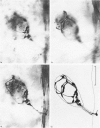
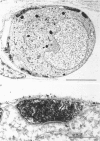
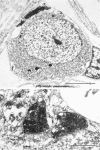
Abstract
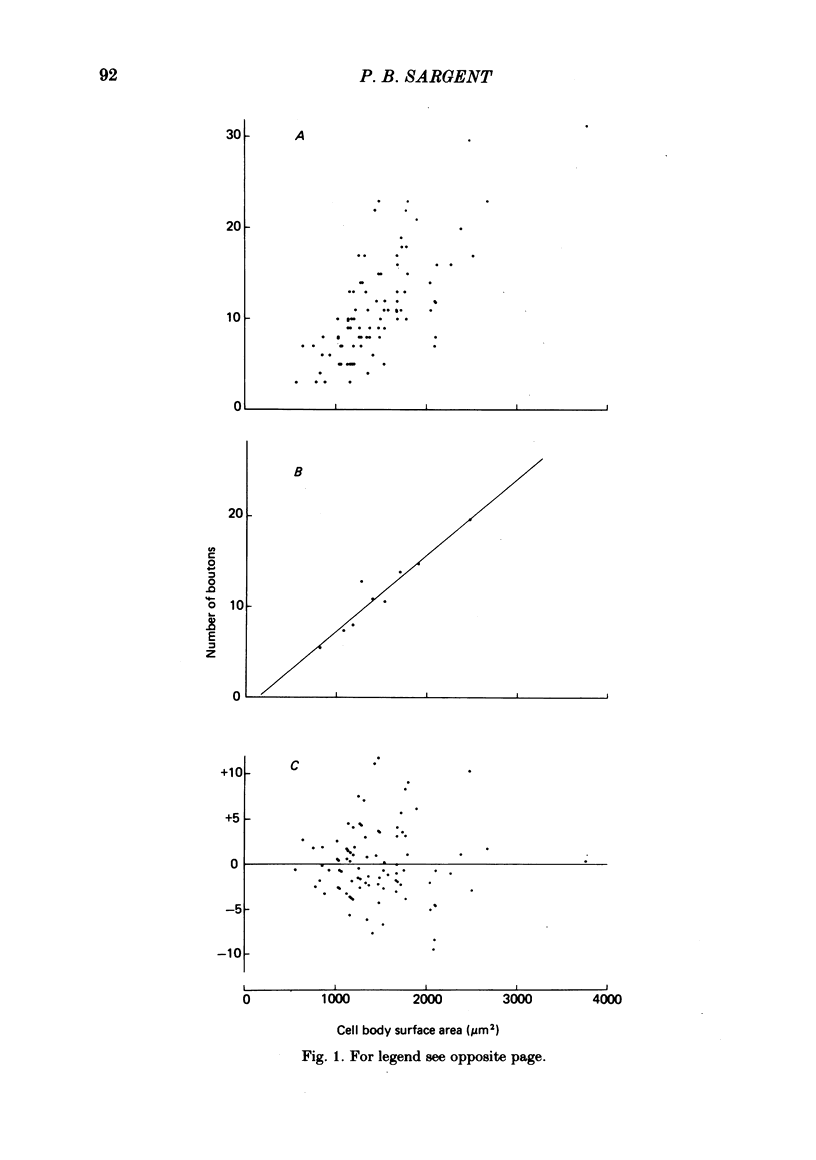
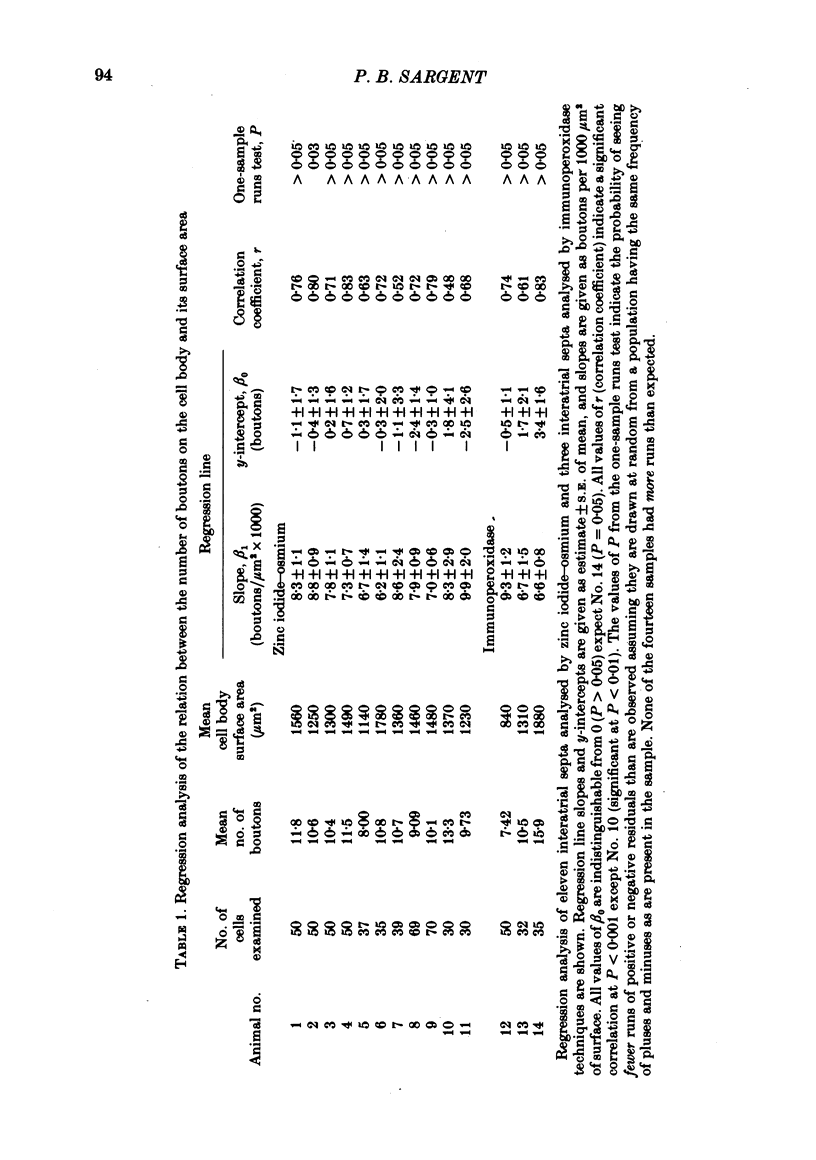
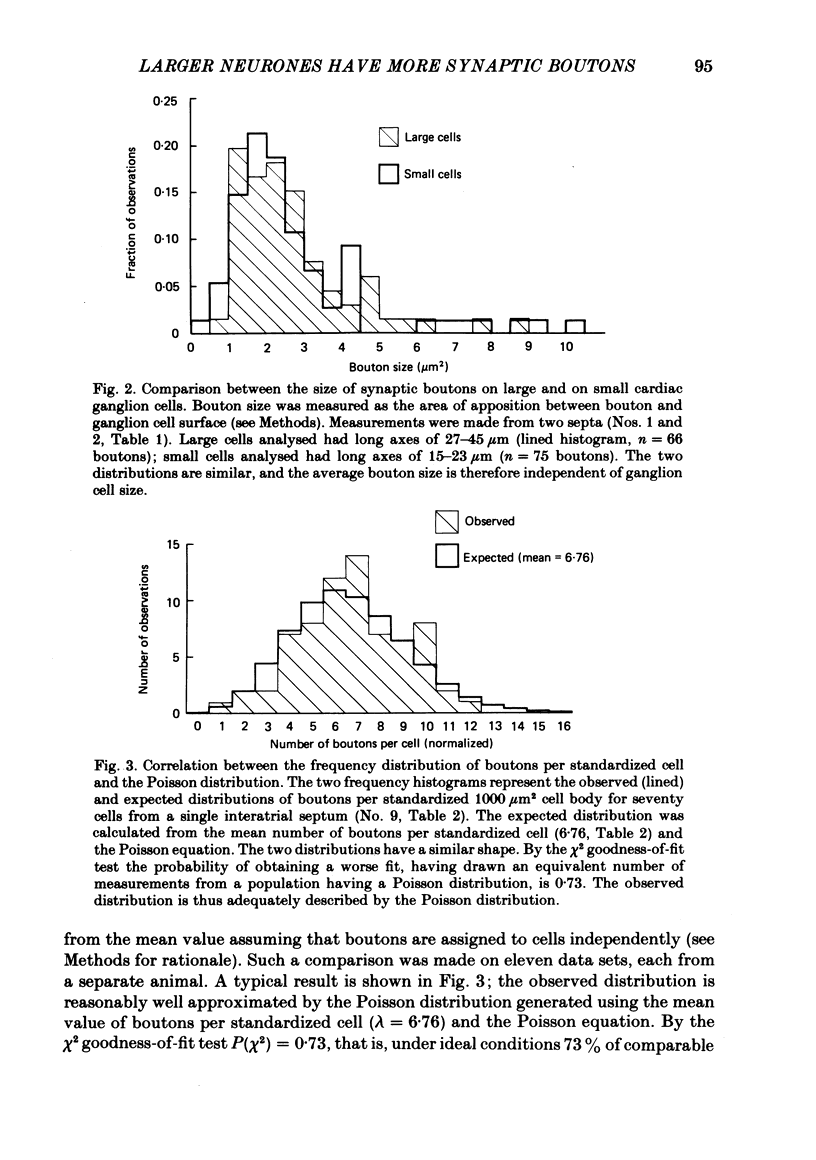
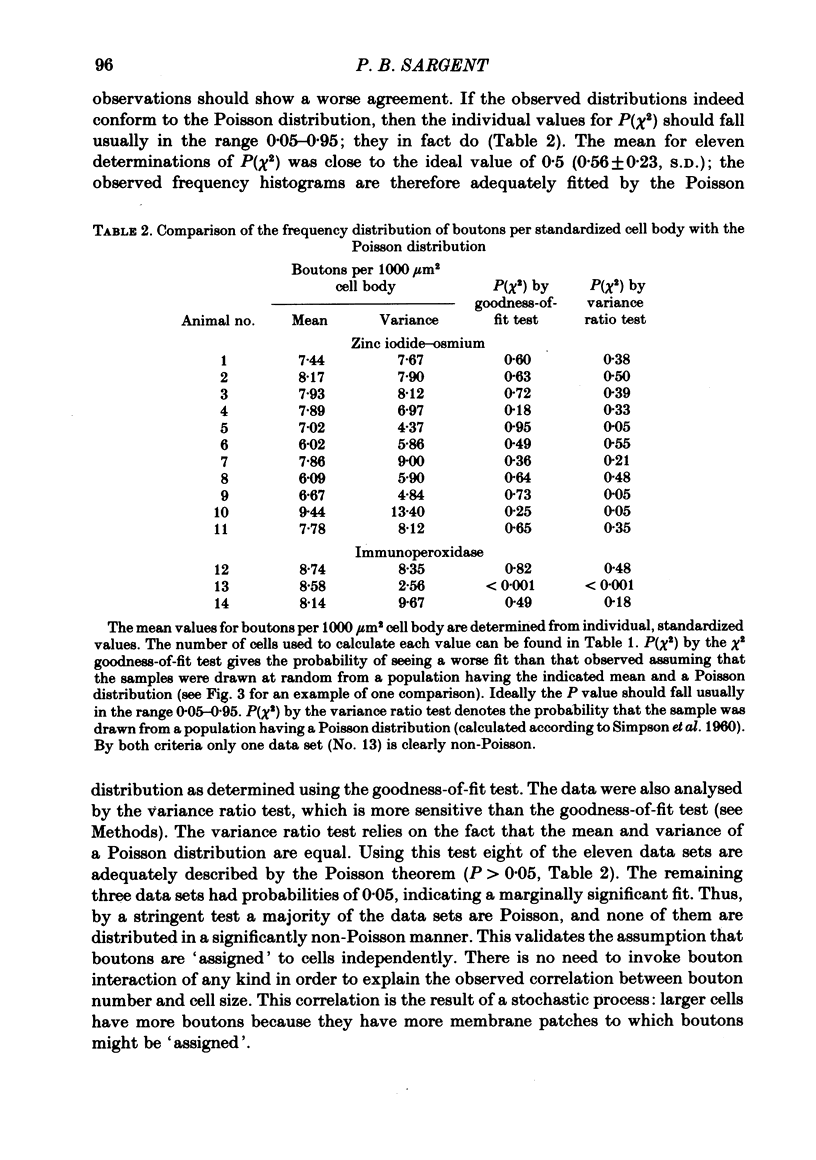
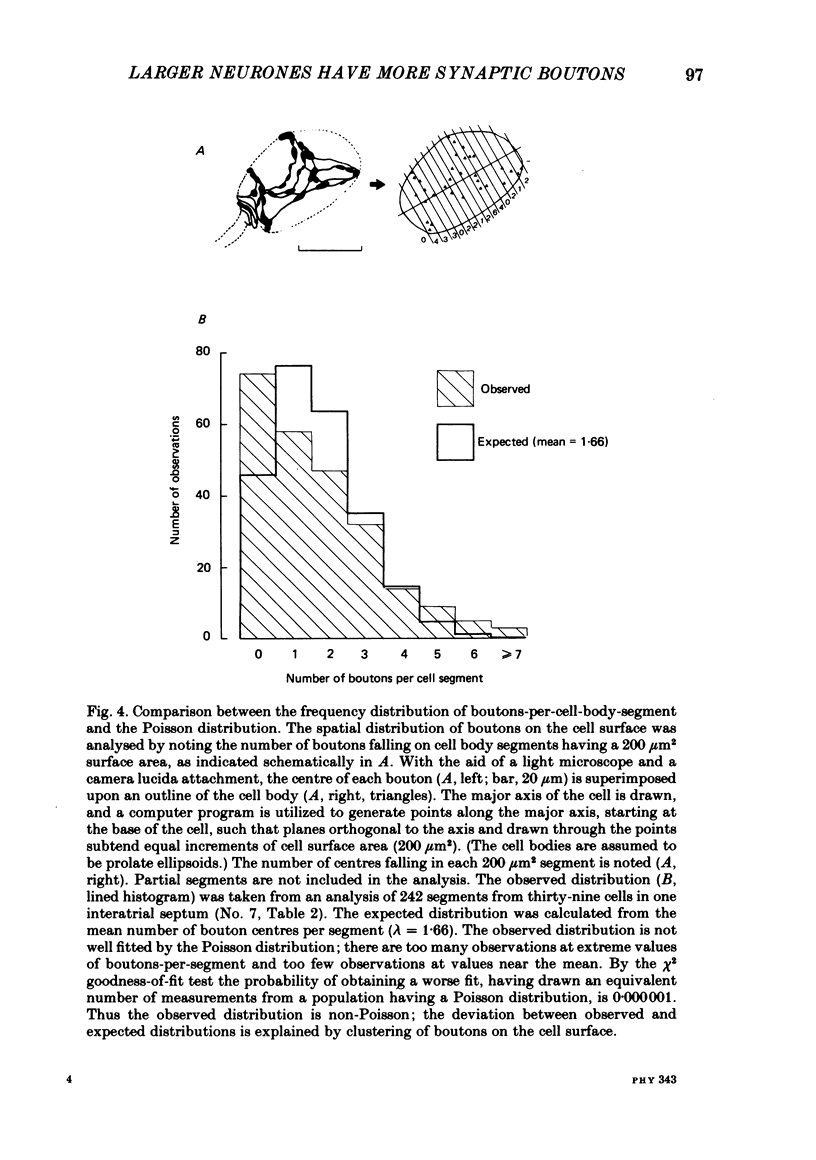
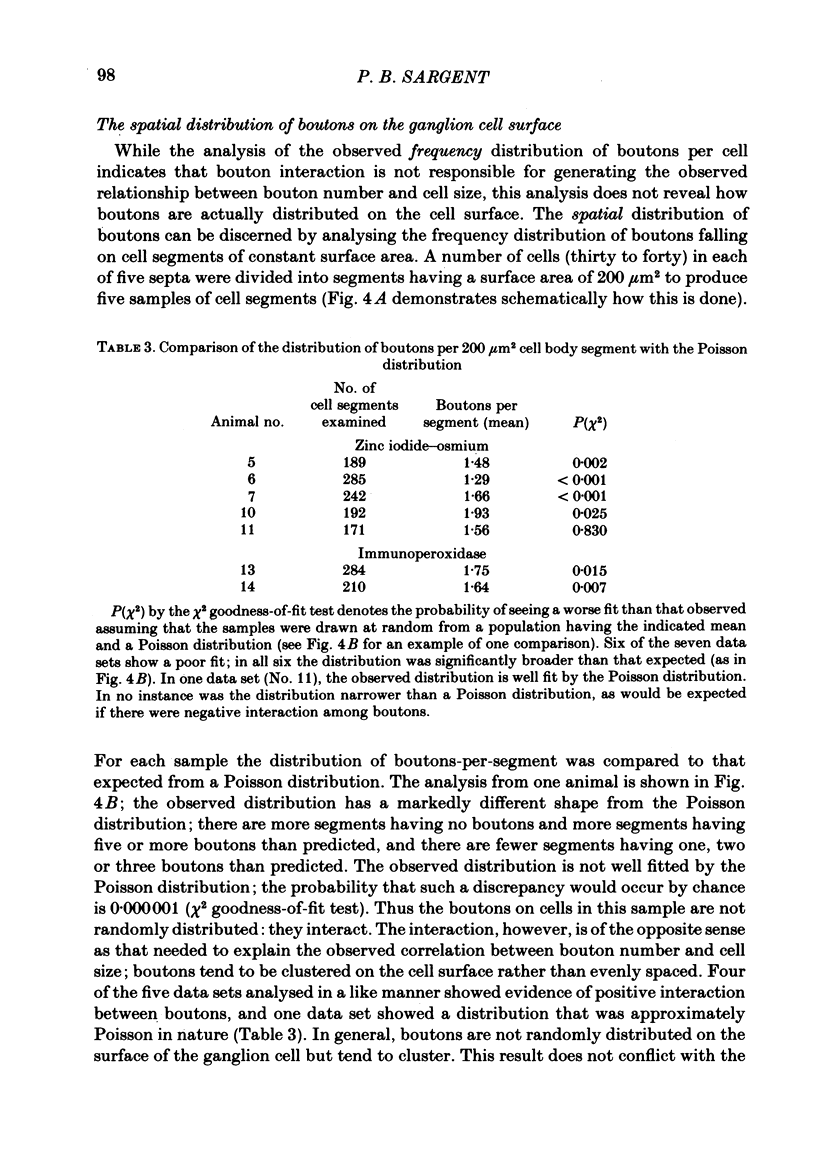
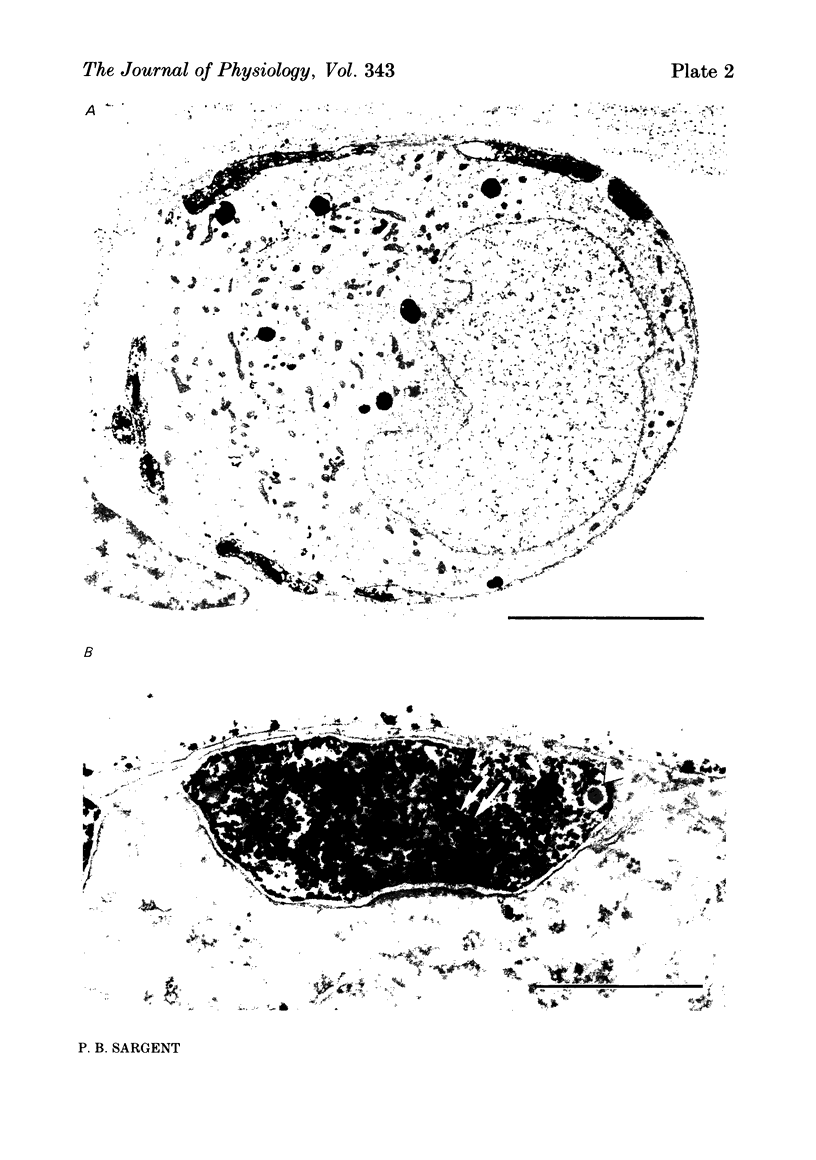
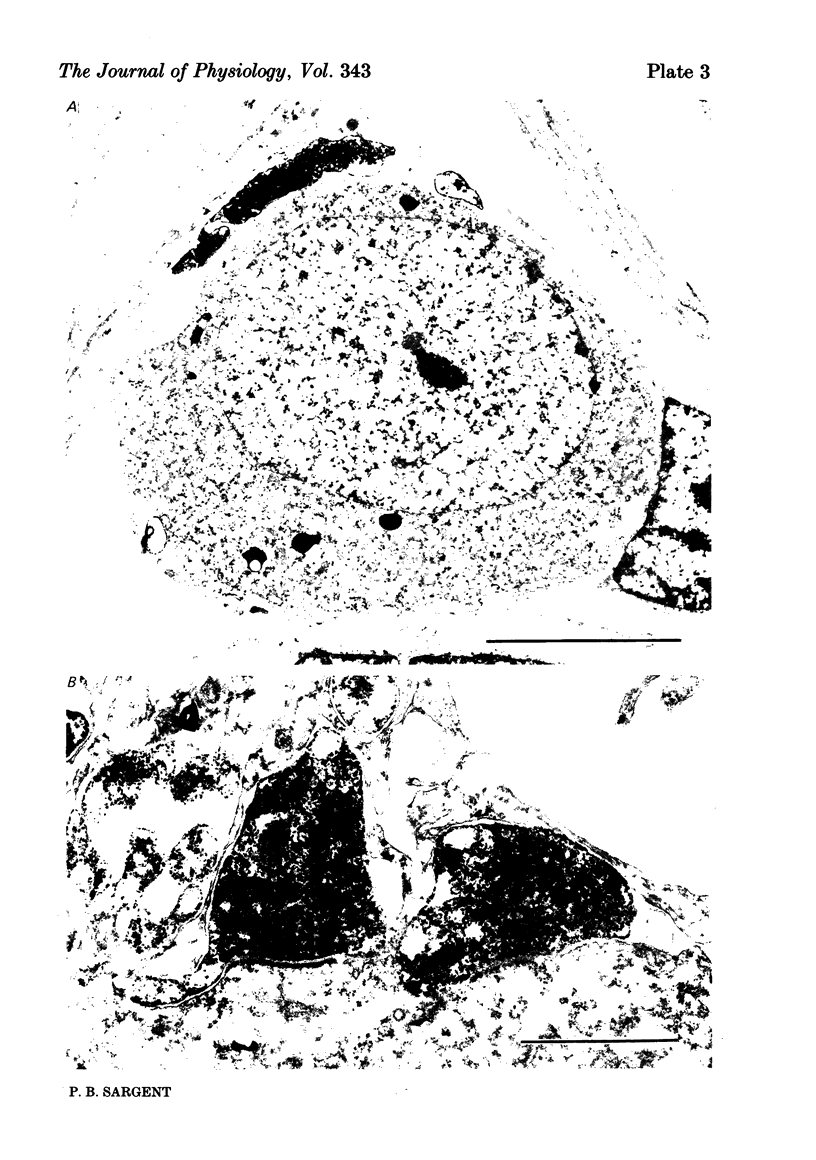
The relationship between the size of parasympathetic neurones and the number of synaptic boutons terminating upon them has been studied in the cardiac ganglion of Xenopus laevis. Synaptic boutons were visualized by impregnation with zinc iodide and osmium (ZIO), which by electron microscopy was shown to stain heavily all synaptic boutons in six preparations. Light microscopic examination of the unipolar ganglion cells in intact tissue reveals that larger neurones have more synaptic boutons. The number of boutons terminating on the cell body is significantly correlated with its surface area. By statistical means it was possible to demonstrate that the relation between bouton number and surface area is linear and that the regression line has a y-intercept not significantly different from zero. Therefore the density of synaptic boutons, one per 127 micron2 of cell body surface, is independent of cell size. The size of synaptic boutons, measured as the area of apposition between bouton and cell body, is similar for small and for large ganglion cells; thus a constant fraction (2%) of the cell body on average is covered by synaptic boutons, regardless of cell size. The correlation between bouton number and cell body surface area is not the result of interaction between boutons. The frequency distribution of boutons per normalized cell was found to be similar to that expected from the Poisson distribution. Thus the probability that a bouton will be 'assigned' to a particular cell is independent of how many other boutons are present. The only factor that appears to influence the number of boutons on the cell body is its size.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akert K., Sandri C. An electron-microscopic study of zinc iodide-osmium impregnation of neurons. I. Staining of synaptic vesicles at cholinergic junctions. Brain Res. 1968 Feb;7(2):286–295. doi: 10.1016/0006-8993(68)90104-2. [DOI] [PubMed] [Google Scholar]
- Angaut-Petit D., Mallart A. Dual innervation of end-plate sites and its consequences for neuromuscular transmission in muscles of adult Xenopus laevis. J Physiol. 1979 Apr;289:203–218. doi: 10.1113/jphysiol.1979.sp012733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Raftos J. The formation and regression of synapses during the re-innervation of axolotl striated muscles. J Physiol. 1977 Feb;265(2):261–295. doi: 10.1113/jphysiol.1977.sp011716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell A. D., Herrera A. A. Physiological regulation of synaptic effectiveness at frog neuromuscular junctions. J Physiol. 1980 Oct;307:301–317. doi: 10.1113/jphysiol.1980.sp013436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS C. The morphology of the myoneural junction as influenced by neurotoxic drugs. Am J Pathol. 1954 May-Jun;30(3):501–519. [PMC free article] [PubMed] [Google Scholar]
- Harris J. B., Ribchester R. R. The relationship between end-plate size and transmitter release in normal and dystrophic muscles of the mouse. J Physiol. 1979 Nov;296:245–265. doi: 10.1113/jphysiol.1979.sp013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Korn H., Mallet A., Triller A., Faber D. S. Transmission at a central inhibitory synapse. II. Quantal description of release, with a physical correlate for binomial n. J Neurophysiol. 1982 Sep;48(3):679–707. doi: 10.1152/jn.1982.48.3.679. [DOI] [PubMed] [Google Scholar]
- Korneliussen H., Waerhaug O. Three morphological types of motor nerve terminals in the rat diaphragm, and their possible innervation of different muscle fiber types. Z Anat Entwicklungsgesch. 1973 May 30;140(1):73–84. doi: 10.1007/BF00520719. [DOI] [PubMed] [Google Scholar]
- Kuno M., Turkanis S. A., Weakly J. N. Correlation between nerve terminal size and transmitter release at the neuromuscular junction of the frog. J Physiol. 1971 Mar;213(3):545–556. doi: 10.1113/jphysiol.1971.sp009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew W. D., Tsavaler L., Reichardt L. F. Identification of a synaptic vesicle-specific membrane protein with a wide distribution in neuronal and neurosecretory tissue. J Cell Biol. 1981 Oct;91(1):257–269. doi: 10.1083/jcb.91.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan U. J., Kuffler S. W. Visual identification of synaptic boutons on living ganglion cells and of varicosities in postganglionic axons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):485–508. doi: 10.1098/rspb.1971.0044. [DOI] [PubMed] [Google Scholar]
- Nicol D., Meinertzhagen I. A. Regulation in the number of fly photoreceptor synapses: the effects of alterations in the number of presynaptic cells. J Comp Neurol. 1982 May 1;207(1):45–60. doi: 10.1002/cne.902070105. [DOI] [PubMed] [Google Scholar]
- Nudell B. M., Grinnell A. D. Inverse relationship between transmitter release and terminal length in synapses on frog muscle fibers of uniform input resistance. J Neurosci. 1982 Feb;2(2):216–224. doi: 10.1523/JNEUROSCI.02-02-00216.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper S. An electrophysiological study of chemical and electrical synapses on neurones in the parasympathetic cardiac ganglion of the mudpuppy, Necturus maculosus: evidence for intrinsic ganglionic innervation. J Physiol. 1976 Jan;254(2):427–454. doi: 10.1113/jphysiol.1976.sp011239. [DOI] [PMC free article] [PubMed] [Google Scholar]