Abstract
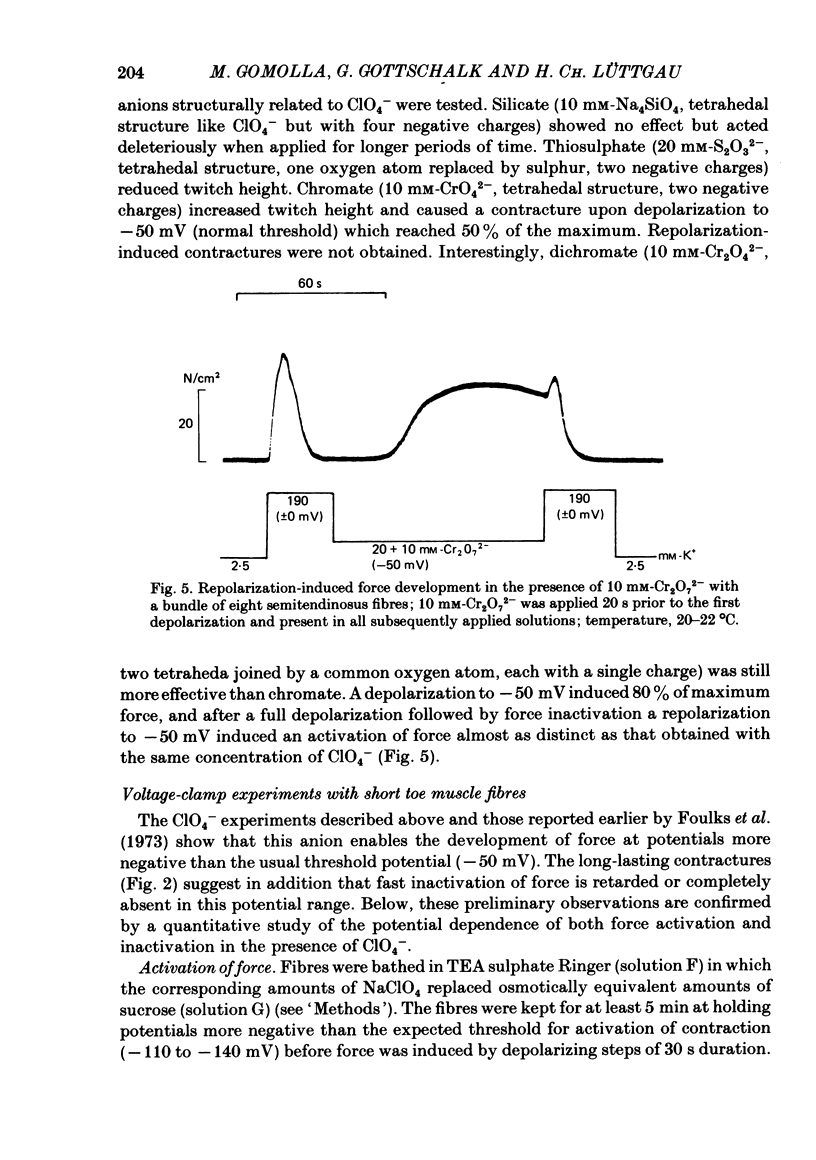
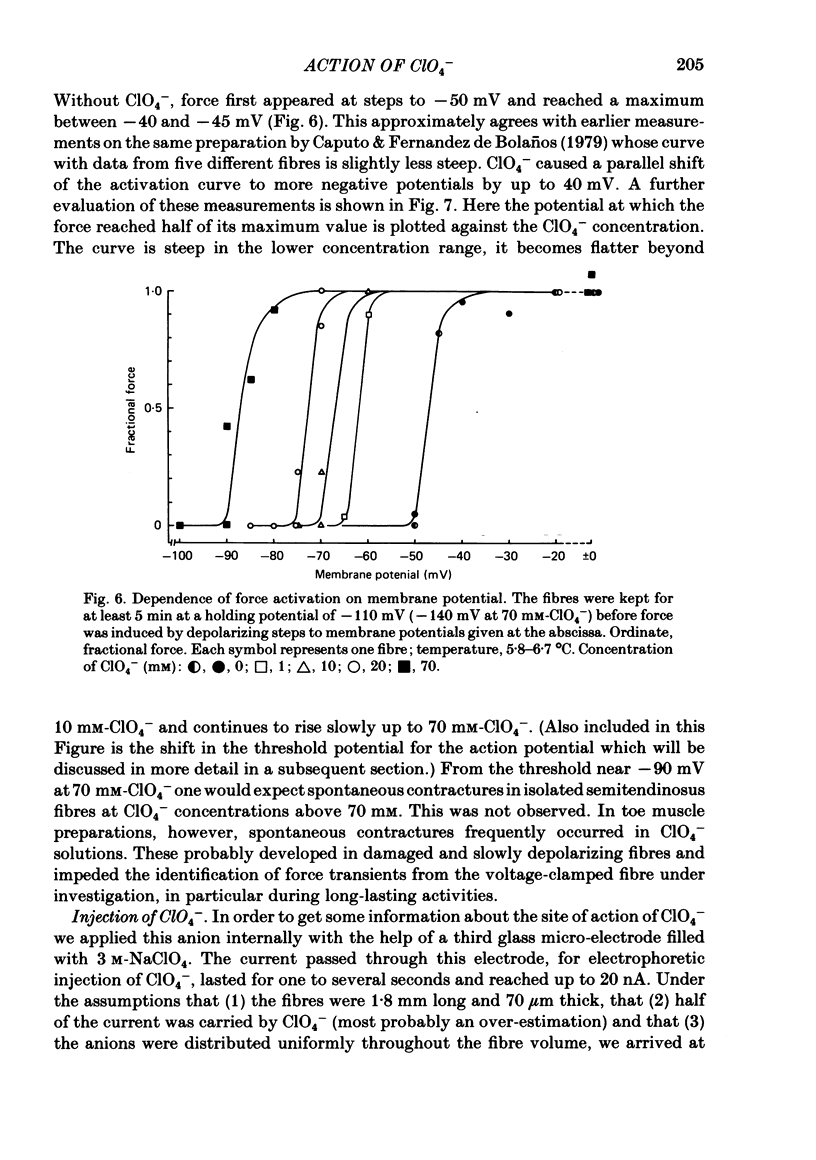
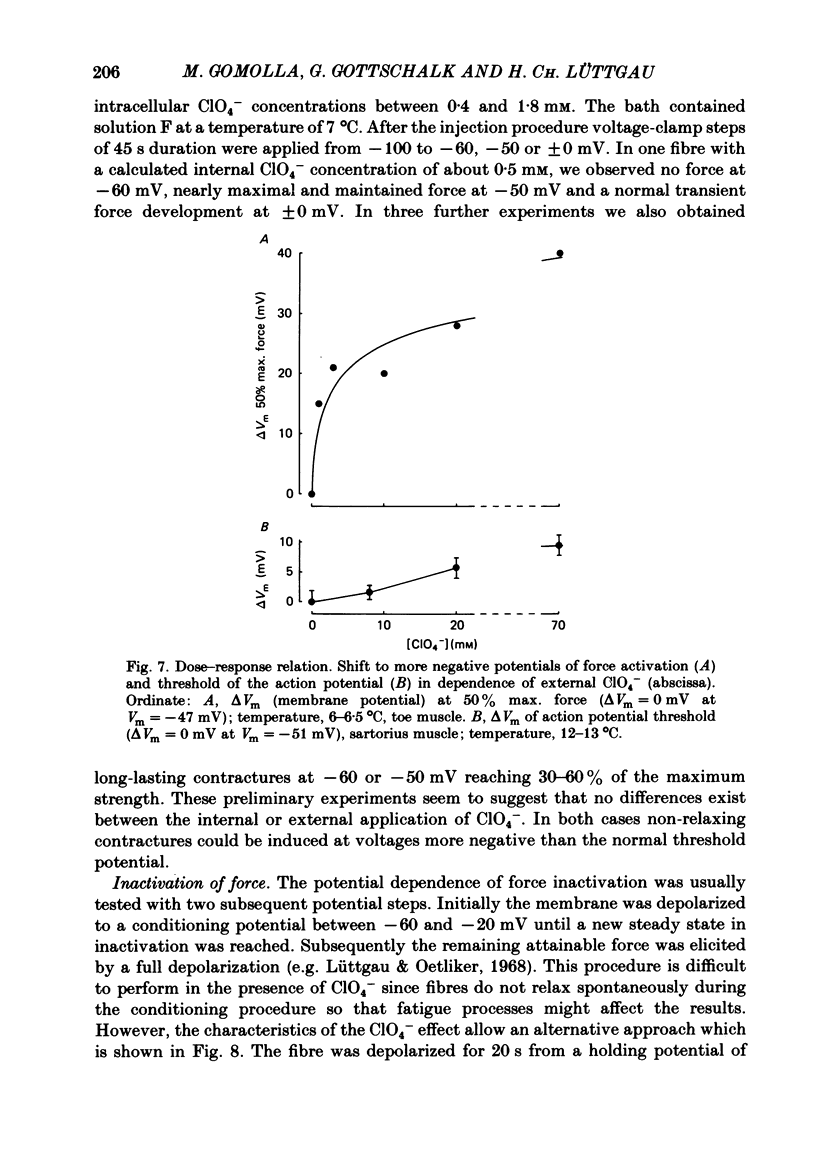
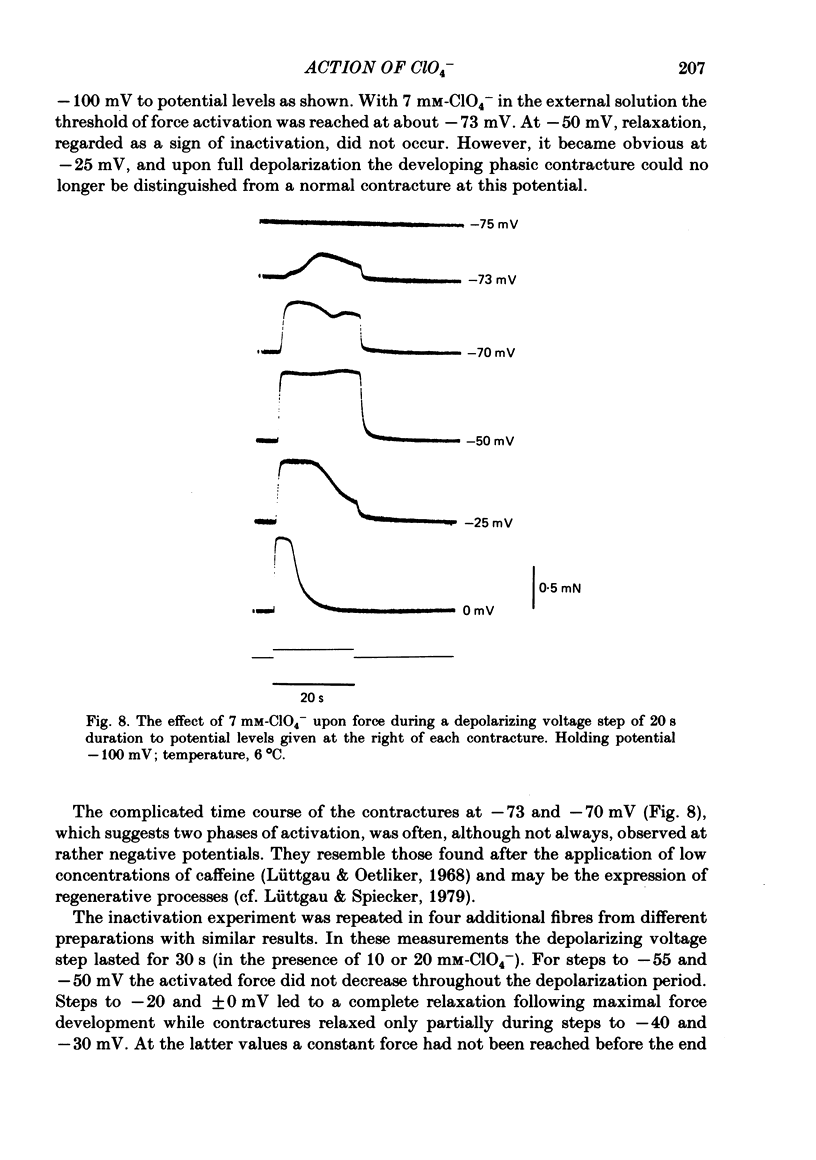
The effect of the perchlorate anion (ClO4-) on the potential dependence of mechanical and electrical parameters was investigated in skeletal muscles fibres of the frog. Two main methods were employed: twitches and K contractures were induced in isolated fibres from the semitendinosus or iliofibularis muscle, and point voltage clamp was applied in sartorius and short toe muscle fibres. Twitch height was unaffected below 10(-4) M-ClO4-, it usually increased several-fold in the concentration range of 10(-3) to 10(-2) M-ClO4- and continued to rise slowly between 10(-2) and 10(-1) M-ClO4-. ClO4- caused a parallel shift of the activation curve, which relates peak force to membrane potential, towards more negative potentials by up to 40 mV (70 mM-ClO4-). The shift in force activation was not accompanied by a corresponding shift in the potential dependence of force inactivation. In the presence of ClO4-, maximum force development upon depolarization to -60 or -50 mV could be maintained for several minutes, suggesting that spontaneous relaxation after full depolarization is due to a potential-dependent inactivation process, and not to an exhaustion of Ca2+ release. ClO4- shifted the threshold for the initiation of the action potential only slightly towards more negative potentials (approximately 10 mV at 70 mM-ClO4-). Little or no shift was observed in the lower concentration range (less than 10 mM) where the threshold of force activation was shifted by about 20 mV. ClO4- slightly depressed the activation of the delayed rectifier without causing any distinct change in its threshold potential. Electrophoretic injection of ClO4- (internal ClO4- concentration ([ClO4-]i) approximately 1 mM) induced similar effects to those following external application of this anion, i.e. a shift of force activation towards more negative potentials. Of several other anions tested, only dichromate, which resembles ClO4-in its tetrahedal structure, similarly caused force activation after repolarization. We conclude that at low concentrations (less than 10 mM) ClO4- rather specifically improves excitation-contraction coupling by direct interference with the gating mechanism which activates Ca release from the sarcoplasmic reticulum. At higher concentrations, it may also influence potential-dependent membrane processes by adsorption to the outer surface of the membrane.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. The effect of internal and external potassium concentration on the membrane potential of frog muscle. J Physiol. 1956 Sep 27;133(3):631–658. doi: 10.1113/jphysiol.1956.sp005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M. D. Modification of sodium channel gating in frog myelinated nerve fibres by Centruroides sculpturatus scorpion venom. J Physiol. 1975 Jan;244(2):511–534. doi: 10.1113/jphysiol.1975.sp010810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C., Fernandez de Bolaños P. Membrane potential, contractile activation and relaxation rates in voltage clamped short muscle fibres of the frog. J Physiol. 1979 Apr;289:175–189. doi: 10.1113/jphysiol.1979.sp012731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C. The effect of low temperature on the excitation-contraction coupling phenomena of frog single muscle fibres. J Physiol. 1972 Jun;223(2):461–482. doi: 10.1113/jphysiol.1972.sp009858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantin L. L. The effect o f calcium on contraction and conductance thresholds in frog skeletal muscle. J Physiol. 1968 Mar;195(1):119–132. doi: 10.1113/jphysiol.1968.sp008450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani J. A., Sanchez J. A., Hille B. Lyotropic anions. Na channel gating and Ca electrode response. J Gen Physiol. 1983 Feb;81(2):255–281. doi: 10.1085/jgp.81.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink R., Wettwer E. Modified K-channel gating by exhaustion and the block by internally applied TEA+ and 4-aminopyridine in muscle. Pflugers Arch. 1978 May 31;374(3):289–292. doi: 10.1007/BF00585607. [DOI] [PubMed] [Google Scholar]
- Foulks J. G., Miller J. A., Perry F. A. Repolarization-induced reactivation of contracture tension in frog skeletal muscle. Can J Physiol Pharmacol. 1973 May;51(5):324–334. doi: 10.1139/y73-049. [DOI] [PubMed] [Google Scholar]
- Foulks J. G., Morishita L. The temperature dependence of ionic influences on contractile function in frog twitch muscle. Can J Physiol Pharmacol. 1980 Jan;58(1):74–84. doi: 10.1139/y80-013. [DOI] [PubMed] [Google Scholar]
- Foulks J. G., Perry F. A. The effects of temperature, local anaesthetics, pH, divalent cations, and group-specific reagents on repriming and repolarization-induced contractures in frog skeletal muscle. Can J Physiol Pharmacol. 1979 Jun;57(6):619–630. doi: 10.1139/y79-095. [DOI] [PubMed] [Google Scholar]
- Frank G. B. Roles of extracellular and "trigger" calcium ions in excitation--contraction coupling in skeletal muscle. Can J Physiol Pharmacol. 1982 Apr;60(4):427–439. doi: 10.1139/y82-063. [DOI] [PubMed] [Google Scholar]
- Gomolla M., Gottschalk G., Lüttgau H. C. Electromechanical coupling II. The effect of perchlorate upon excitation-contraction coupling in frog skeletal muscle fibres. Z Naturforsch C. 1982 Jul-Aug;37(7-8):707–708. doi: 10.1515/znc-1982-7-822. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Potassium contractures in single muscle fibres. J Physiol. 1960 Sep;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The effect of nitrate and other anions on the mechanical response of single muscle fibres. J Physiol. 1960 Sep;153:404–412. doi: 10.1113/jphysiol.1960.sp006542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistracher P., Hunt C. C. The relation of membrane changes ot contraction in twitch muscle fibres. J Physiol. 1969 May;201(3):589–611. doi: 10.1113/jphysiol.1969.sp008774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. Y., Stanfield P. R. Actions of some anions on electrical properties and mechanical threshold of frog twitch muscle. J Physiol. 1968 Sep;198(2):291–309. doi: 10.1113/jphysiol.1968.sp008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttgau H. C., Oetliker H. The action of caffeine on the activation of the contractile mechanism in straited muscle fibres. J Physiol. 1968 Jan;194(1):51–74. doi: 10.1113/jphysiol.1968.sp008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttgau H. C., Spiecker W. The effects of calcium deprivation upon mechanical and electrophysiological parameters in skeletal muscle fibres of the frog. J Physiol. 1979 Nov;296:411–429. doi: 10.1113/jphysiol.1979.sp013013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias R. T., Levis R. A., Eisenberg R. S. Electrical models of excitation-contraction coupling and charge movement in skeletal muscle. J Gen Physiol. 1980 Jul;76(1):1–31. doi: 10.1085/jgp.76.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S., Bruder A., Chen S., Moser C. Chaotropic anions and the surface potential of bilayer membranes. Biochim Biophys Acta. 1975 Jun 25;394(2):304–313. doi: 10.1016/0005-2736(75)90267-9. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]


