Abstract
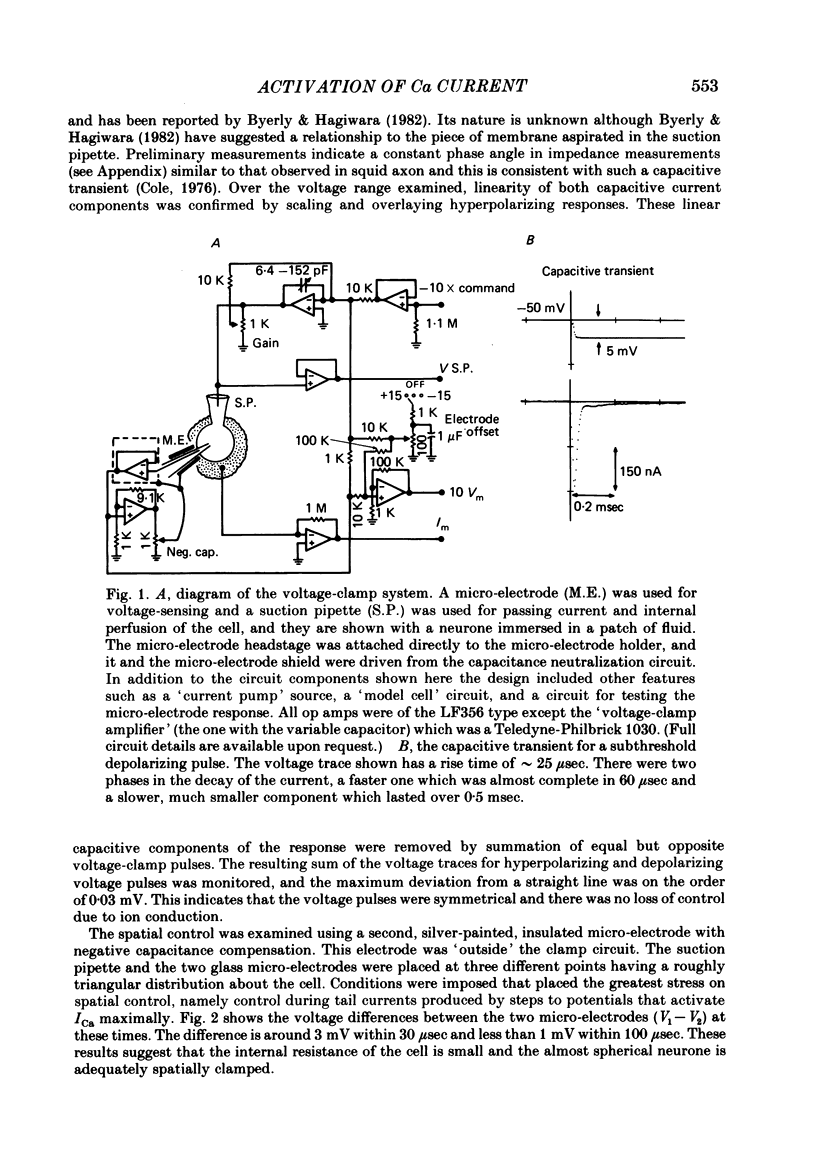
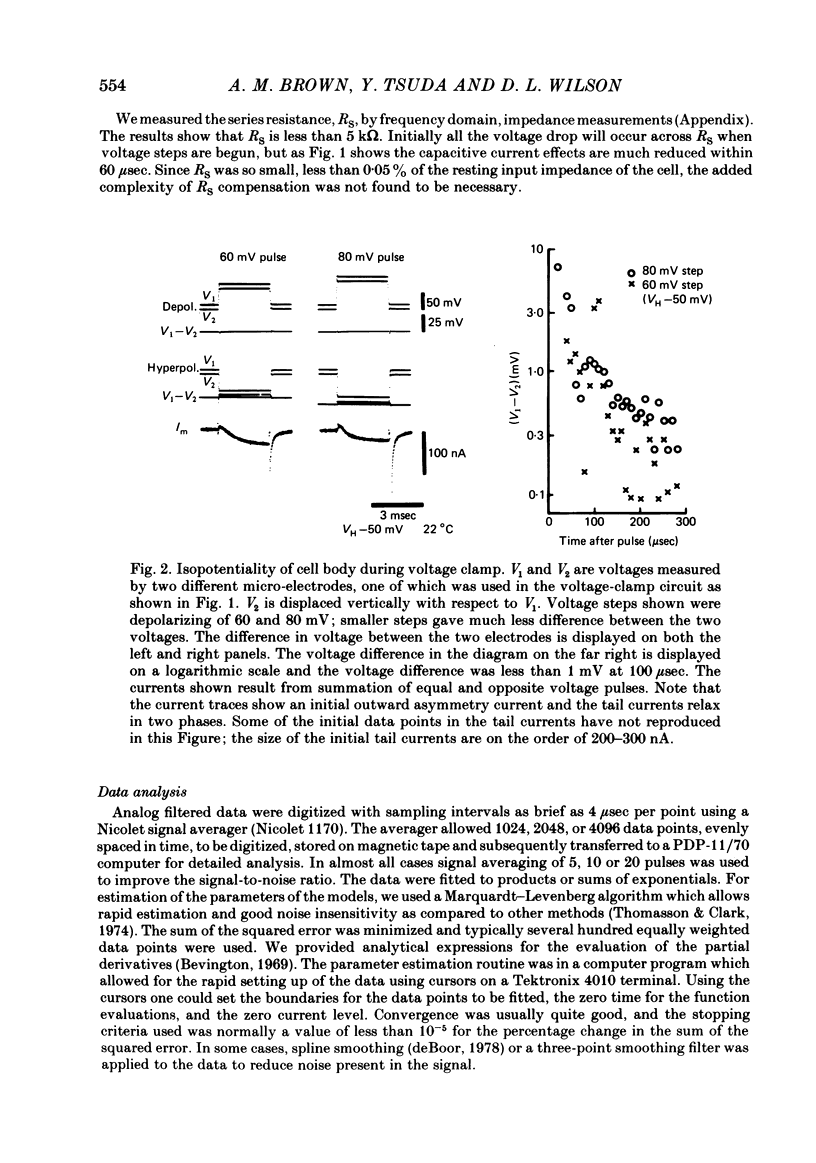
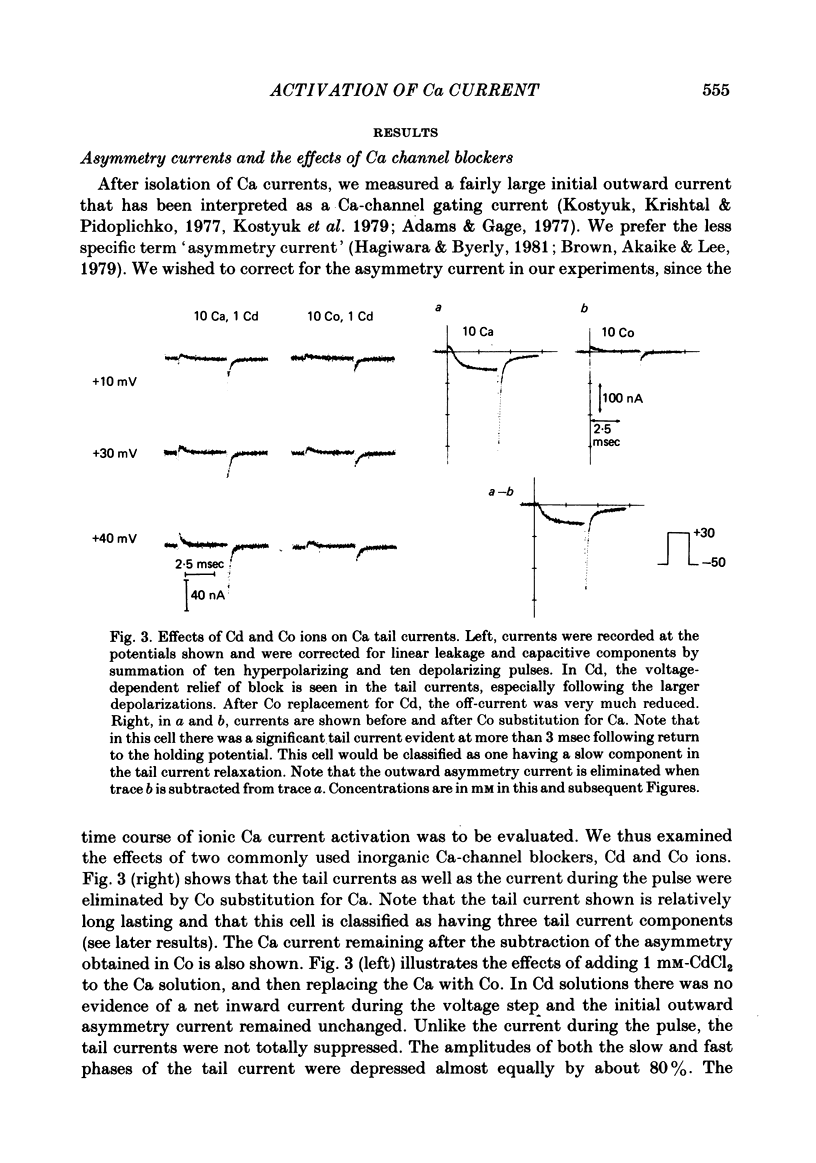
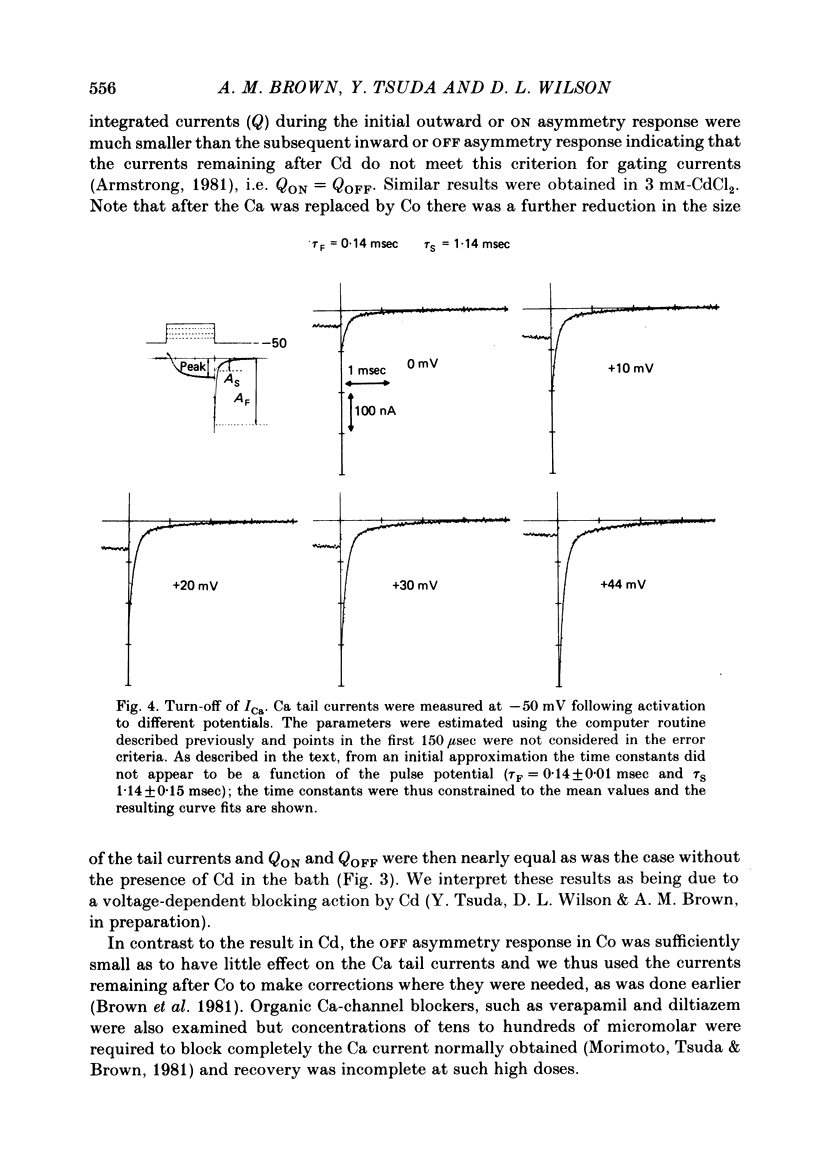
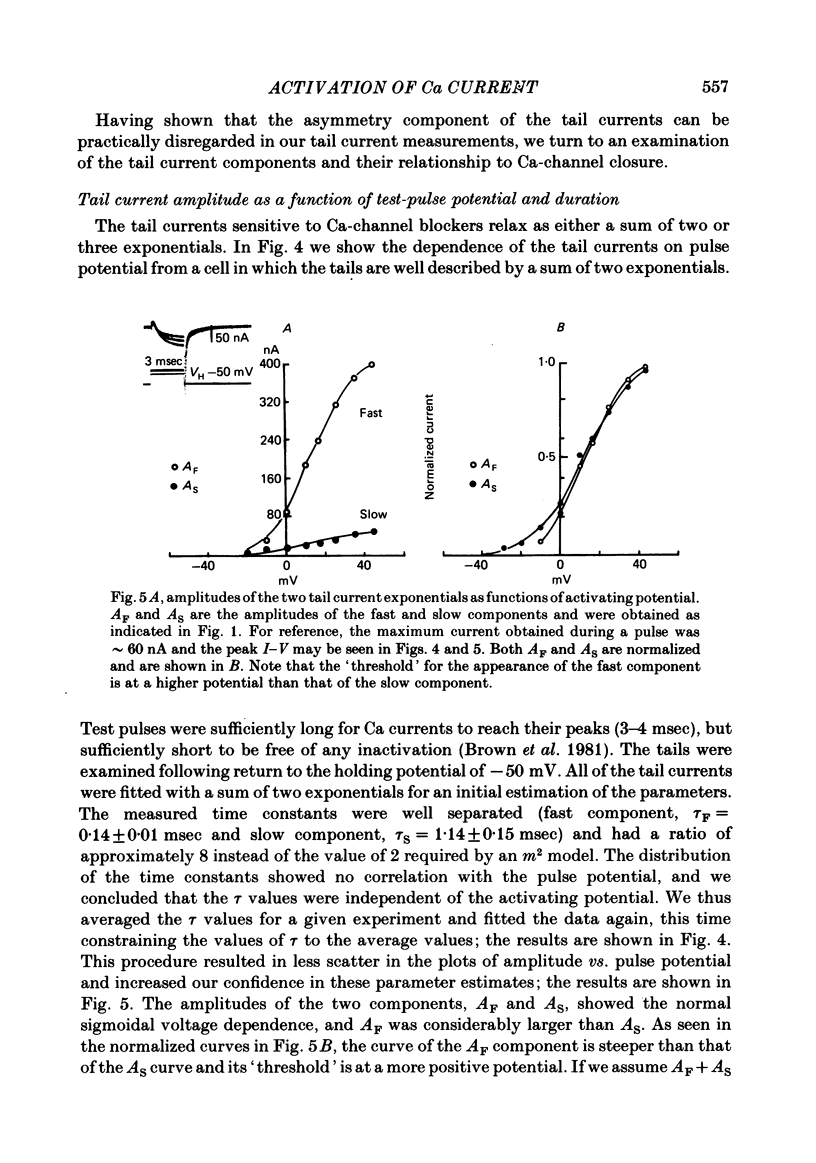
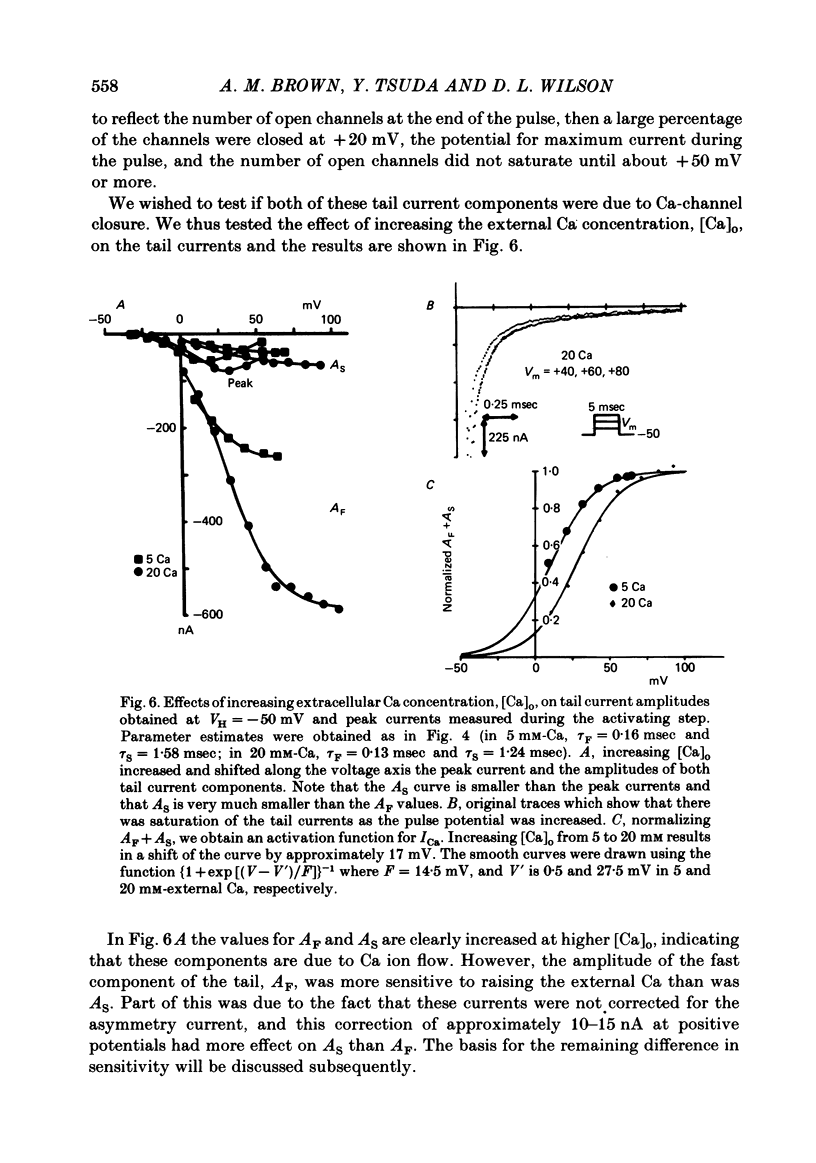
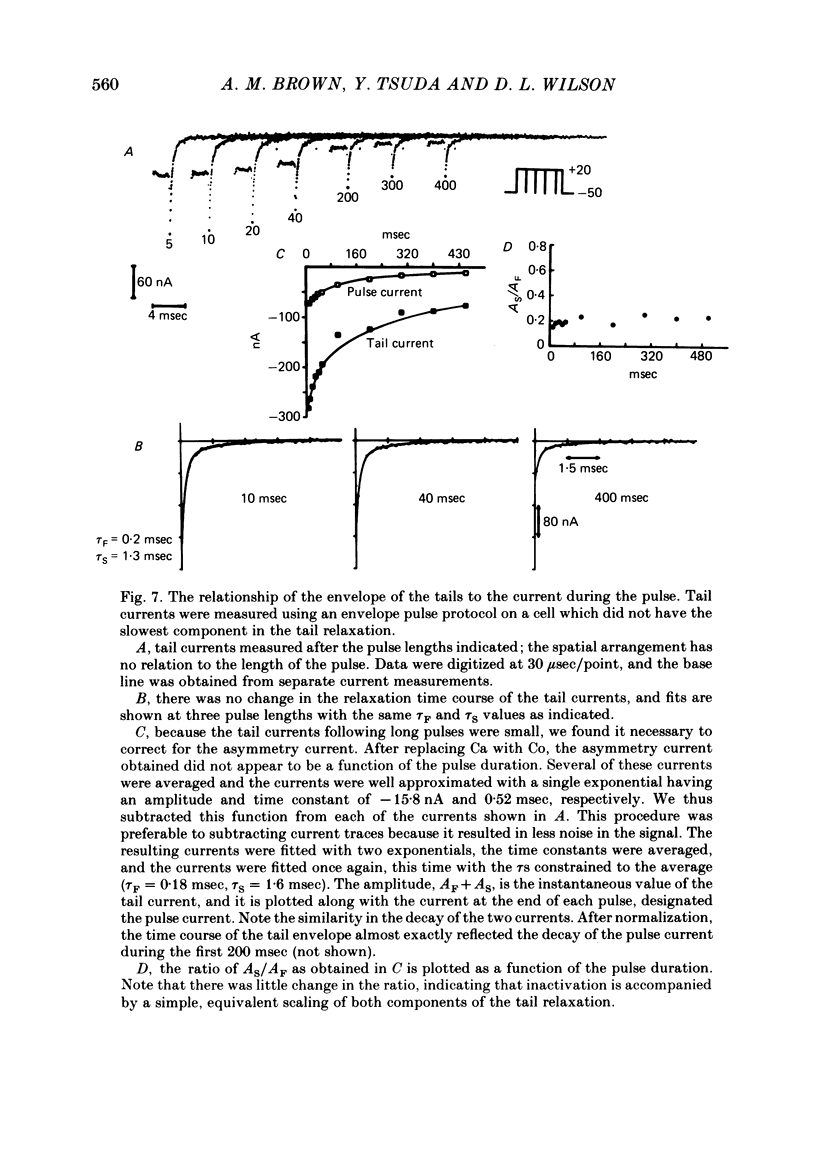
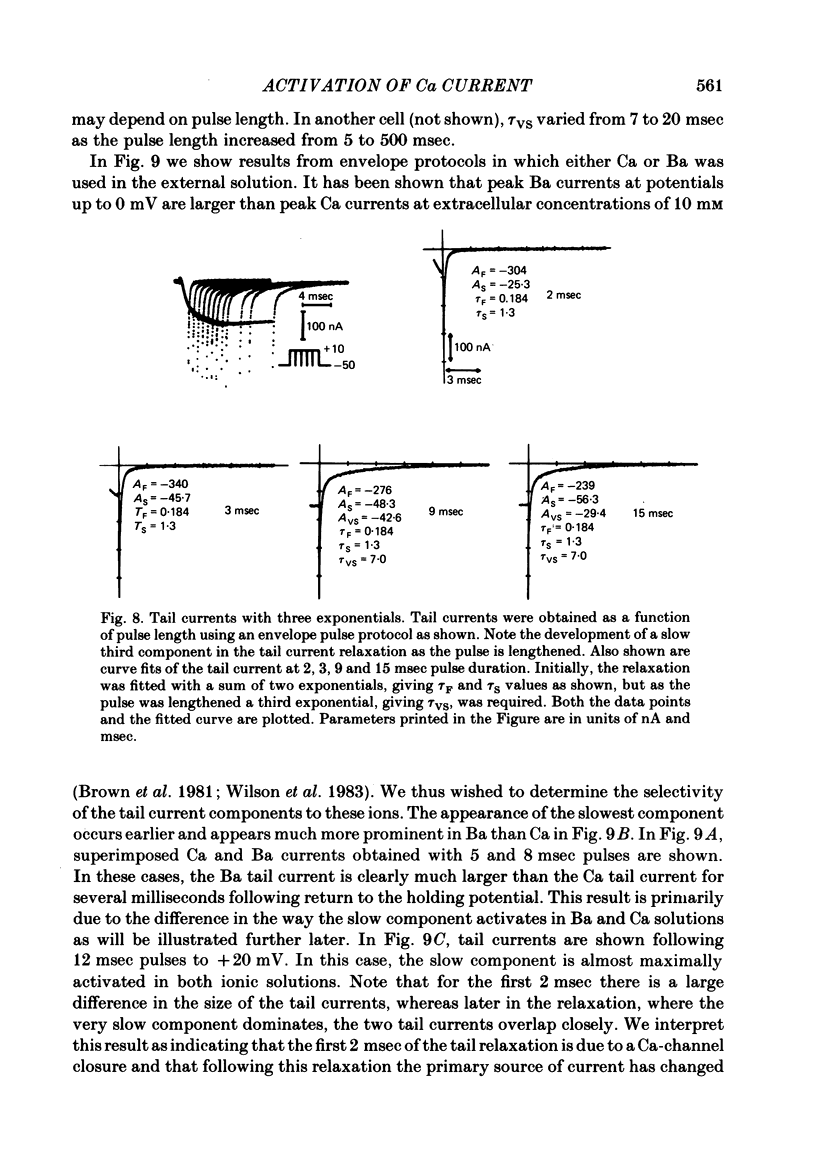
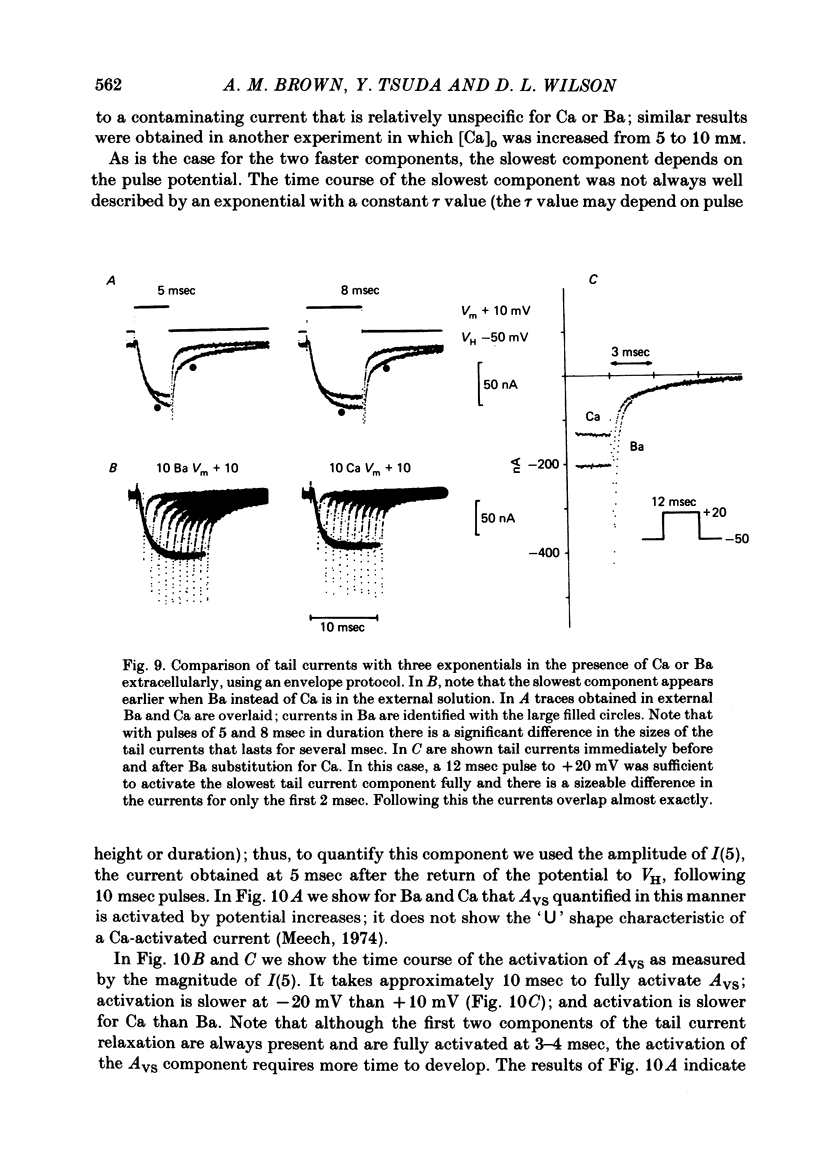
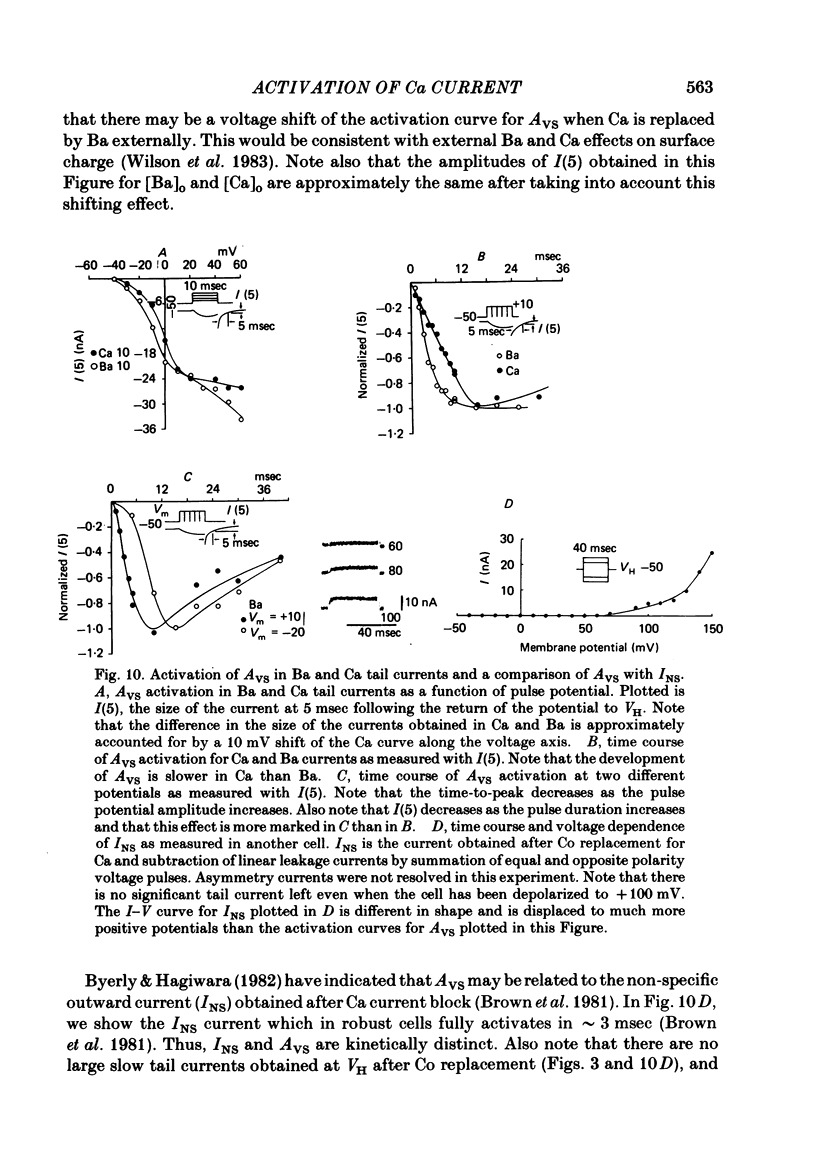
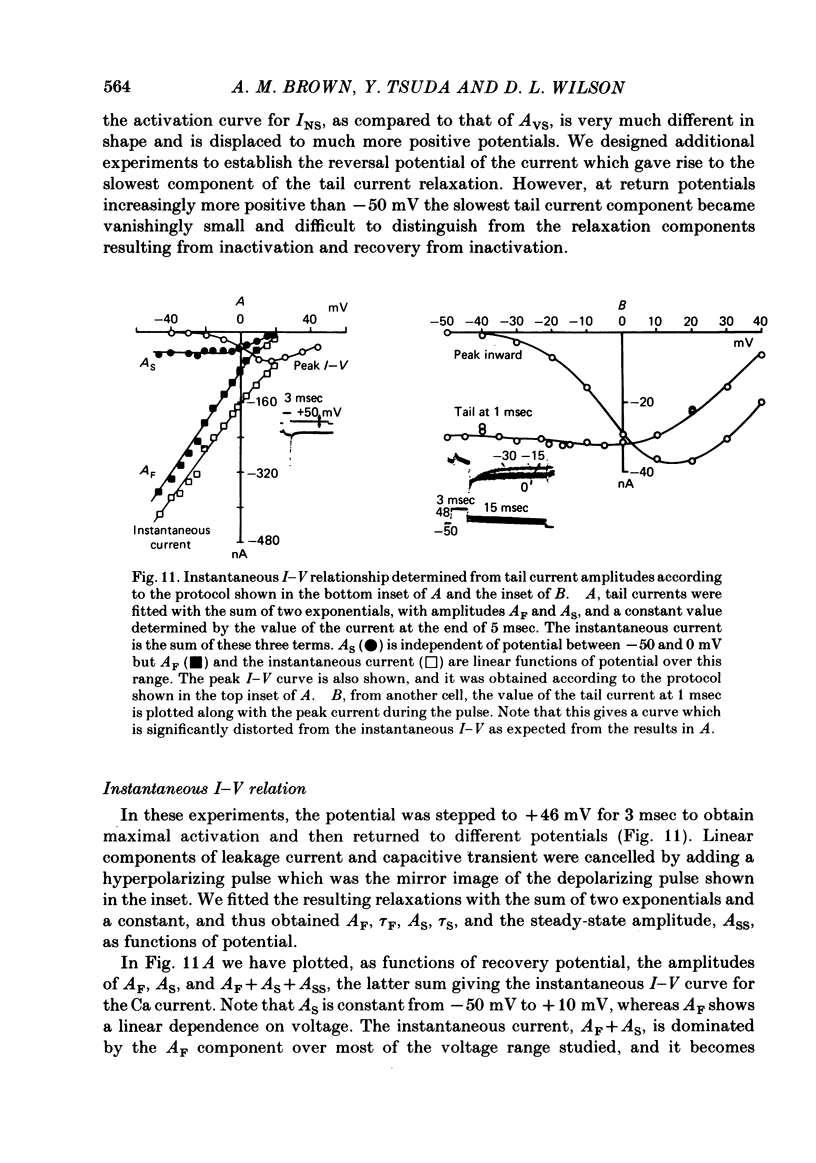
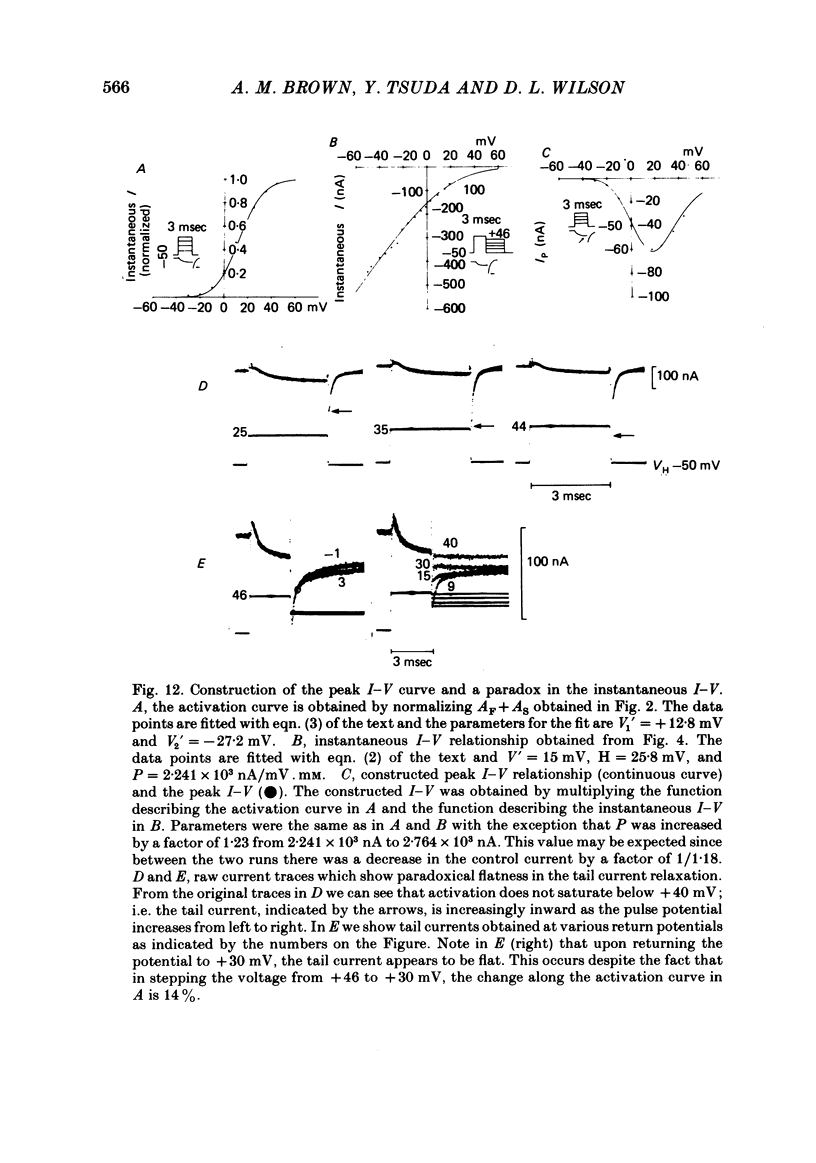
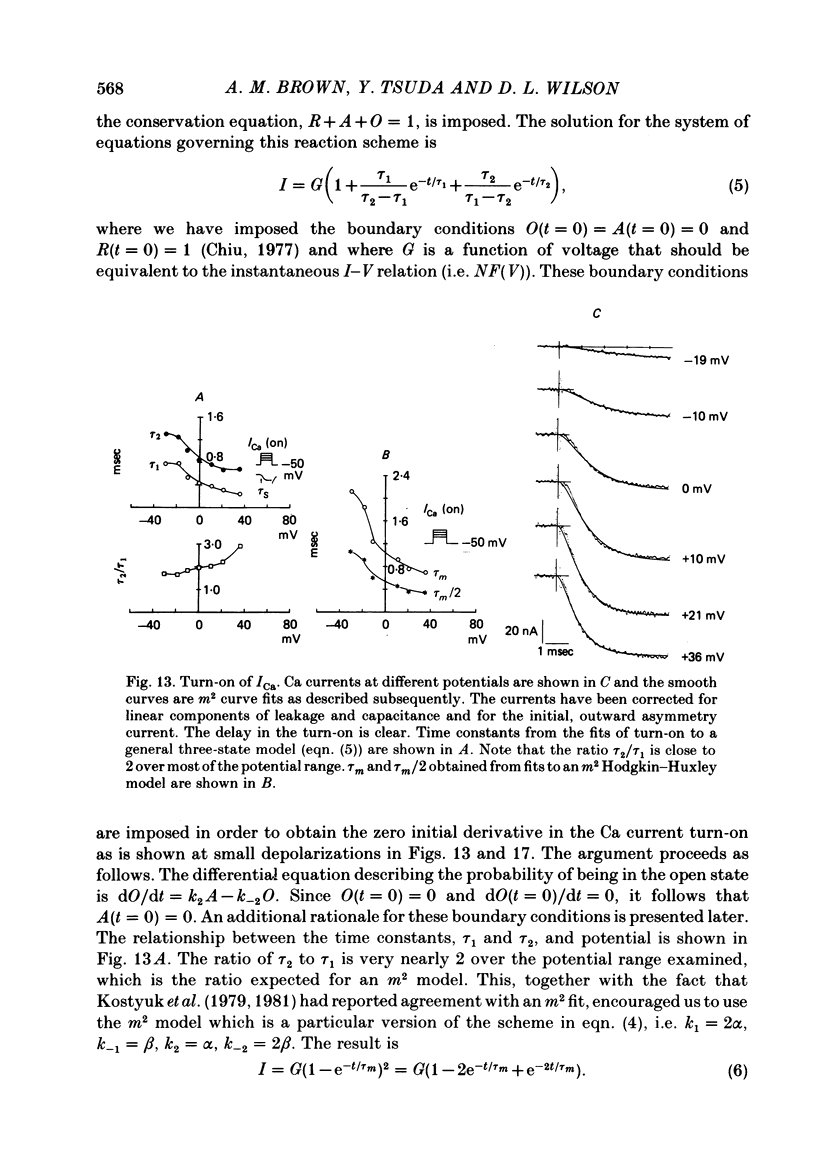
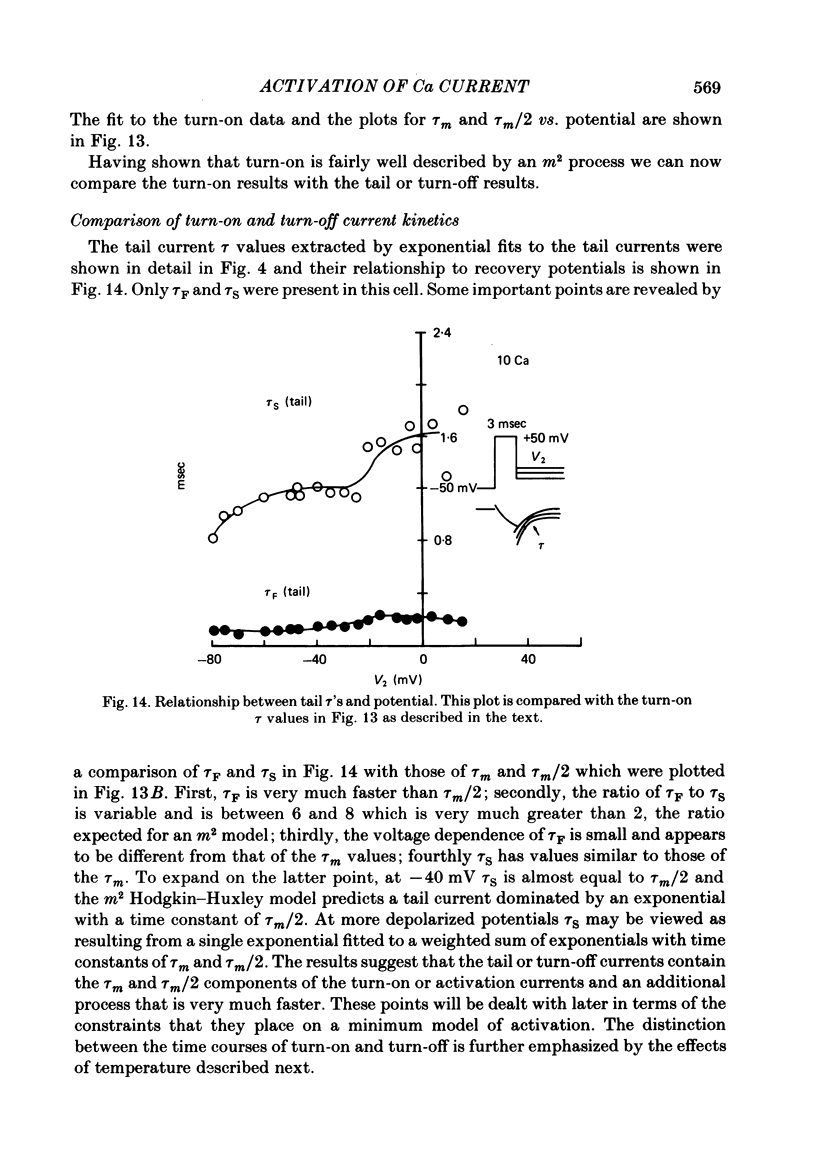
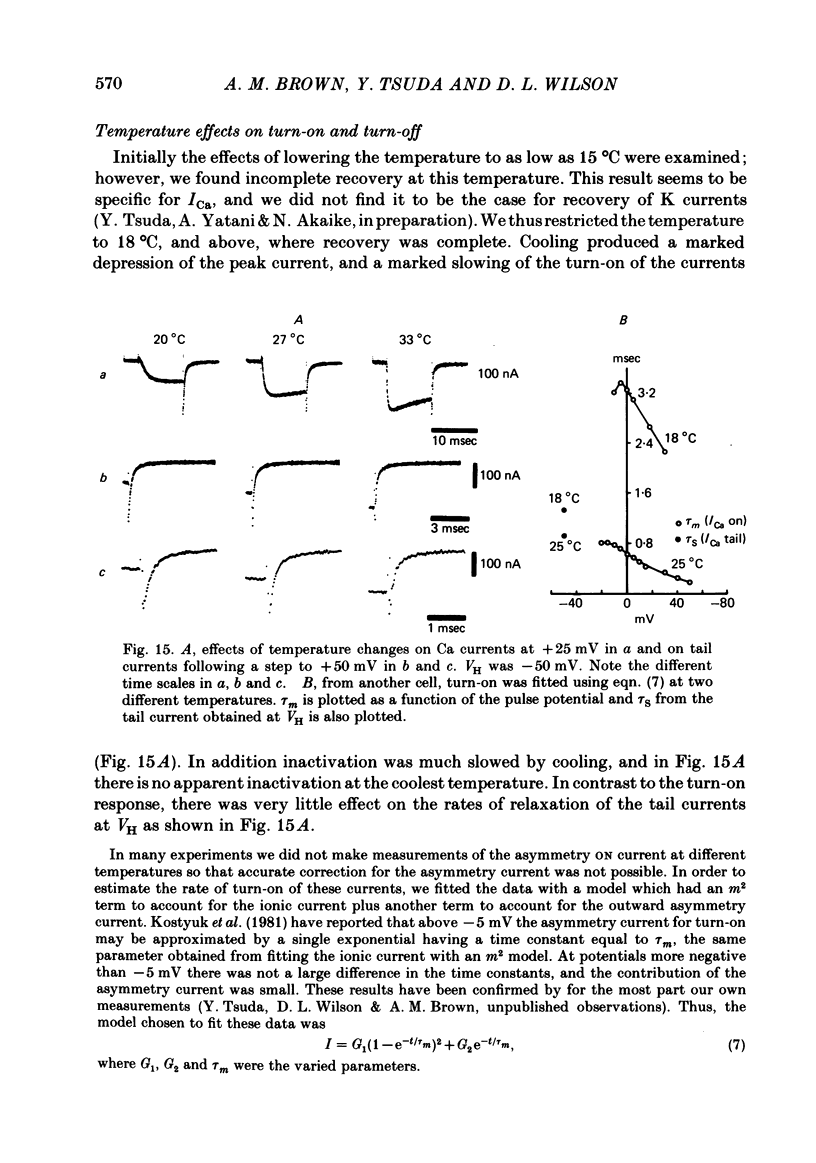
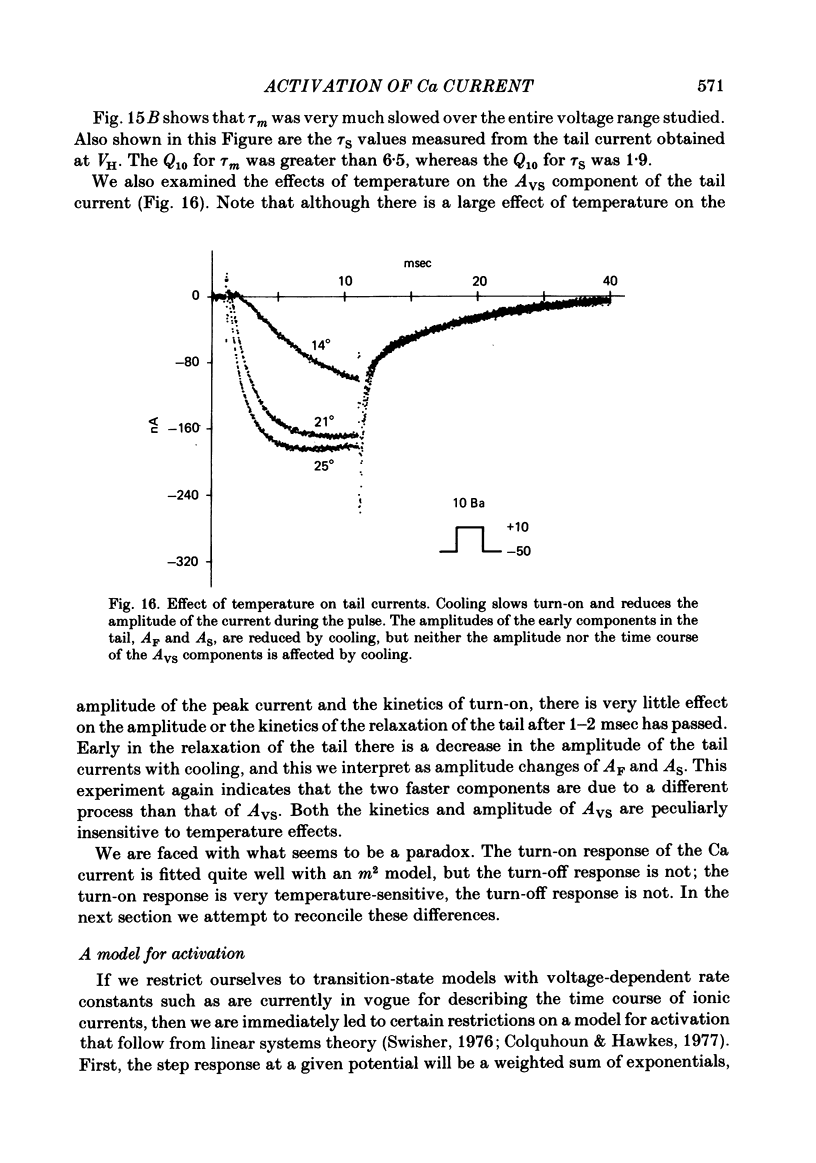
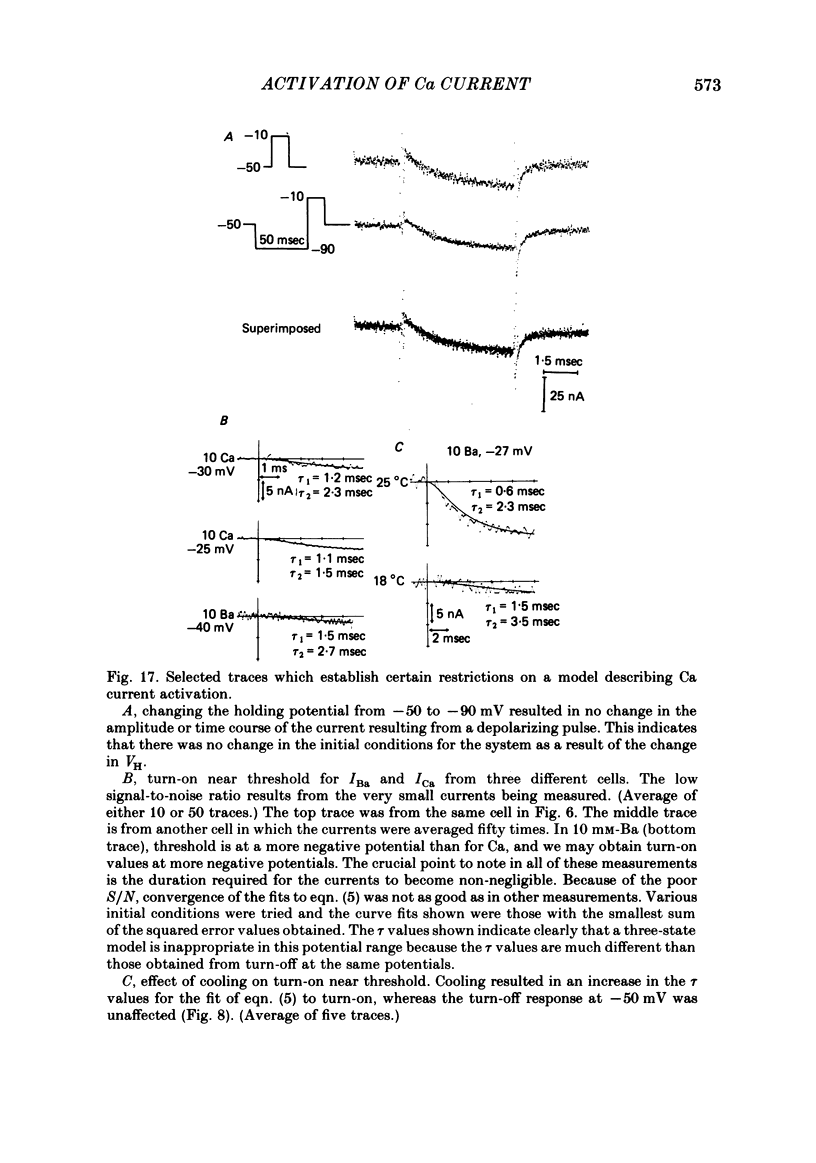
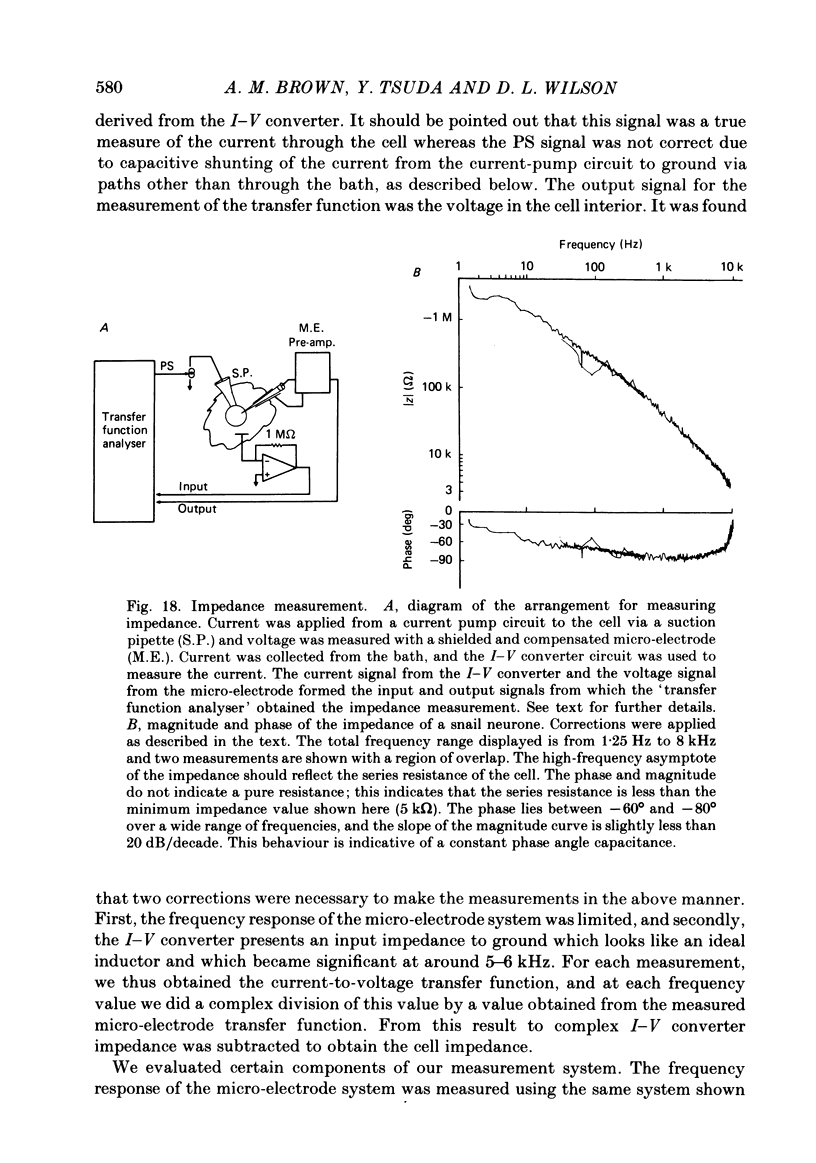
Turn-on of Ca currents, or activation, was compared with turn-off, or deactivation. The experiments were done on nerve cell bodies of Helix aspersa separated by dissection, voltage-clamped and internally perfused using a combined suction pipette--micro-electrode method. Ca currents were isolated by suppression of Na and K currents. The turn-off or tail currents were large and fast; this required that the limitations of the voltage clamp be established. A second micro-electrode was inserted to determine temporal and spatial control of potential, and it was found that the cells were essentially equipotential within 60 microsec during the largest tail currents. Series resistance was less than 5 k omega as measured by a small-perturbation, pseudorandom noise-current signal and presented negligible error in the measurements. Activation was complicated by the presence of asymmetry currents which required evaluation. This was done after Co replacement for Ca. The asymmetry currents were sufficiently small for their contribution to the tail currents to be ignored. Addition of Cd to Ca solutions could not be used since relatively large inward tail currents persisted in the presence of Cd. Tail currents were fitted by sums of two or three exponentials; each was sensitive to Ca-channel blockers but only two were due to closure of Ca channels. The two faster components with time constants tau F and tau S, for fast and slow respectively, were produced by brief, 3.0 msec voltage pulses and were present in all cells. The third and slowest component with time constant tau VS, for very slow, activated much more slowly and was not always present. The amplitudes of tau F and tau S were reduced by cooling and were increased when Ca was replaced by Ba extracellularly or when the external Ca concentration was increased. Hence, these components were due to closure of Ca channels. The third component activated faster in Ba solution. When fully activated it had the same amplitude in either Ba or Ca solution despite the differences in amplitude of Ba and Ca currents during the voltage-clamp step. The amplitude of the third component was also not changed by increasing external Ca concentration; hence it was not due to closure of Ca channels. Cooling also had very little effect. The third component was abolished by Ca blockers, but it does not appear to be related to previously described Ca-activated currents.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Lee K. S., Brown A. M. The calcium current of Helix neuron. J Gen Physiol. 1978 May;71(5):509–531. doi: 10.1085/jgp.71.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Sodium channels and gating currents. Physiol Rev. 1981 Jul;61(3):644–683. doi: 10.1152/physrev.1981.61.3.644. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Camerer H., Kunze D. L., Lux H. D. Similarity of unitary Ca2+ currents in three different species. Nature. 1982 Sep 9;299(5879):156–158. doi: 10.1038/299156a0. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Morimoto K., Tsuda Y., wilson D. L. Calcium current-dependent and voltage-dependent inactivation of calcium channels in Helix aspersa. J Physiol. 1981 Nov;320:193–218. doi: 10.1113/jphysiol.1981.sp013944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L., Hagiwara S. Calcium currents in internally perfused nerve cell bodies of Limnea stagnalis. J Physiol. 1982 Jan;322:503–528. doi: 10.1113/jphysiol.1982.sp014052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y. Inactivation of sodium channels: second order kinetics in myelinated nerve. J Physiol. 1977 Dec;273(3):573–596. doi: 10.1113/jphysiol.1977.sp012111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole K. S. Electrical properties of the squid axon sheath. Biophys J. 1976 Feb;16(2 Pt 1):137–142. doi: 10.1016/s0006-3495(76)85670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. S., Engel E. The spatial variation of membrane potential near a small source of current in a spherical cell. J Gen Physiol. 1970 Jun;55(6):736–757. doi: 10.1085/jgp.55.6.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman H. M., Poussart D., Moore L. E. Complex admittance of Na+ conduction in squid axon. J Membr Biol. 1979 Oct 5;50(1):43–63. doi: 10.1007/BF01868787. [DOI] [PubMed] [Google Scholar]
- Goldman L., Hahin R. Initial conditions and the kinetics of the sodium conductance in Myxicola giant axons. II. Relaxation experiments. J Gen Physiol. 1978 Dec;72(6):879–898. doi: 10.1085/jgp.72.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hahin R., Goldman L. Initial conditions and the kinetics of the sodium conductance in Myxicola giant axons. I. effects on the time-course of the sodium conductance. J Gen Physiol. 1978 Dec;72(6):863–877. doi: 10.1085/jgp.72.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G. Calcium ionic channels in electrically excitable membrane. Neuroscience. 1980;5(6):945–959. doi: 10.1016/0306-4522(80)90178-5. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Pidoplichko V. I. Asymmetrical displacement currents in nerve cell membrane and effect of internal fluoride. Nature. 1977 May 5;267(5606):70–72. doi: 10.1038/267070a0. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Pidoplichko V. I. Calcium inward current and related charge movements in the membrane of snail neurones. J Physiol. 1981 Jan;310:403–421. doi: 10.1113/jphysiol.1981.sp013557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Pidoplichko V. I., Shakhovalov YuA Kinetics of calcium inward current activation. J Gen Physiol. 1979 May;73(5):675–680. doi: 10.1085/jgp.73.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Akaike N., Brown A. M. The suction pipette method for internal perfusion and voltage clamp of small excitable cells. J Neurosci Methods. 1980 Feb;2(1):51–78. doi: 10.1016/0165-0270(80)90045-x. [DOI] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents in squid giant synapse. Biophys J. 1981 Mar;33(3):289–321. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux H. D., Nagy K. Single channel Ca2+ currents in Helix pomatia neurons. Pflugers Arch. 1981 Sep;391(3):252–254. doi: 10.1007/BF00596179. [DOI] [PubMed] [Google Scholar]
- Meech R. W. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974 Mar;237(2):259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saimi Y., Kung C. Are ions involved in the gating of calcium channels? Science. 1982 Oct 8;218(4568):153–156. doi: 10.1126/science.6289432. [DOI] [PubMed] [Google Scholar]
- Wilson D. L., Morimoto K., Tsuda Y., Brown A. M. Interaction between calcium ions and surface charge as it relates to calcium currents. J Membr Biol. 1983;72(1-2):117–130. doi: 10.1007/BF01870319. [DOI] [PubMed] [Google Scholar]


