Abstract
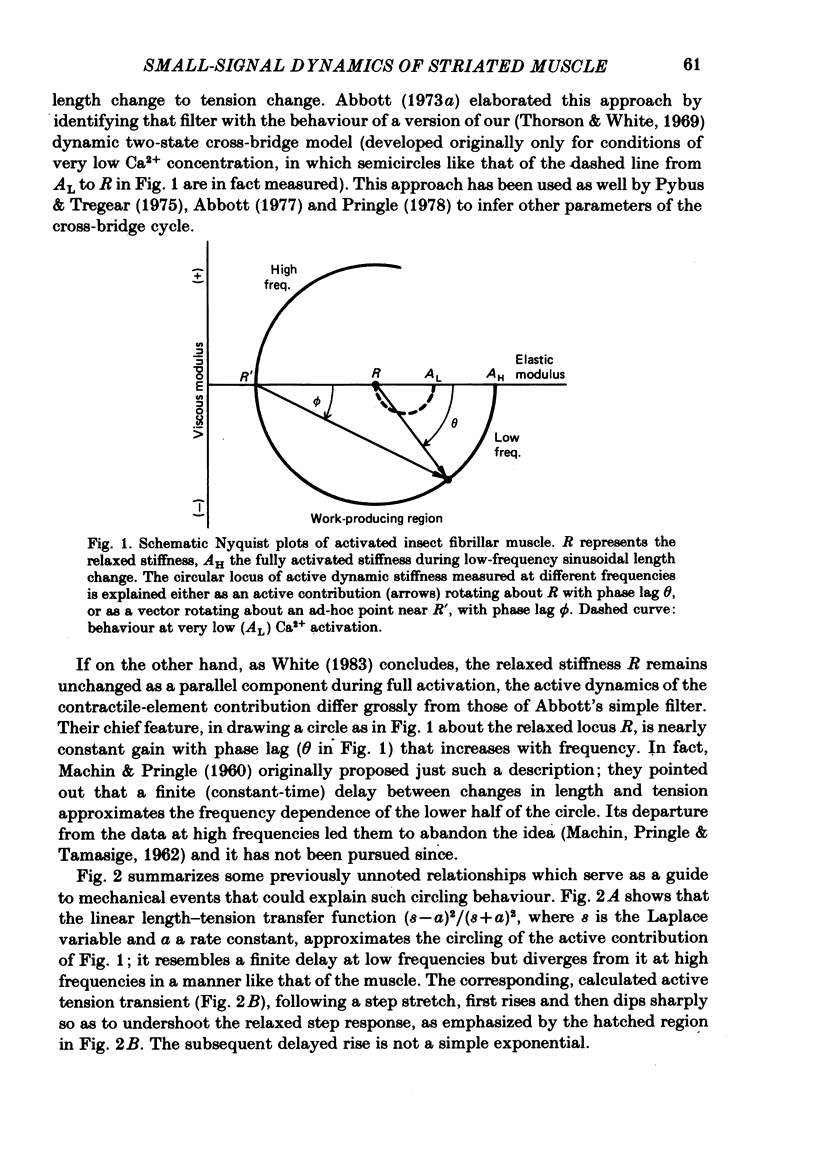
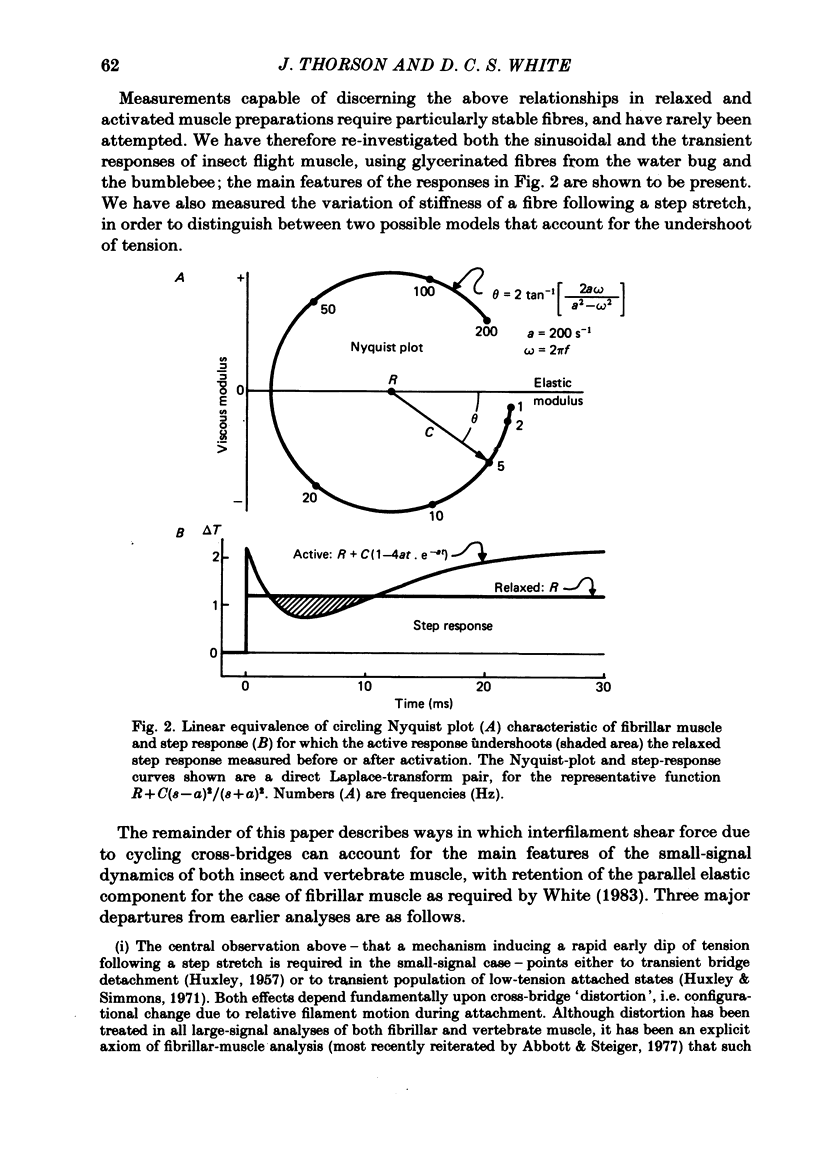
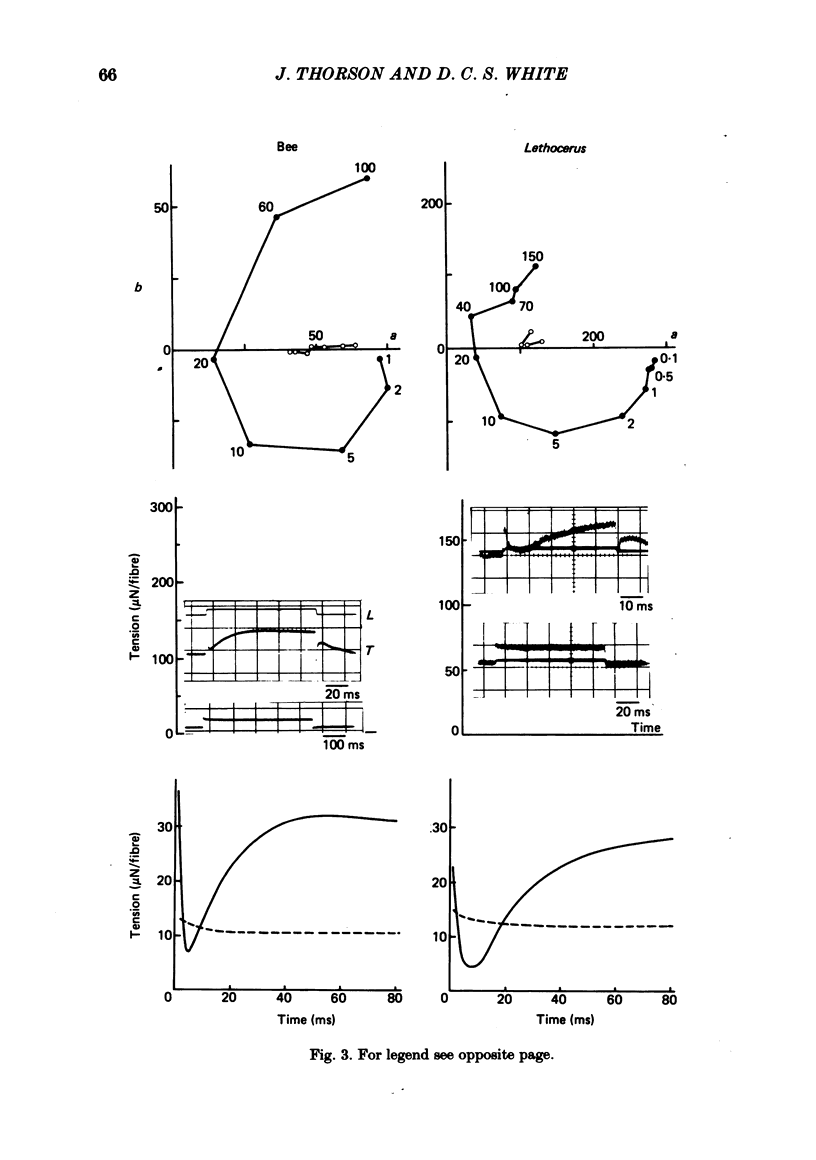
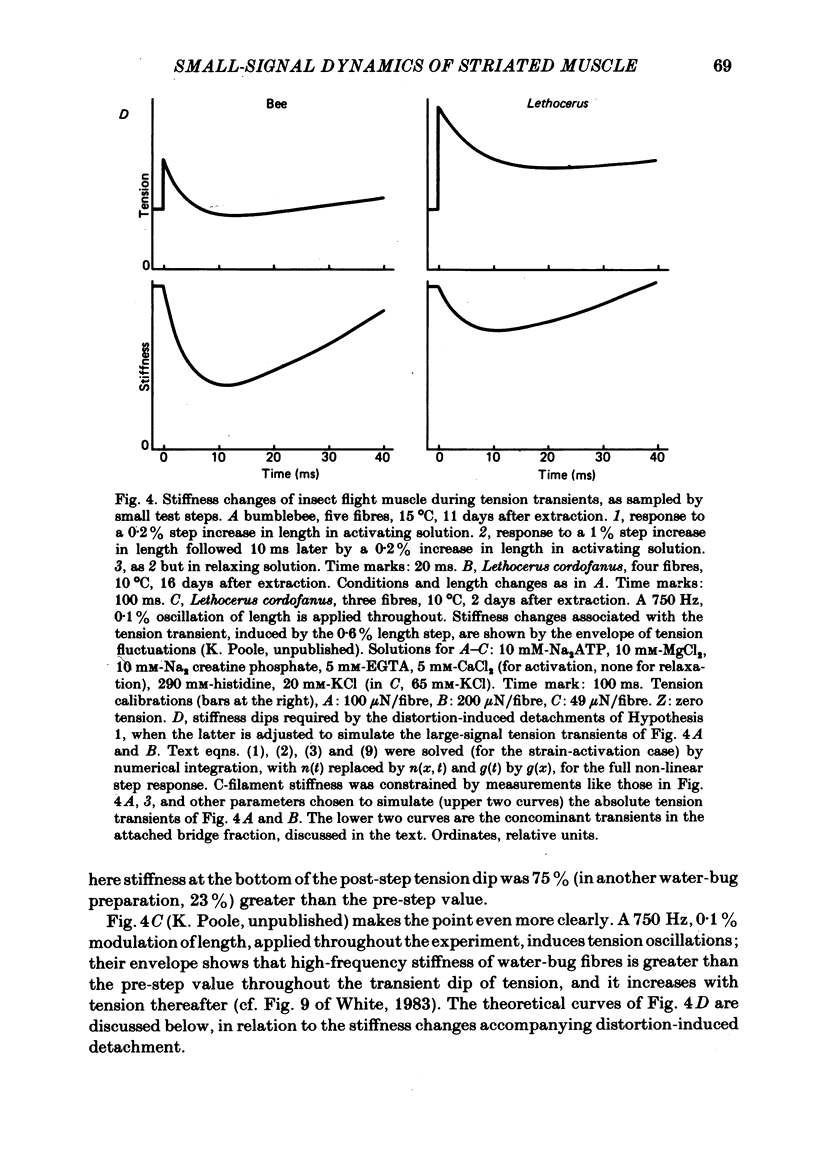
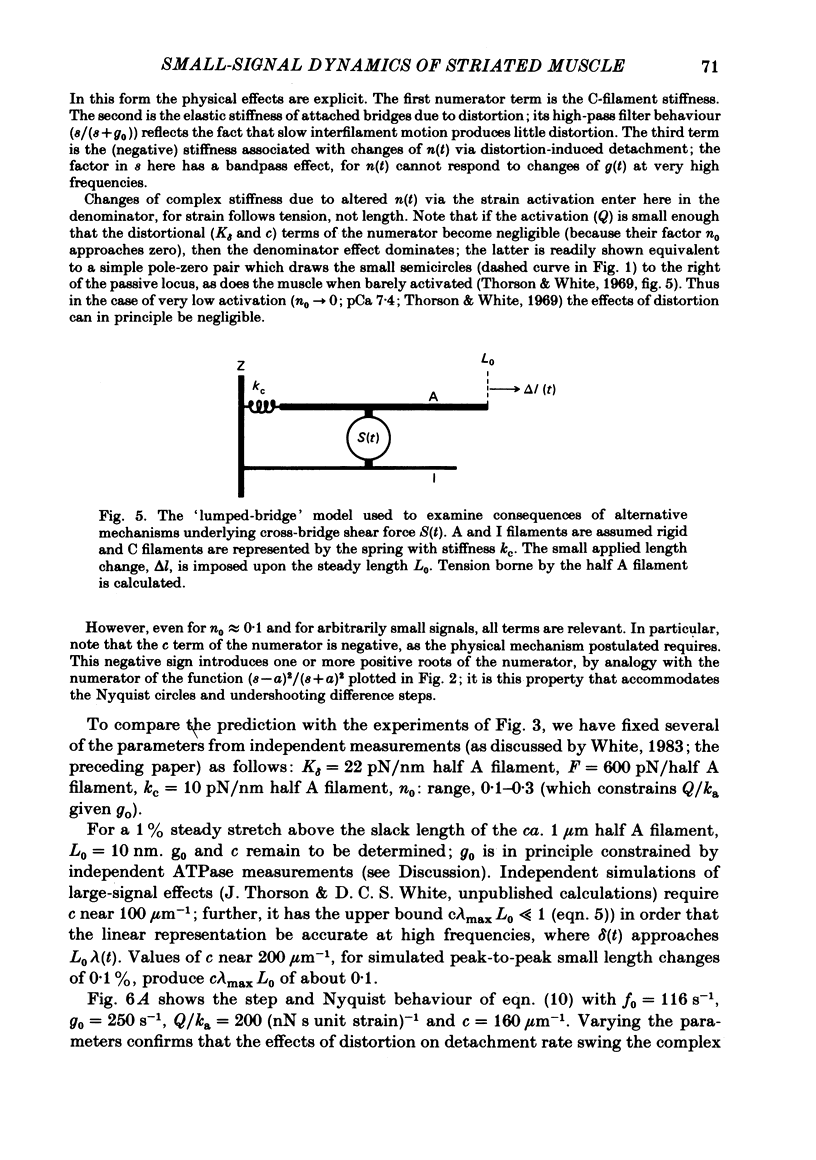
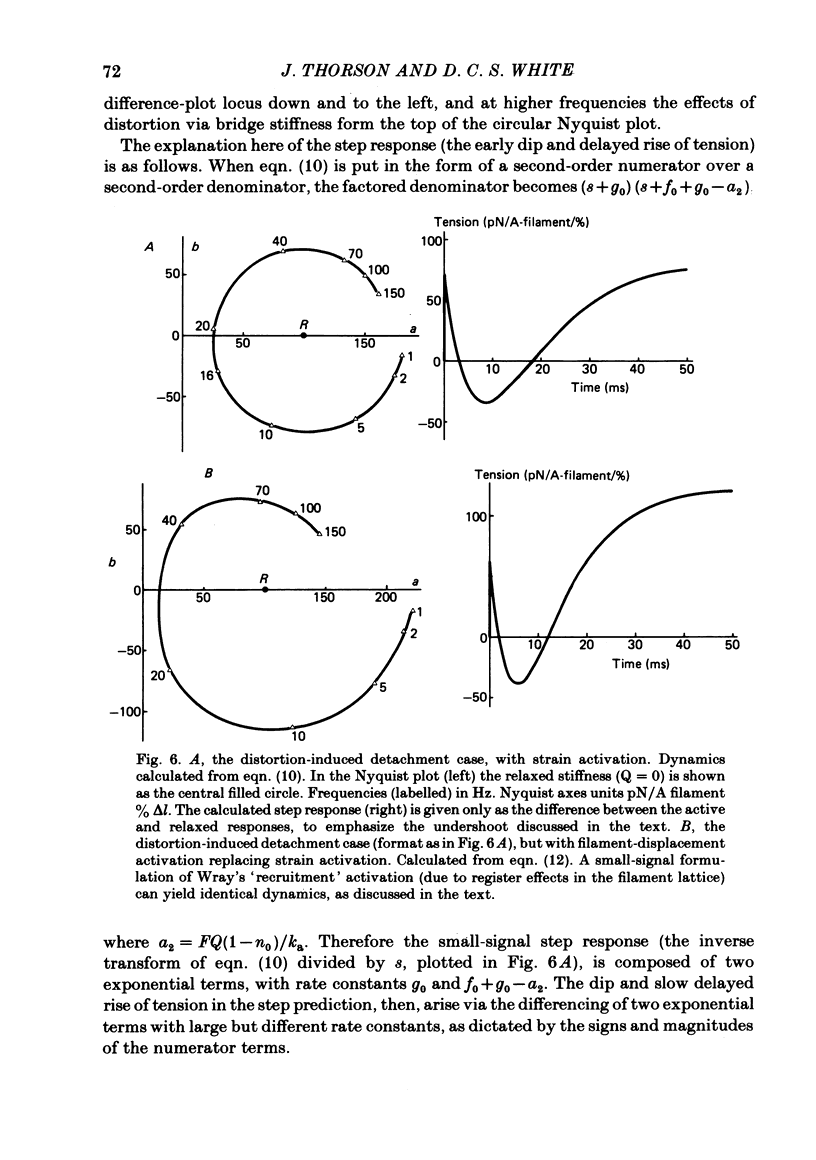
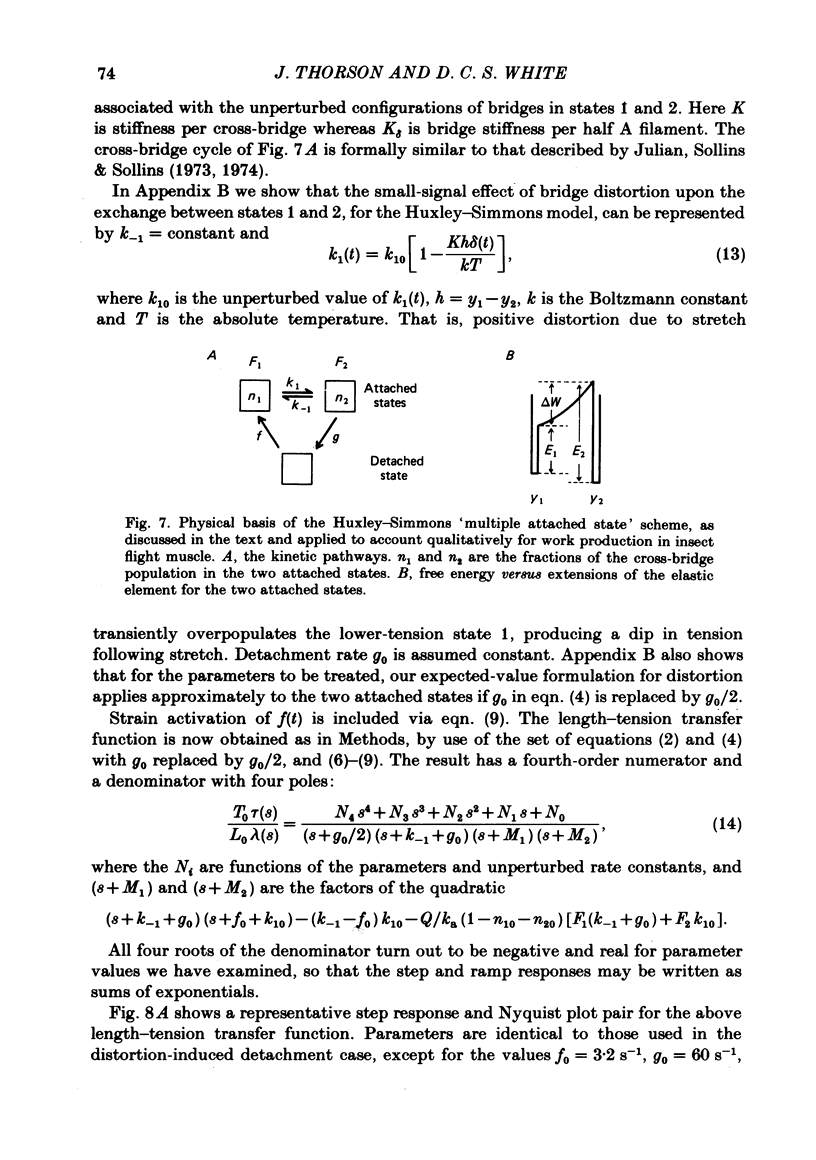
The mechanism of the active tension response of insect fibrillar muscle to step changes and small oscillations of length was re-investigated, following White's demonstration (1983) that the high relaxed stiffness evidently persists during activation and cannot be neglected as had previously been assumed. White's result makes earlier explanations of the small-signal response untenable; the experimental and theoretical studies described here lead to a new class of explanations at the cross-bridge level. The response of an activated muscle to a fast stretch consists of a synchronous tension increase that is followed first by a rapid decay of tension and then by a delayed rise ('stretch activation'). It was shown in glycerinated fibre preparations from the water bug and the bumblebee that subtraction of the relaxed tension response from the active response results in a prominent undershoot of the tension level preceding the step, before the delayed rise of tension. The responses of the same fibres to sinusoidal oscillations, in the frequency range 1-150 Hz, showed an equivalent behaviour, with the active locus circling the relaxed locus in a Nyquist plot, as described by Machin & Pringle (1960). Stiffness was determined during the tension response to a large step (of 1%) by recording the immediate change of tension to a small test step (0.2%), applied at various times after the conditioning step. In the majority of preparations stiffness remained constant or increased during the undershoot of tension. Step and sinusoidal responses with the above features cannot be explained at all by an active component resembling a simple exponential delay. We show, however, that such features are predicted if certain small-signal effects of cross-bridge distortion (previously and erroneously assumed negligible in insect flight muscle for the small-signal case) are incorporated in models of the cross-bridge cycle. Two alternative hypotheses for the effects of distortion are examined: (i) distortion-induced detachments and (ii) distortion-modulated transitions among multiple attached states (Huxley & Simmons, 1971). For the first we also show that the results do not differ qualitatively whether one assumes strain, interfilament displacement or 'bridge recruitment' as the physical correlate of stretch activation. Both of the above explanations account, at least qualitatively, for the observed rapid decay and undershoot of tension following a step increase of length, and for the circling of the active Nyquist-plot loci about the passive locus. The explanation based on distortion-induced detachments, however, appears to be inconsistent with the stiffness measurements.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott R. H., Cage P. E. Periodicity in insect flight muscle stretch activation [proceedings]. J Physiol. 1979 Apr;289:32P–33P. [PubMed] [Google Scholar]
- Abbott R. H., Steiger G. J. Temperature and amplitude dependence of tension transients in glycerinated skeletal and insect fibrillar muscle. J Physiol. 1977 Mar;266(1):13–42. doi: 10.1113/jphysiol.1977.sp011754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott R. H. The effects of fibre length and calcium ion concentration on the dynamic response of glycerol extracted insect fibrillar muscle. J Physiol. 1973 Jun;231(2):195–208. doi: 10.1113/jphysiol.1973.sp010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplain R. A., Frommelt B. On the contractile mechanism of insect fibrillar flight muscle. I. The dynamics and energetics of the linearized system. Kybernetik. 1968 Jul;5(1):1–17. doi: 10.1007/BF00288894. [DOI] [PubMed] [Google Scholar]
- Cuminetti R., Rossmanith G. Small amplitude non-linearities in the mechanical response of an asynchronous flight muscle. J Muscle Res Cell Motil. 1980 Sep;1(3):345–356. doi: 10.1007/BF00711935. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. The relation between stiffness and filament overlap in stimulated frog muscle fibres. J Physiol. 1981 Feb;311:219–249. doi: 10.1113/jphysiol.1981.sp013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Hill T. L. Theoretical formalism for the sliding filament model of contraction of striated muscle. Part I. Prog Biophys Mol Biol. 1974;28:267–340. doi: 10.1016/0079-6107(74)90020-0. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Julian F. J., Sollins K. R., Sollins M. R. A model for the transient and steady-state mechanical behavior of contracting muscle. Biophys J. 1974 Jul;14(7):546–562. doi: 10.1016/S0006-3495(74)85934-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M., Brandt P. W. Sinusoidal analysis: a high resolution method for correlating biochemical reactions with physiological processes in activated skeletal muscles of rabbit, frog and crayfish. J Muscle Res Cell Motil. 1980 Sep;1(3):279–303. doi: 10.1007/BF00711932. [DOI] [PubMed] [Google Scholar]
- Kawai M. Head rotation or dissociation? A study of exponential rate processes in chemically skinned rabbit muscle fibers when MgATP concentration is changed. Biophys J. 1978 Apr;22(1):97–103. doi: 10.1016/S0006-3495(78)85473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACHIN K. E., PRINGLE J. W. The physiology of insect fibrillar muscle. III. The effect of sinusoidal changes of length on a beetle flight muscle. Proc R Soc Lond B Biol Sci. 1960 Jun 14;152:311–330. doi: 10.1098/rspb.1960.0041. [DOI] [PubMed] [Google Scholar]
- Pringle J. W. The Croonian Lecture, 1977. Stretch activation of muscle: function and mechanism. Proc R Soc Lond B Biol Sci. 1978 May 5;201(1143):107–130. doi: 10.1098/rspb.1978.0035. [DOI] [PubMed] [Google Scholar]
- Pybus J., Tregear R. T. The relationship of adenosine triphosphatase activity to tension and power output of insect flight muscle. J Physiol. 1975 May;247(1):71–89. doi: 10.1113/jphysiol.1975.sp010921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorson J., Biederman-Thorson M. Distributed relaxation processes in sensory adaptation. Science. 1974 Jan 18;183(4121):161–172. doi: 10.1126/science.183.4121.161. [DOI] [PubMed] [Google Scholar]
- Thorson J., White D. C. Distributed representations for actin-myosin interaction in the oscillatory contraction of muscle. Biophys J. 1969 Mar;9(3):360–390. doi: 10.1016/S0006-3495(69)86392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C. The elasticity of relaxed insect fibrillar flight muscle. J Physiol. 1983 Oct;343:31–57. doi: 10.1113/jphysiol.1983.sp014880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Thorson J. Phosphate starvation and the nonlinear dynamics of insect fibrillar flight muscle. J Gen Physiol. 1972 Sep;60(3):307–336. doi: 10.1085/jgp.60.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]


