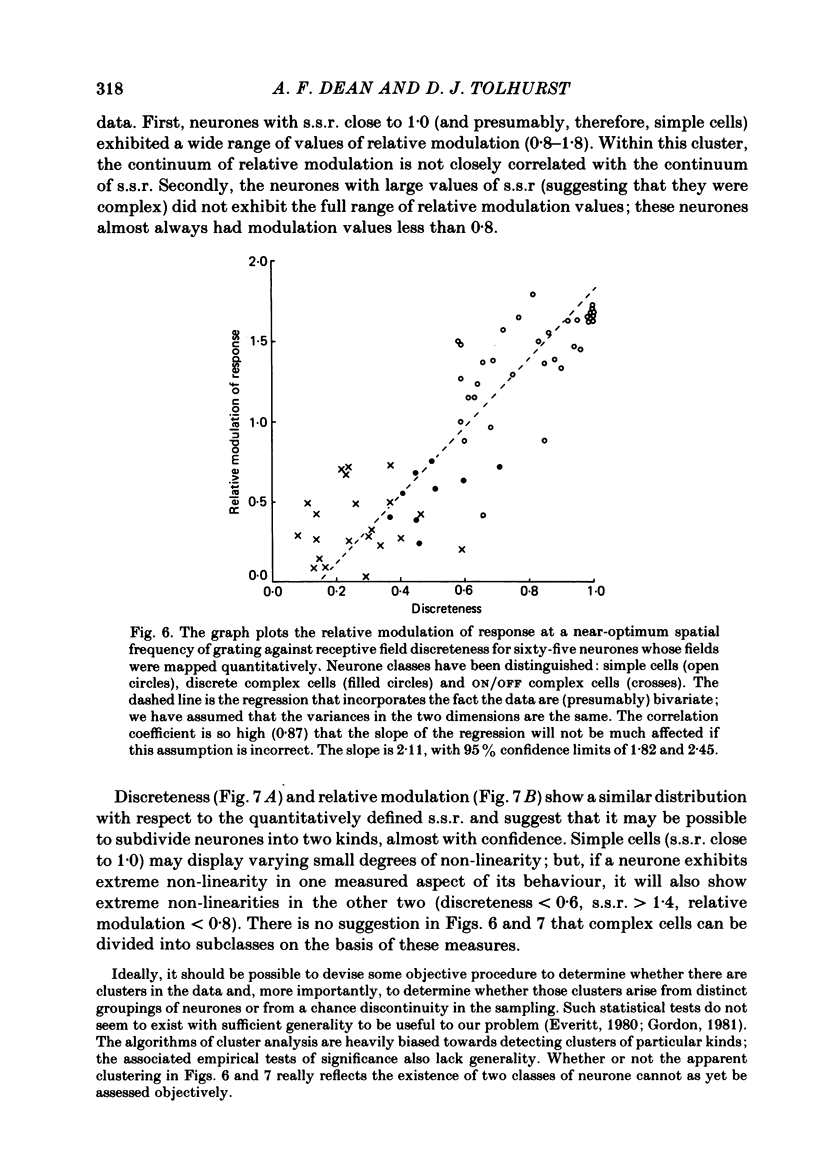
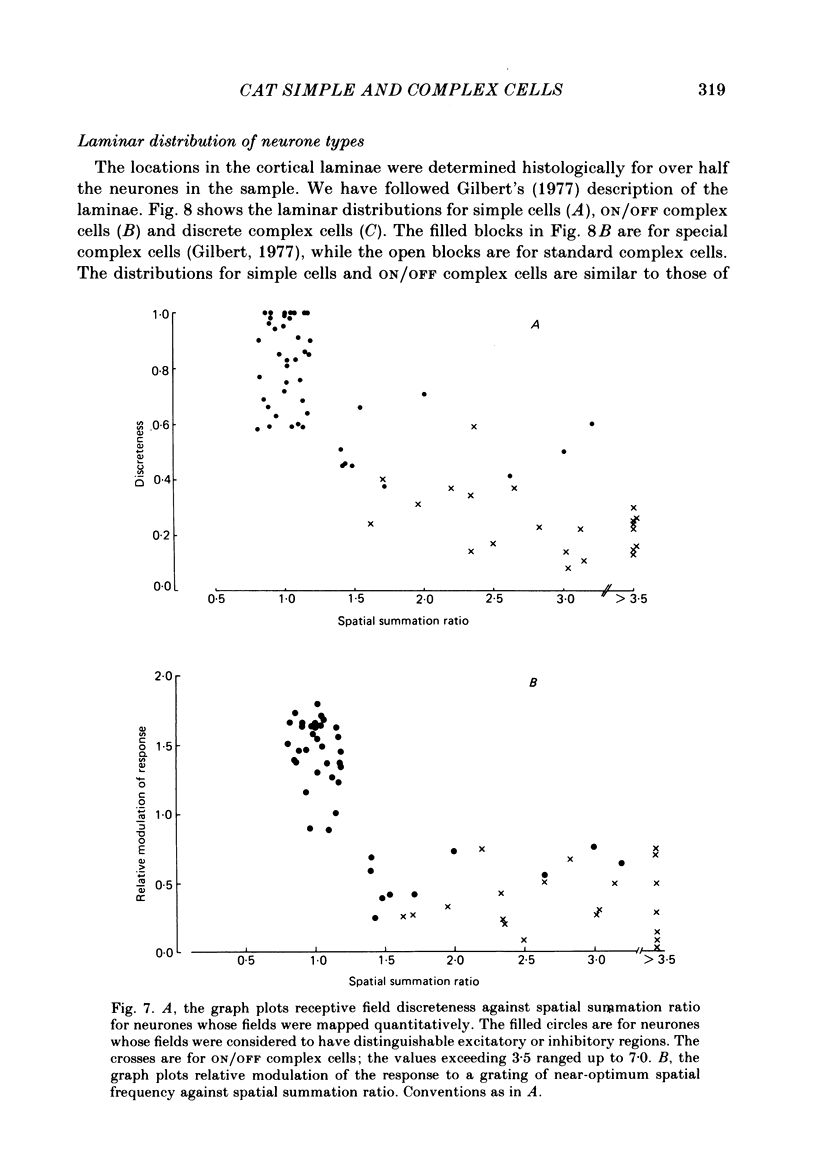
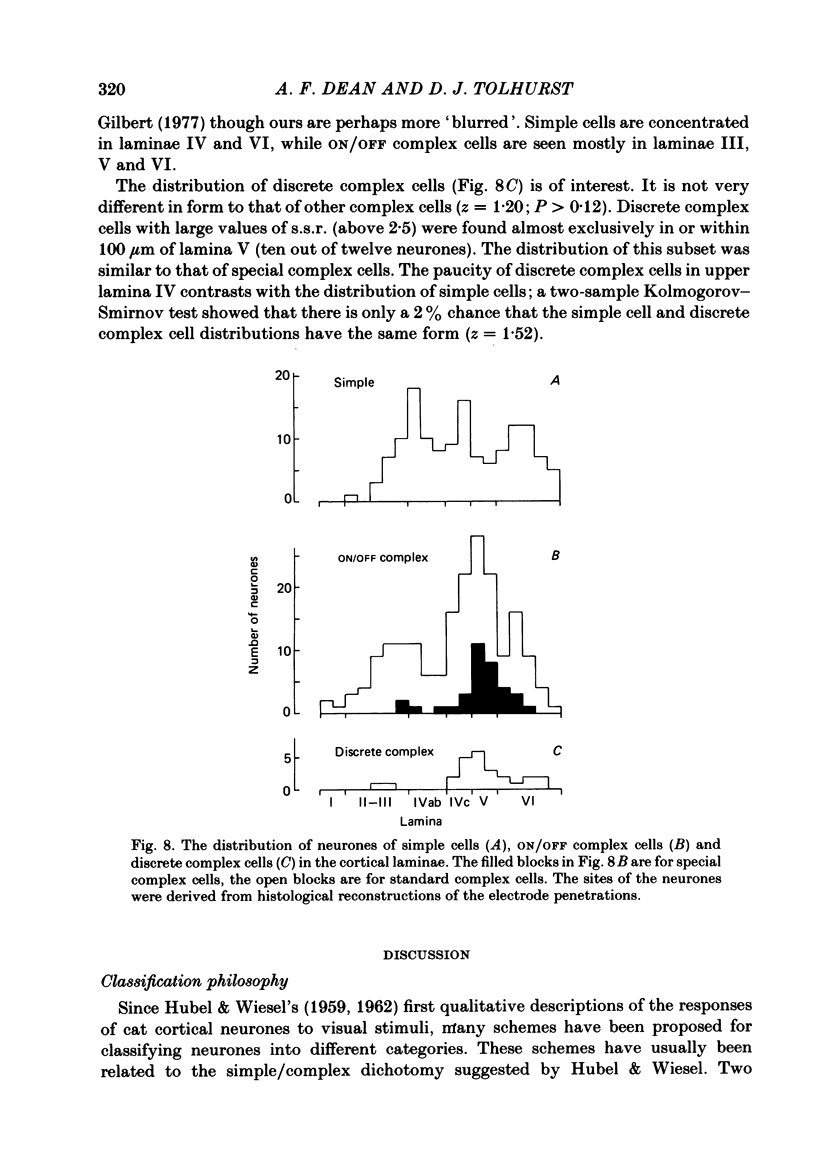
The behaviour of neurones in cat striate cortex was examined in response to moving sinusoidal gratings and flashed bright and dark lines. The responses were summarized by three indices: discreteness was a measure of the degree of separation of inhibitory and excitatory regions in the receptive field; spatial summation ratio showed the degree of spatial summation within each region; relative modulation was a measure of the degree of modulation in the response to a moving grating. Some neurones had receptive fields with completely discrete excitatory and inhibitory regions; others responded equally to stimulus onset and offset throughout their receptive fields; however, some had overlapping excitatory and inhibitory regions. The degree of overlap varied continuously from complete separation to complete overlap. For neurones with discrete receptive fields, the widths of the regions were compared with the width of the bars in a grating of optimum spatial frequency to assess the degree of spatial summation within the regions. Most neurones with discrete receptive fields showed roughly predictable spatial summation, in that the two width measures agreed; but about 10% of them had receptive field regions that were too large by a factor of over two. The neurones which showed incomplete spatial summation also had considerable overlap of their excitatory and inhibitory regions. The waveforms of the responses to moving gratings of optimal spatial frequency were examined. The degree of modulation in the response was continuously distributed between low values typical of complex cells and high values typical of simple cells; the distribution was not bimodal. The degree of response modulation was closely correlated with the degree to which the excitatory and inhibitory regions in the receptive field were discrete. Both the degree of spatial summation and the degree of response modulation have been previously proposed as means for distinguishing simple and complex cells. In the present study, the continuity of the distributions of both indices ensured that neither index alone could be used to class all neurones unequivocally. However, a criterion based on two indices did allow classification. Simple and complex cells showed distinctive behaviour. However, complex cells with distinguishable excitatory and inhibitory regions in their receptive fields were not distinctly different from other complex cells.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews B. W., Pollen D. A. Relationship between spatial frequency selectivity and receptive field profile of simple cells. J Physiol. 1979 Feb;287:163–176. doi: 10.1113/jphysiol.1979.sp012652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Green D. G. Optical and retinal factors affecting visual resolution. J Physiol. 1965 Dec;181(3):576–593. doi: 10.1113/jphysiol.1965.sp007784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W., Pearlman A. L. Cat colour vision: evidence for more than one cone process. J Physiol. 1970 Nov;211(1):125–137. doi: 10.1113/jphysiol.1970.sp009270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Valois R. L., Albrecht D. G., Thorell L. G. Spatial frequency selectivity of cells in macaque visual cortex. Vision Res. 1982;22(5):545–559. doi: 10.1016/0042-6989(82)90113-4. [DOI] [PubMed] [Google Scholar]
- Dean A. F. The relationship between response amplitude and contrast for cat striate cortical neurones. J Physiol. 1981 Sep;318:413–427. doi: 10.1113/jphysiol.1981.sp013875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D. Laminar differences in receptive field properties of cells in cat primary visual cortex. J Physiol. 1977 Jun;268(2):391–421. doi: 10.1113/jphysiol.1977.sp011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezer V. D., Tsherbach T. A., Gauselman V. E., Bondarko V. M. Linear and non-linear properties of simple and complex receptive fields in area 17 of the cat visual cortex. A model of the field. Biol Cybern. 1980;37(4):195–208. doi: 10.1007/BF00337038. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields of single neurones in the cat's striate cortex. J Physiol. 1959 Oct;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry G. H., Goodwin A. W., Bishop P. O. Spatial summation of responses in receptive fields of single cells in cat striate cortex. Exp Brain Res. 1978 Jun 19;32(2):245–266. doi: 10.1007/BF00239730. [DOI] [PubMed] [Google Scholar]
- Henry G. H. Receptive field classes of cells in the striate cortex of the cat. Brain Res. 1977 Sep 9;133(1):1–28. doi: 10.1016/0006-8993(77)90045-2. [DOI] [PubMed] [Google Scholar]
- Kulikowski J. J., Bishop P. O., Kato H. Spatial arrangements of responses by cells in the cat visual cortex to light and dark bars and edges. Exp Brain Res. 1981;44(4):371–385. doi: 10.1007/BF00238830. [DOI] [PubMed] [Google Scholar]
- Kulikowski J. J., Bishop P. O. Linear analysis of the responses of simple cells in the cat visual cortex. Exp Brain Res. 1981;44(4):386–400. doi: 10.1007/BF00238831. [DOI] [PubMed] [Google Scholar]
- Kulikowski J. J., Bishop P. O. Silent periodic cells in the cat striate cortex. Vision Res. 1982;22(1):191–200. doi: 10.1016/0042-6989(82)90182-1. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A. The visual cortex as a spatial frequency analyser. Vision Res. 1973 Jul;13(7):1255–1267. doi: 10.1016/0042-6989(73)90201-0. [DOI] [PubMed] [Google Scholar]
- Maffei L., Morrone C., Pirchio M., Sandini G. Responses of visual cortical cells to periodic and non-periodic stimuli. J Physiol. 1979 Nov;296:27–47. doi: 10.1113/jphysiol.1979.sp012989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill E. G., Ainsworth A. Glass-coated platinum-plated tungsten microelectrodes. Med Biol Eng. 1972 Sep;10(5):662–672. doi: 10.1007/BF02476084. [DOI] [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Receptive field organization of complex cells in the cat's striate cortex. J Physiol. 1978 Oct;283:79–99. doi: 10.1113/jphysiol.1978.sp012489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Spatial summation in the receptive fields of simple cells in the cat's striate cortex. J Physiol. 1978 Oct;283:53–77. doi: 10.1113/jphysiol.1978.sp012488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. A., Rosenquist A. C. Visual receptive fields of single striate corical units projecting to the superior colliculus in the cat. Brain Res. 1974 Feb 15;67(1):27–42. doi: 10.1016/0006-8993(74)90295-9. [DOI] [PubMed] [Google Scholar]
- Pollen D. A., Andrews B. W., Feldon S. E. Spatial frequency selectivity of periodic complex cells in the visual cortex of the cat. Vision Res. 1978;18(6):665–682. doi: 10.1016/0042-6989(78)90146-3. [DOI] [PubMed] [Google Scholar]
- Rose D. Responses of single units in cat visual cortex to moving bars of light as a function of bar length. J Physiol. 1977 Sep;271(1):1–23. doi: 10.1113/jphysiol.1977.sp011987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman S. M., Watkins D. W., Wilson J. R. Further differences in receptive field properties of simple and complex cells in cat striate cortex. Vision Res. 1976;16(9):919–927. doi: 10.1016/0042-6989(76)90221-2. [DOI] [PubMed] [Google Scholar]
- Tolhurst D. J., Thompson I. D. On the variety of spatial frequency selectivities shown by neurons in area 17 of the cat. Proc R Soc Lond B Biol Sci. 1981 Oct 14;213(1191):183–199. doi: 10.1098/rspb.1981.0061. [DOI] [PubMed] [Google Scholar]
- Tolhurst D. J., Thompson I. D. Organization of neurones preferring similar spatial frequencies in cat striate cortex. Exp Brain Res. 1982;48(2):217–227. doi: 10.1007/BF00237217. [DOI] [PubMed] [Google Scholar]


