Abstract
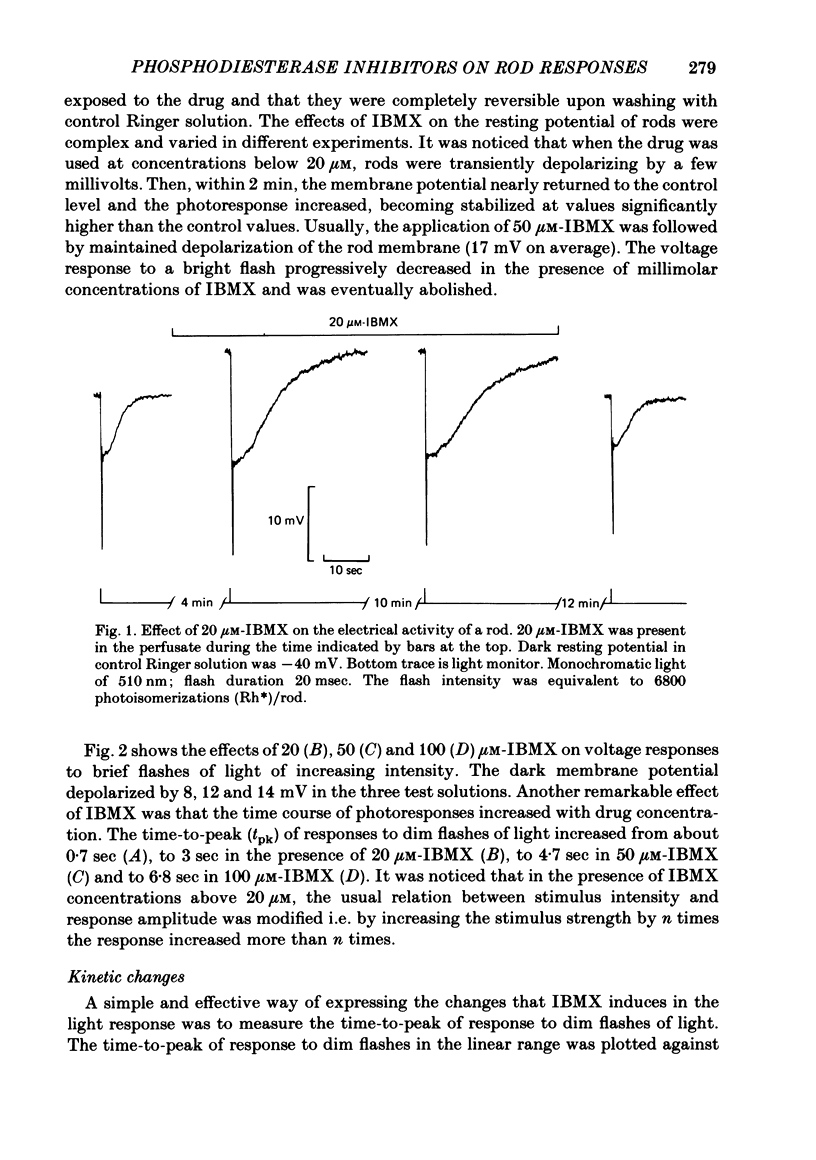
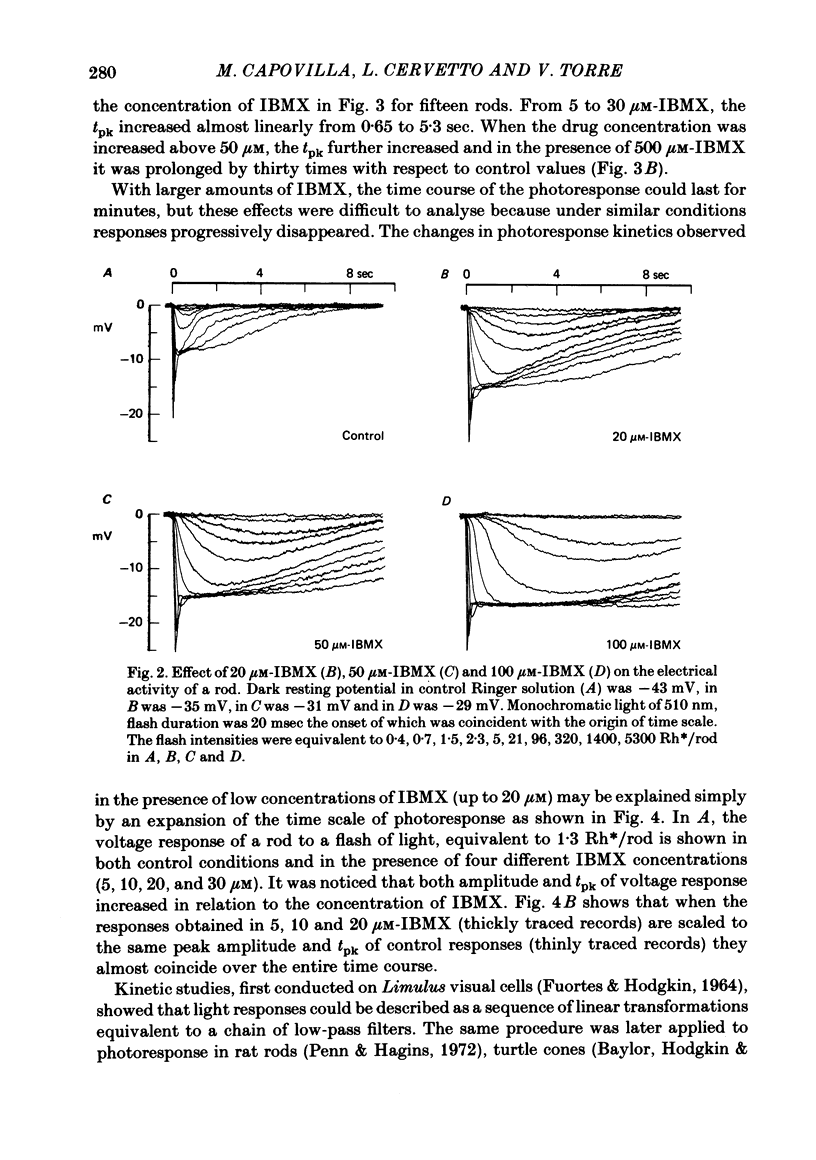
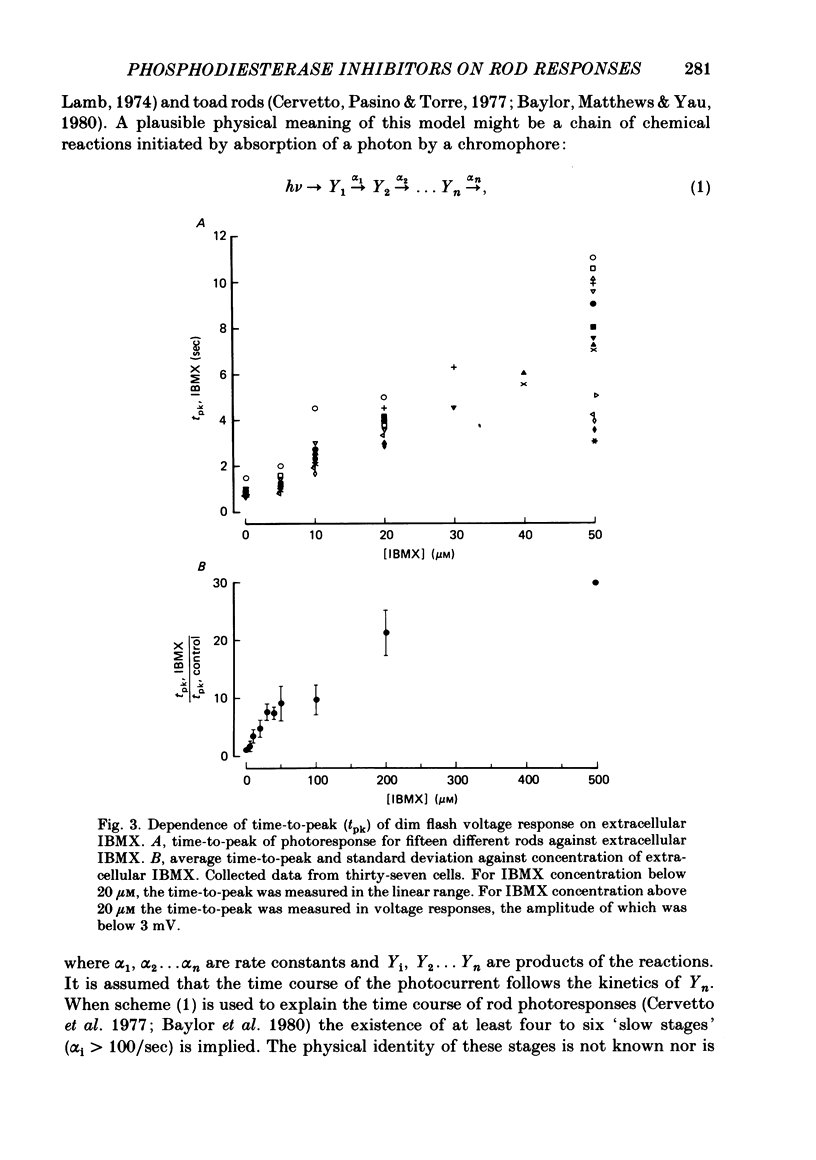
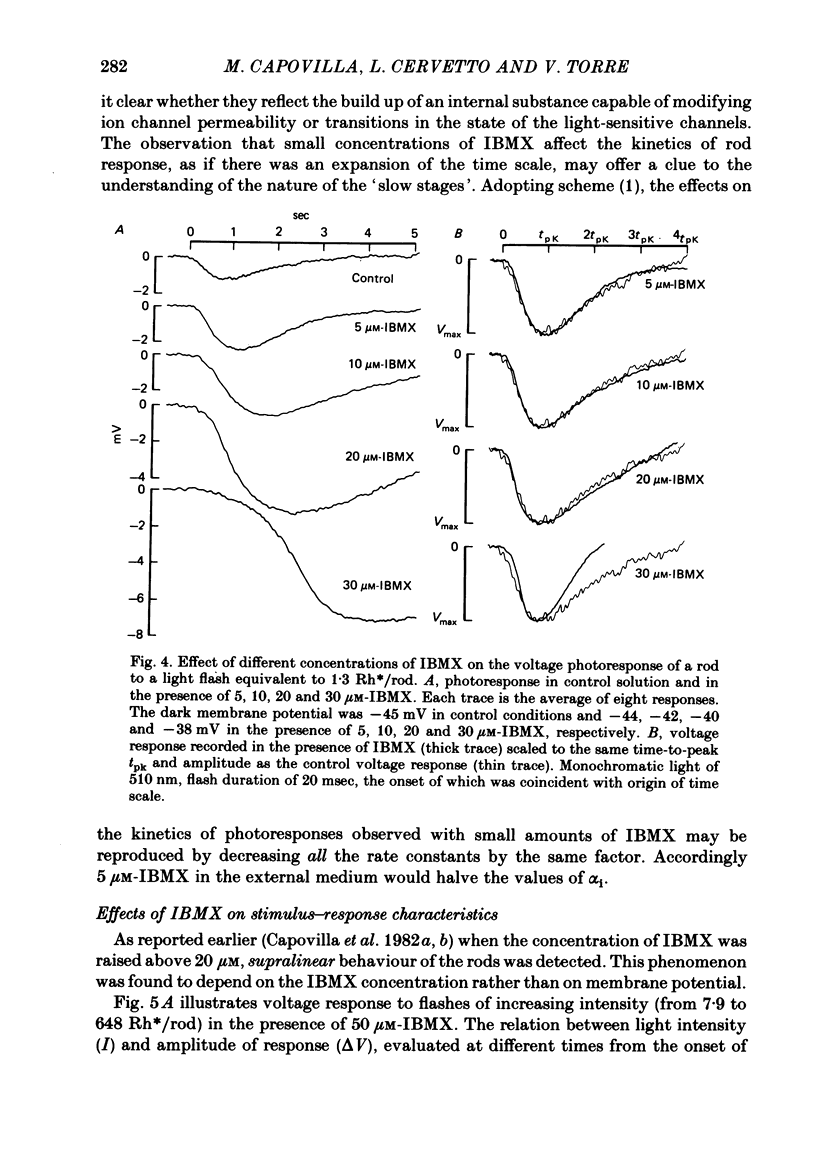
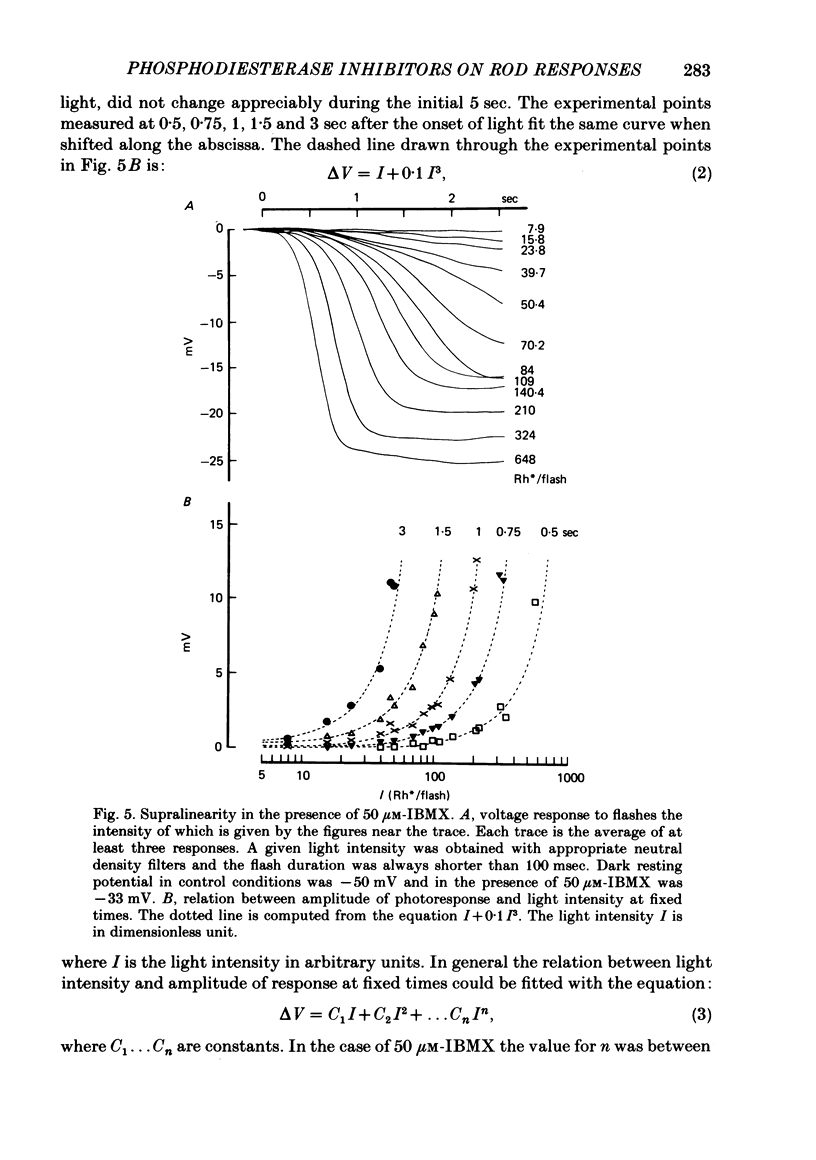
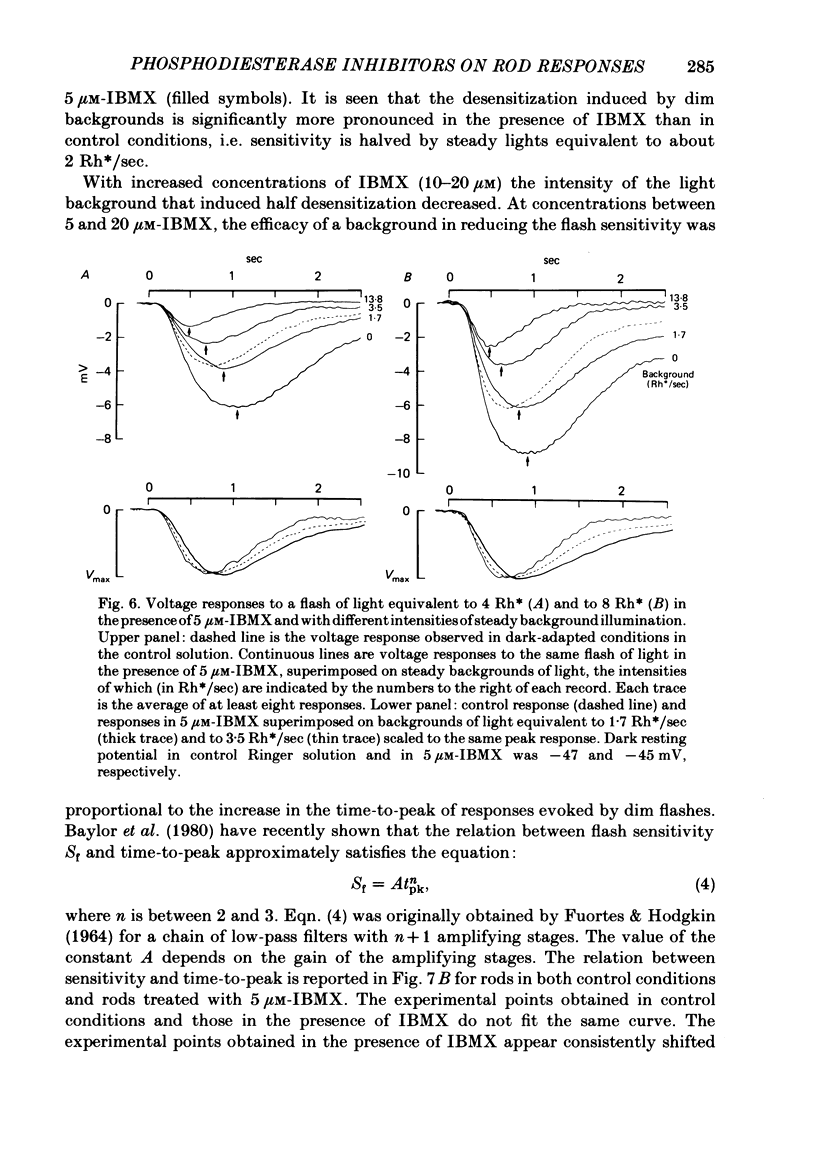
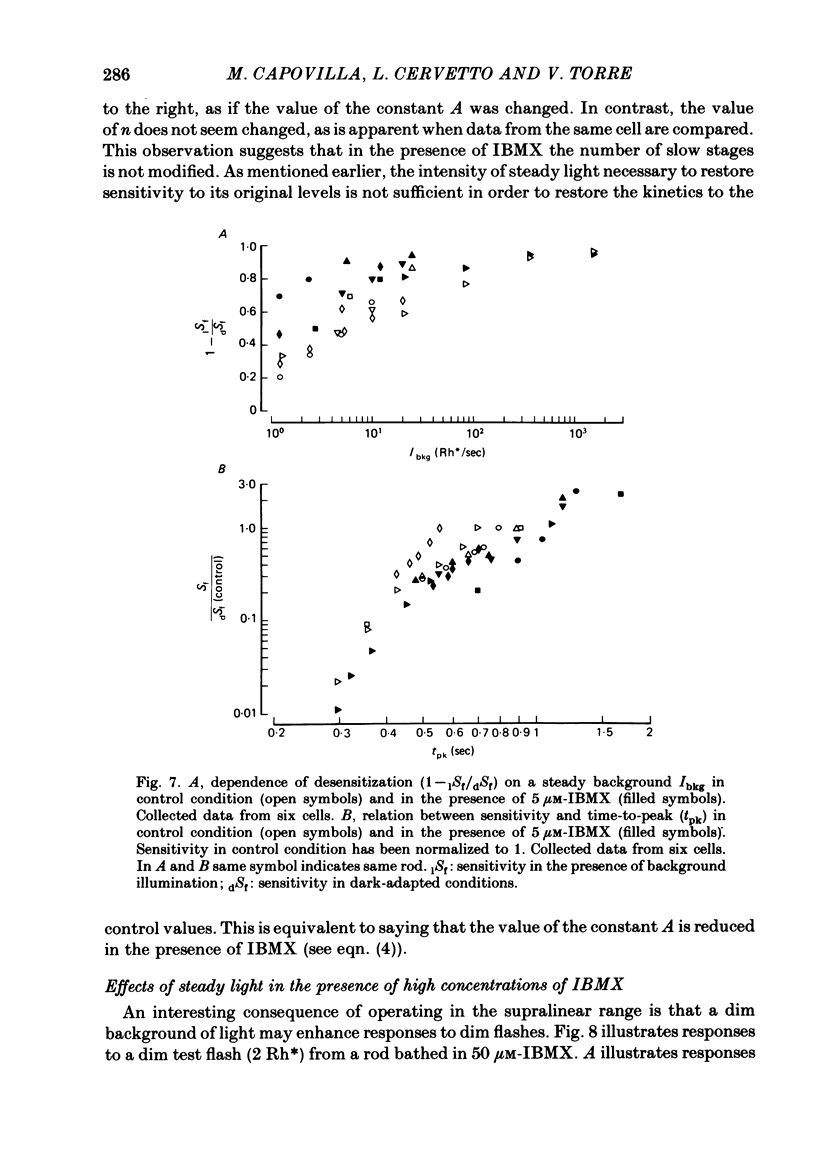
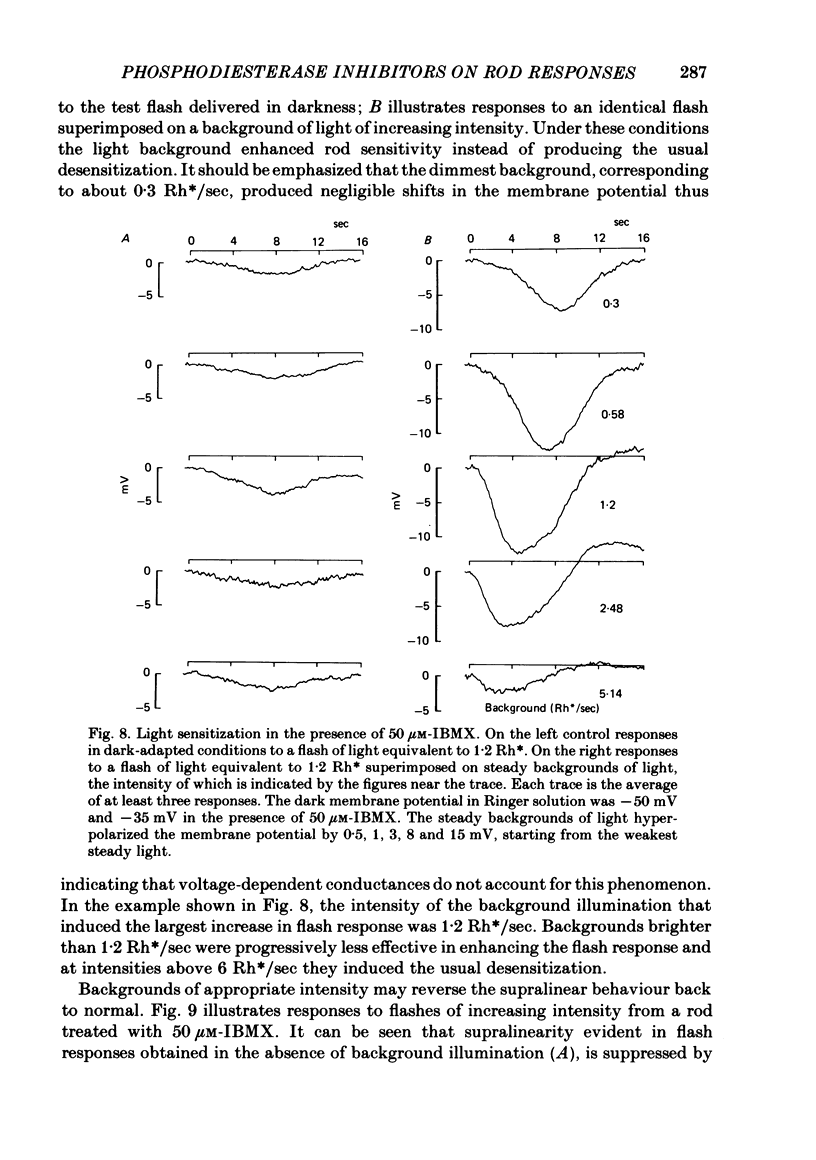
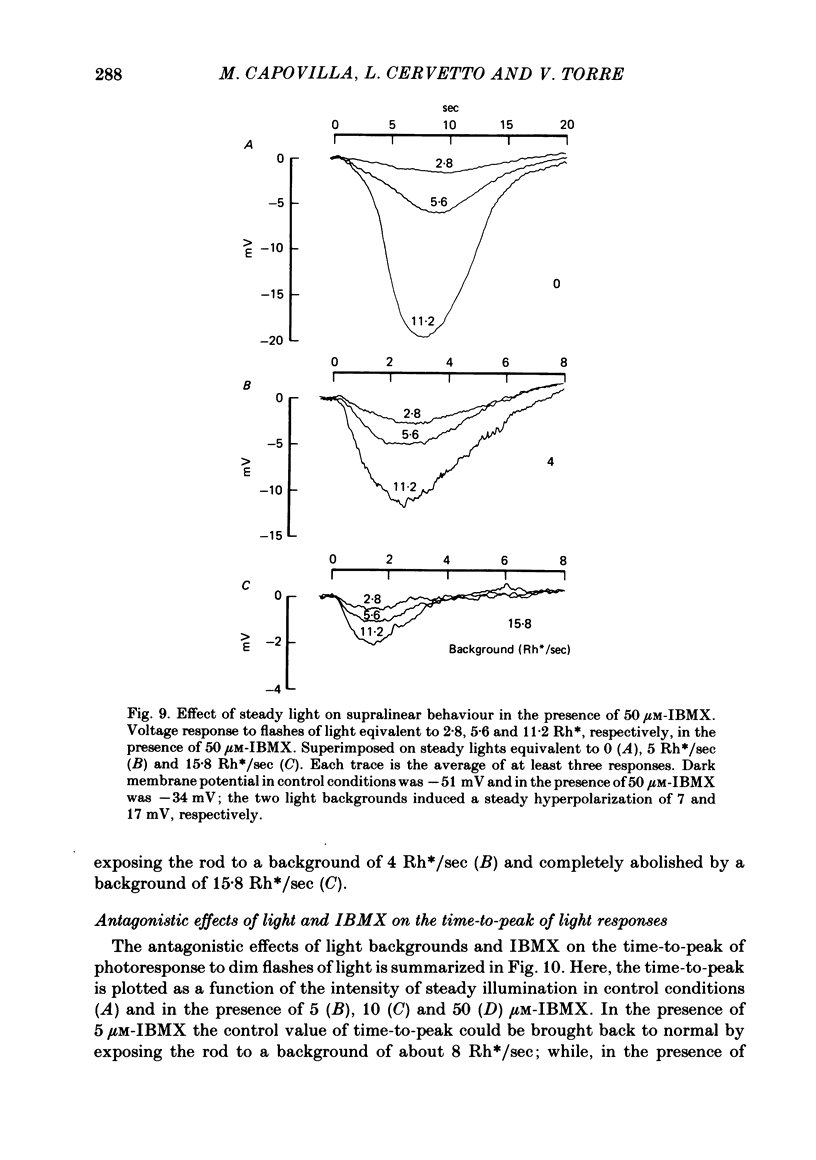
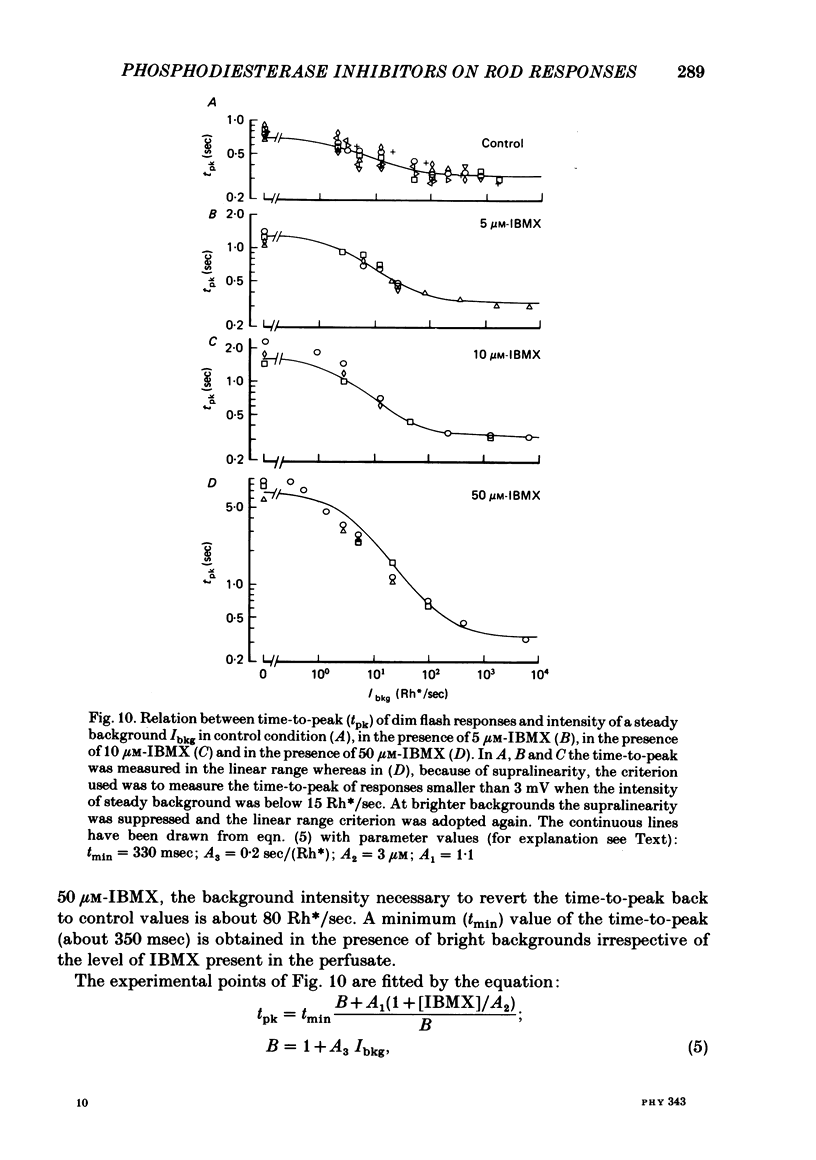
The membrane potential of toad rods was recorded during addition of small amounts of phosphodiesterase inhibitors to the extracellular medium. Separate application of 3-isobutyl-1-methylxanthine (IBMX), caffeine, theophylline, papaverine and RO 20-1724 slowed down the time course of rod photo-response to dim flashes of light. These changes were associated with a two to six-fold increase in the amplitude of photoresponse. The effects on kinetics may be described simply by an expansion of the photoresponse time scale. When the drug concentration was raised above a certain level, the rods showed supralinear behaviour whereby doubling of the intensity of a dim flash could increase the response more than two-fold. Under similar conditions rods also showed light sensitization whereby responses to dim flashes were enhanced in the presence of dim backgrounds. Taking the drug concentration that induced a two-fold increase in the time-to-peak, IBMX was found the most effective compound, followed by papaverine, RO 20-1724, theophylline and caffeine with relative effectivities 1, 1/2, 1/7, 1/40 and 1/100. Sensitivity, kinetics and supralinear behaviour may be restored to normal by steady background illumination while still in the presence of IBMX. However the intensity of the steady light, needed to restore the sensitivity to control levels, is not sufficient to accelerate the kinetics back to control values. In the presence of 50 microM-IBMX a dim steady background of light enhanced the response to dim flashes. When the intensity of the light background was increased rods were desensitized and the supralinear behaviour disappeared. The antagonism between the effects of IBMX and the effects of background illumination on the kinetics of photoresponse suggests that phosphodiesterase activity controls the time course of light response in vertebrate rods.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwell D., Wilson M. Behaviour of the rod network in the tiger salamander retina mediated by membrane properties of individual rods. J Physiol. 1980 Dec;309:287–315. doi: 10.1113/jphysiol.1980.sp013509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Macleish P. R., Schwartz E. A. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J Physiol. 1979 Nov;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Yau K. W. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980 Dec;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignetti E., Cavaggioni A., Sorbi R. T. Light-activated hydrolysis of GTP and cyclic GMP in the rod outer segments. J Physiol. 1978 Jun;279:55–69. doi: 10.1113/jphysiol.1978.sp012330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie A. E., Bownds D. Biochemical correlates of adaptation processes in isolated frog photoreceptor membranes. J Gen Physiol. 1976 Jul;68(1):1–11. doi: 10.1085/jgp.68.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capovilla M., Cervetto L., Pasino E., Torre V. The sodium current underlying the responses of toad rods to light. J Physiol. 1981 Aug;317:223–242. doi: 10.1113/jphysiol.1981.sp013822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capovilla M., Cervetto L., Torre V. Antagonism between steady light and phosphodiesterase inhibitors on the kinetics of rod photoresponses. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6698–6702. doi: 10.1073/pnas.79.21.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capovilla M., Cervetto L., Torre V. Effects of changing external potassium and chloride concentrations on the photoresponses of Bufo bufo rods. J Physiol. 1980 Oct;307:529–551. doi: 10.1113/jphysiol.1980.sp013452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., Pasino E., Torre V. Electrical responses of rods in the retina of Bufo marinus. J Physiol. 1977 May;267(1):17–51. doi: 10.1113/jphysiol.1977.sp011799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chader G. J., Herz L. R., Fletcher R. T. Light activation of phosphodiesterase activity in retinal rod outer segments. Biochim Biophys Acta. 1974 Jun 28;347(3):491–493. doi: 10.1016/0005-2728(74)90086-3. [DOI] [PubMed] [Google Scholar]
- Detwiler P. B., Hodgkin A. L., McNaughton P. A. Temporal and spatial characteristics of the voltage response of rods in the retina of the snapping turtle. J Physiol. 1980 Mar;300:213–250. doi: 10.1113/jphysiol.1980.sp013159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUORTES M. G., HODGKIN A. L. CHANGES IN TIME SCALE AND SENSITIVITY IN THE OMMATIDIA OF LIMULUS. J Physiol. 1964 Aug;172:239–263. doi: 10.1113/jphysiol.1964.sp007415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L. Sensitivity of toad rods: Dependence on wave-length and background illumination. J Physiol. 1976 Sep;261(1):71–101. doi: 10.1113/jphysiol.1976.sp011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goridis C., Virmaux N. Light-regulated guanosine 3',5'-monophosphate phosphodiesterase of bovine retina. Nature. 1974 Mar 1;248(5443):57–58. doi: 10.1038/248057a0. [DOI] [PubMed] [Google Scholar]
- Hagins W. A., Penn R. D., Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970 May;10(5):380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A. The visual process: Excitatory mechanisms in the primary receptor cells. Annu Rev Biophys Bioeng. 1972;1:131–158. doi: 10.1146/annurev.bb.01.060172.001023. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L., Bownds M. D. Visual transduction in vertebrate photoreceptors. Annu Rev Neurosci. 1979;2:17–34. doi: 10.1146/annurev.ne.02.030179.000313. [DOI] [PubMed] [Google Scholar]
- Kwok-Keung Fung B., Stryer L. Photolyzed rhodopsin catalyzes the exchange of GTP for bound GDP in retinal rod outer segments. Proc Natl Acad Sci U S A. 1980 May;77(5):2500–2504. doi: 10.1073/pnas.77.5.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., McNaughton P. A., Yau K. W. Spatial spread of activation and background desensitization in toad rod outer segments. J Physiol. 1981;319:463–496. doi: 10.1113/jphysiol.1981.sp013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki N., Keirns J. J., Marcus F. R., Freeman J., Bitensky M. W. Regulation of cyclic nucleotide concentrations in photoreceptors: an ATP-dependent stimulation of cyclic nucleotide phosphodiesterase by light. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3820–3824. doi: 10.1073/pnas.70.12.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen W. G., Torre V. High-pass filtering of small signals by retinal rods. Ionic studies. Biophys J. 1983 Mar;41(3):325–339. doi: 10.1016/S0006-3495(83)84444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Kinetics of the photocurrent of retinal rods. Biophys J. 1972 Aug;12(8):1073–1094. doi: 10.1016/S0006-3495(72)86145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaramayya A., Virmaux N., Mandel P. On the mechanism of light activation of retinal rod outer segments cyclic GMP phosphodiesterase (light activation-influence of bleached rhodopsin and KF-deinhibition). Exp Eye Res. 1977 Aug;25(2):163–169. doi: 10.1016/0014-4835(77)90128-2. [DOI] [PubMed] [Google Scholar]
- Torre V., Owen W. G. High-pass filtering of small signals by the rod network in the retina of the toad, Bufo marinus. Biophys J. 1983 Mar;41(3):305–324. doi: 10.1016/S0006-3495(83)84443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre V. The contribution of the electrogenic sodium-potassium pump to the electrical activity of toad rods. J Physiol. 1982 Dec;333:315–341. doi: 10.1113/jphysiol.1982.sp014456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald G. Molecular basis of visual excitation. Science. 1968 Oct 11;162(3850):230–239. doi: 10.1126/science.162.3850.230. [DOI] [PubMed] [Google Scholar]
- Woodruff M. L., Bownds M. D. Amplitude, kinetics, and reversibility of a light-induced decrease in guanosine 3',5'-cyclic monophosphate in frog photoreceptor membranes. J Gen Physiol. 1979 May;73(5):629–653. doi: 10.1085/jgp.73.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., McNaughton P. A., Hodgkin A. L. Effect of ions on the light-sensitive current in retinal rods. Nature. 1981 Aug 6;292(5823):502–505. doi: 10.1038/292502a0. [DOI] [PubMed] [Google Scholar]


