Abstract
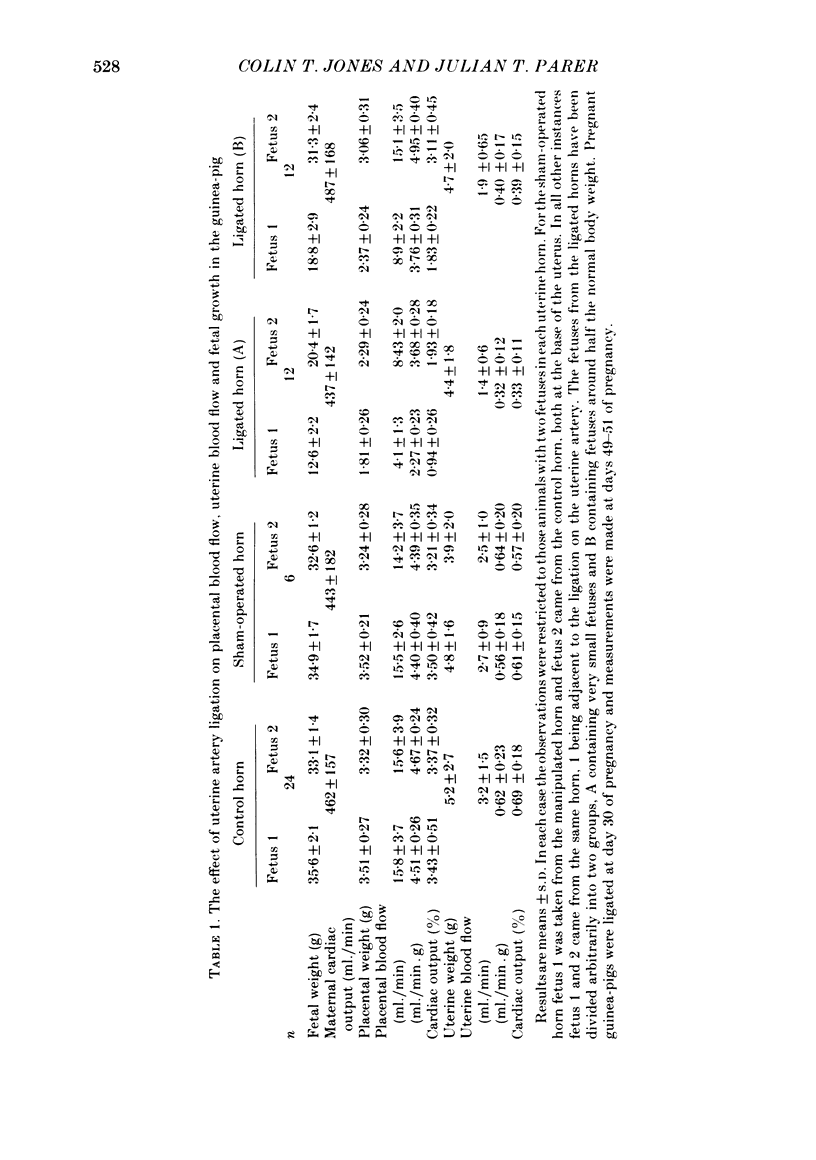
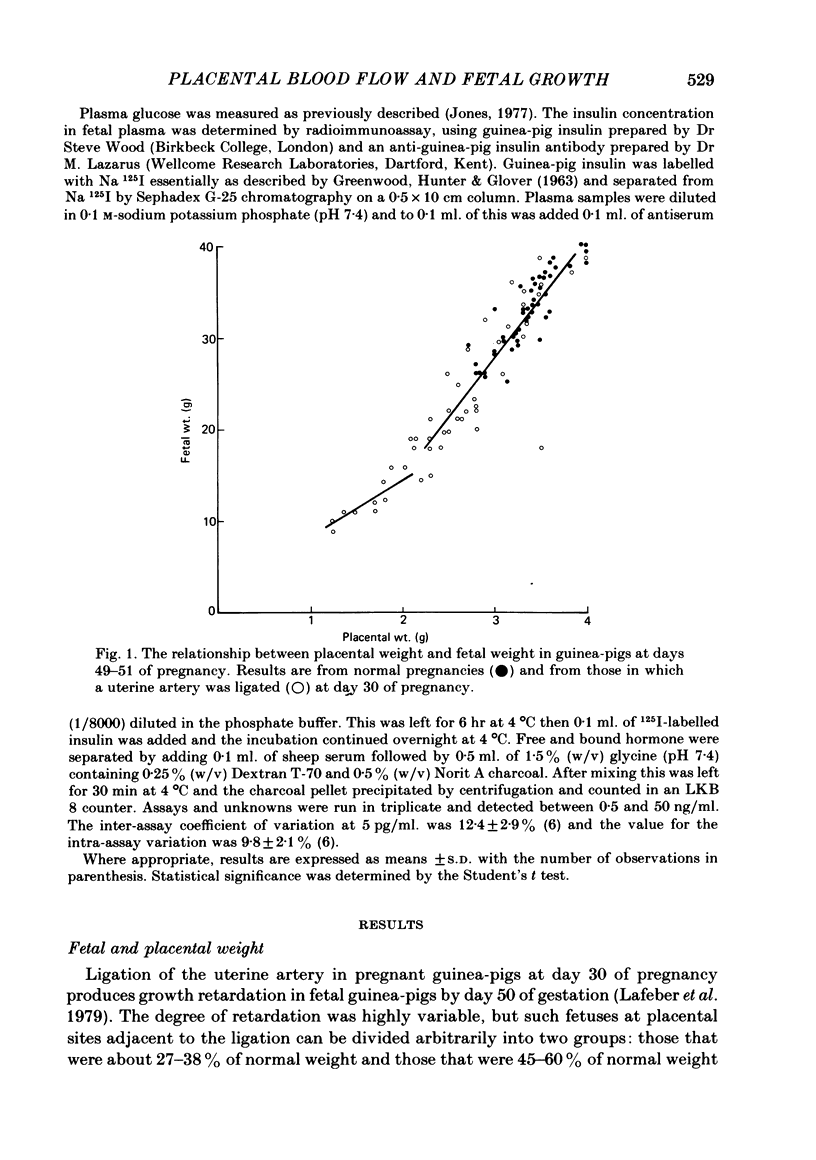
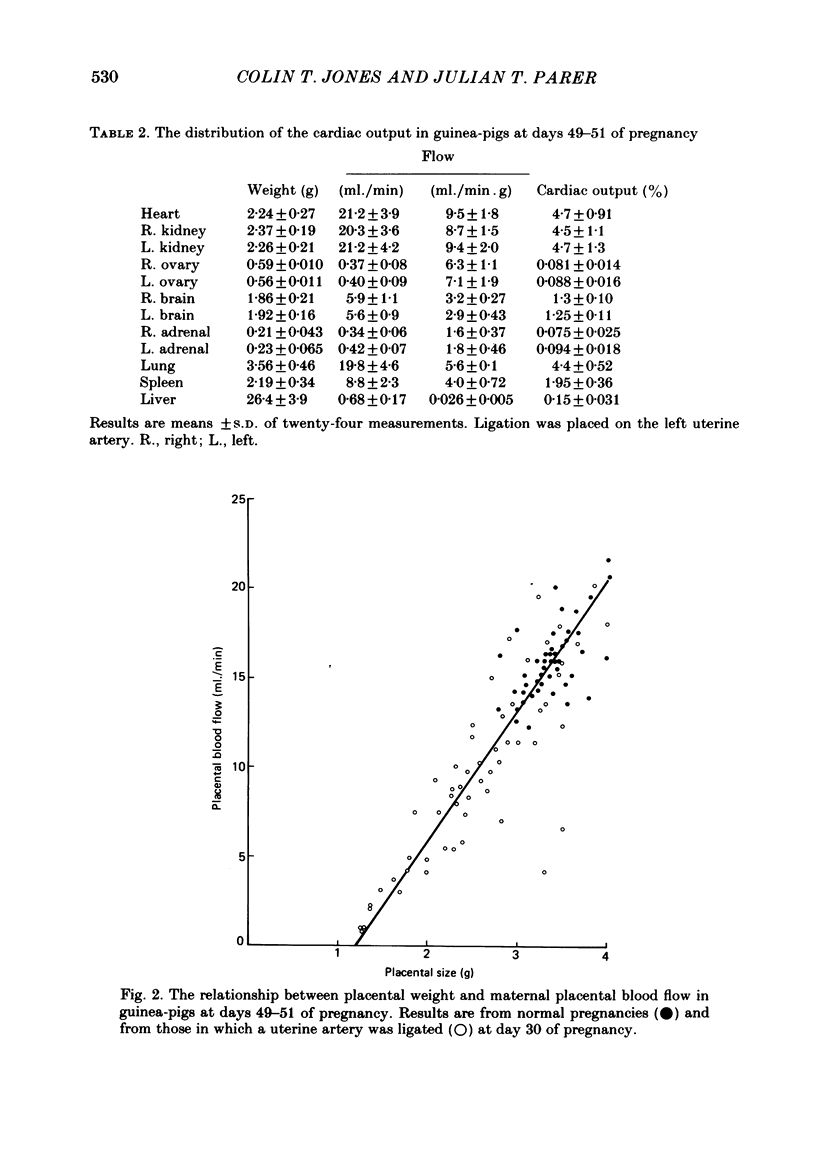
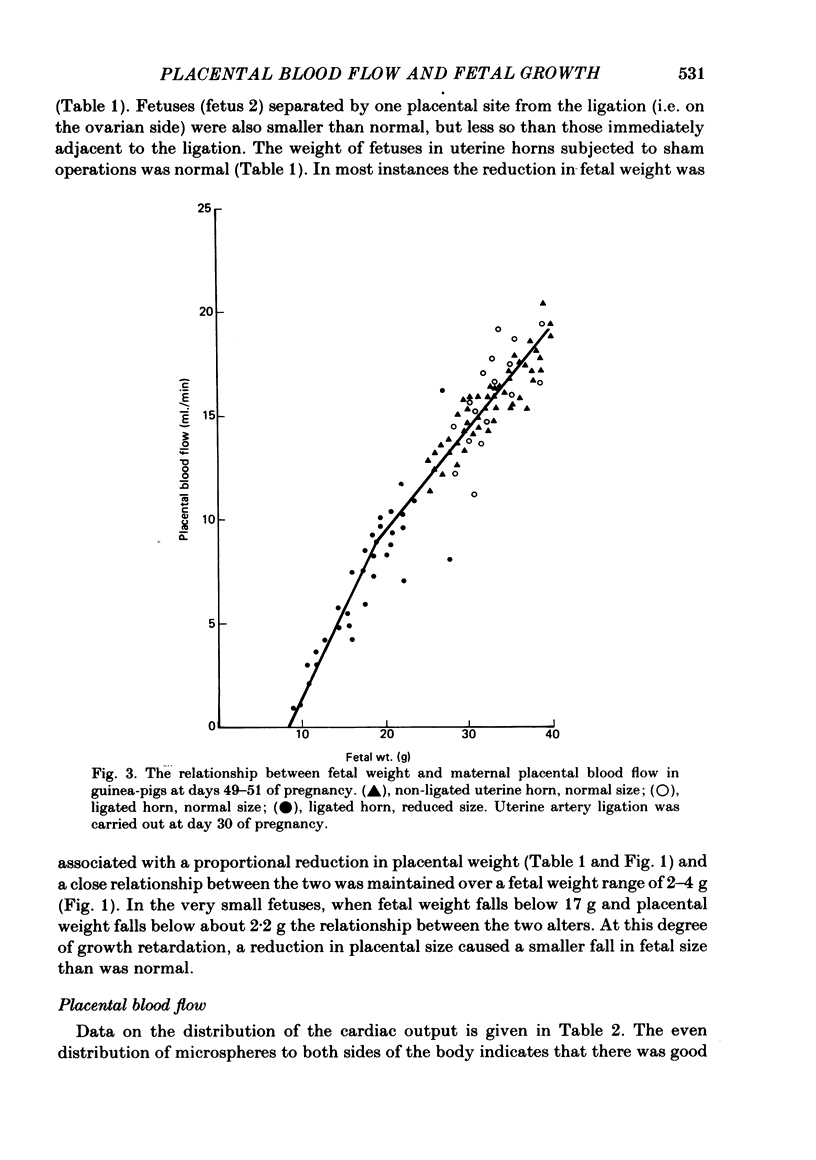
The distribution of the cardiac output and the maternal placental blood flow has been measured in guinea-pigs at days 49-51 of pregnancy using radioactively labelled microspheres. In some instances uterine blood flow was reduced chronically by ligating one uterine artery at day 30 of pregnancy. Between 3 and 4% of the cardiac output passed normally to placenta, and this could be reduced to less than 2% after uterine artery ligation. The result of the ligation was to reduce fetal and placental weight by up to 70%. Fetal and placental weight showed a close linear correlation in controls and in pregnancies with uterine artery ligation. However, when placental size was reduced below 60% of control, fetal weight was less affected by a reduction in placental weights than normal. Placental blood flow and placental size exhibited a close linear relationship over the whole range of values, but there was limiting placental weight which approached 1.3 g as placental blood flow approached zero. Thus a reduced placental size, particularly below about 50%, was associated with a proportionately greater reduction in maternal placental blood flow. Maternal placental blood flow or the percentage of maternal cardiac output to the placenta and fetal weight also showed a well-correlated linear relationship. However, when placental blood flow was below about 50% of control values further reduction had a less than normal effect upon fetal growth. Small fetuses were hypoglycaemic and hypoinsulinaemic and the degree of each was dependent upon the extent of the reduction in fetal weight and in maternal placental blood flow. In fetuses that were below about 40% of normal size, and in which placental blood was below about 30% of control, fetal weight was less sensitive to falls in blood glucose, which in turn was more sensitive than normal to a fall in maternal placental blood flow. The results indicate that over the range of 50-100% of normal fetal growth, maternal placental blood flow and probably nutrient supply to the fetus vary in parallel. Hence over this range fetal and placental growth rates are determined in part by placental blood flow. At placental blood flow rates and fetal growth rates below 40% of normal fetal growth is less dependent upon placental blood flow than usual, presumably because of a reduced dependence upon glucose metabolism for growth. This would appear to be essential, since as maternal placental blood flow is reduced to low values the placenta has to utilize an increasing proportion of the available glucose.
Full text
PDF


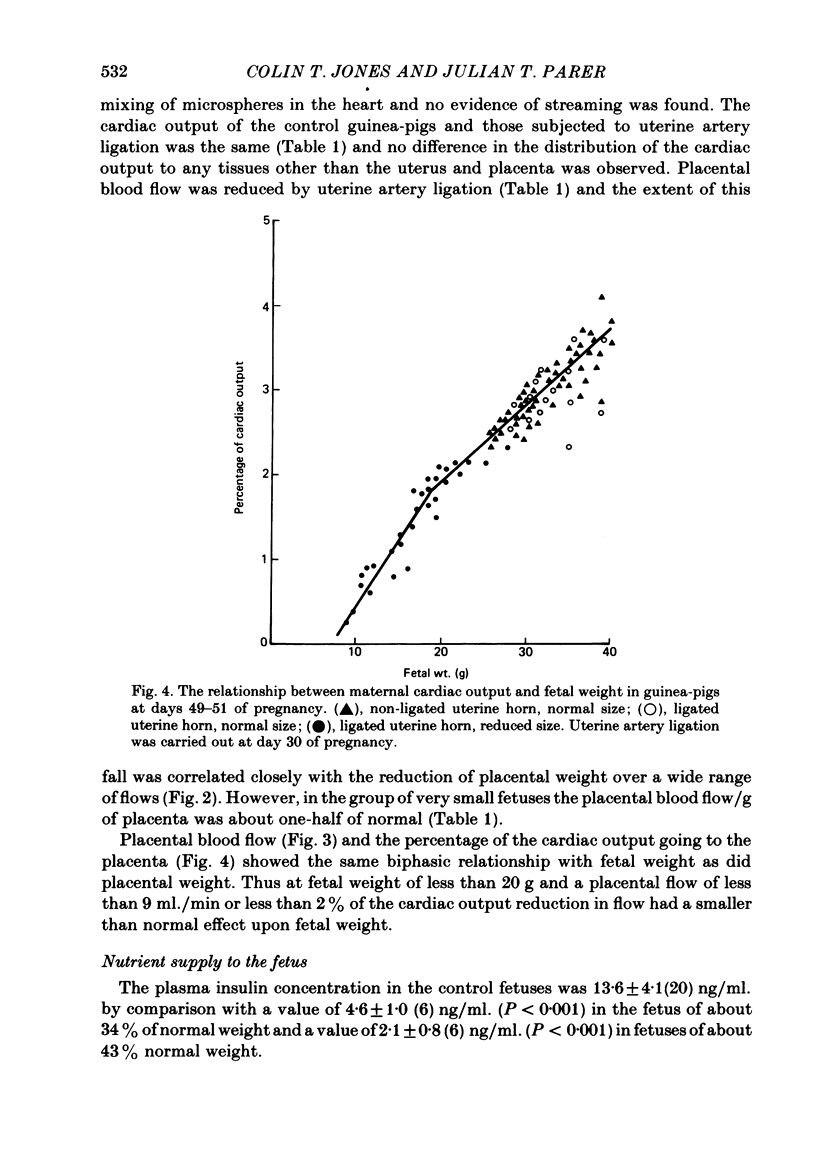
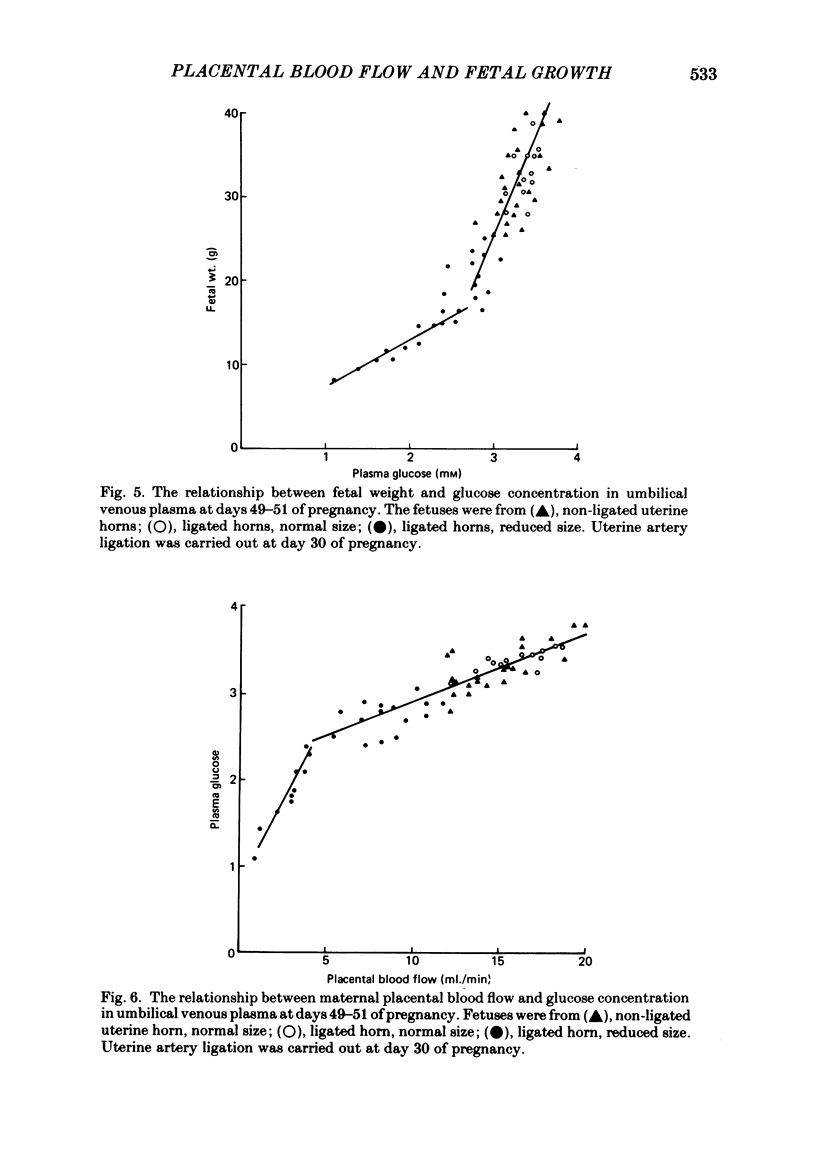




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruce N. W., Abdul-Karim R. W. Relationships between fetal weight, placental weight and maternal placental circulation in the rabbit at different stages of gestation. J Reprod Fertil. 1973 Jan;32(1):15–24. doi: 10.1530/jrf.0.0320015. [DOI] [PubMed] [Google Scholar]
- Bruce N. W. The effect on fetal development and utero-placental blood flow of ligating a uterine artery in the rat near term. Teratology. 1977 Dec;16(3):327–331. doi: 10.1002/tera.1420160312. [DOI] [PubMed] [Google Scholar]
- Duncan S. L., Lewis B. V. Maternal placental and myometrial blood flow in the pregnant rabbit. J Physiol. 1969 Jun;202(2):471–481. doi: 10.1113/jphysiol.1969.sp008821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard J. R., Chanez C., Kervran A., Tordet-Caridroit C., Assan R. Studies on experimental hypotrophy in the rat. III. Plasma insulin and glucagon. Biol Neonate. 1976;29(5-6):262–266. doi: 10.1159/000240873. [DOI] [PubMed] [Google Scholar]
- Hohenauer L., Oh W. Body composition in experimental intrauterine growth retardation in the rat. J Nutr. 1969 Sep;99(1):23–26. doi: 10.1093/jn/99.1.23. [DOI] [PubMed] [Google Scholar]
- Jones C. T. The development of some metabolic responses to hypoxia in the foetal sheep. J Physiol. 1977 Mar;265(3):743–762. doi: 10.1113/jphysiol.1977.sp011741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollée L. A., Monnens L. A., Trijbels J. M., Veerkamp J. H., Janssen A. J. Experimental intrauterine growth retardation in the rat. Evaluation of the Wigglesworth model. Early Hum Dev. 1979 Sep;3(3):295–300. doi: 10.1016/0378-3782(79)90038-0. [DOI] [PubMed] [Google Scholar]
- Kulhanek J. F., Meschia G., Makowski E. L., Battaglia F. C. Changes in DNA content and urea permeability of the sheep placenta. Am J Physiol. 1974 May;226(5):1257–1263. doi: 10.1152/ajplegacy.1974.226.5.1257. [DOI] [PubMed] [Google Scholar]
- Leduc B. Maternal placental blood flow and gestational age in rabbits. Am J Obstet Gynecol. 1972 Feb 1;112(3):374–378. doi: 10.1016/0002-9378(72)90480-2. [DOI] [PubMed] [Google Scholar]
- Meschia G., Battaglia F. C., Hay W. W., Sparks J. W. Utilization of substrates by the ovine placenta in vivo. Fed Proc. 1980 Feb;39(2):245–249. [PubMed] [Google Scholar]
- Myers R. E., Hill D. E., Holt A. B., Scott R. E., Mellits E. D., Cheek D. B. Fetal growth retardation produced by experimental placental insufficiency in the rhesus monkey. I. Body weight, organ size. Biol Neonate. 1971;18(5):379–394. doi: 10.1159/000240380. [DOI] [PubMed] [Google Scholar]
- Robinson J. S., Hart I. C., Kingston E. J., Jones C. T., Thorburn G. D. Studies on the growth of the fetal sheep. The effects of reduction of placental size on hormone concentration in fetal plasma. J Dev Physiol. 1980 Aug;2(4):239–248. [PubMed] [Google Scholar]
- Robinson J. S., Kingston E. J., Jones C. T., Thorburn G. D. Studies on experimental growth retardation in sheep. The effect of removal of a endometrial caruncles on fetal size and metabolism. J Dev Physiol. 1979 Oct;1(5):379–398. [PubMed] [Google Scholar]
- Rosenfeld C. R., Morriss F. H., Jr, Makowski E. L., Meschia G., Battaglia F. C. Circulatory changes in the reproductive tissues of ewes during pregnancy. Gynecol Invest. 1974;5(5-6):252–268. doi: 10.1159/000301658. [DOI] [PubMed] [Google Scholar]
- Roux J. M., Tordet-Caridroit C., Chanez C. Studies on experimental hypotrophy in the rat. I. Chemical composition of the total body and some organs in the rat foetus. Biol Neonate. 1970;15(56):342–347. doi: 10.1159/000240239. [DOI] [PubMed] [Google Scholar]
- Sasaki Y., Wagner H. N., Jr Measurement of the distribution of cardiac output in unanesthetized rats. J Appl Physiol. 1971 Jun;30(6):879–884. doi: 10.1152/jappl.1971.30.6.879. [DOI] [PubMed] [Google Scholar]
- Simmons M. A., Battaglia F. C., Meschia G. Placental transfer of glucose. J Dev Physiol. 1979 Jun;1(3):227–243. [PubMed] [Google Scholar]
- Smith T. L., Hutchins P. M. Anesthetic effects on hemodynamics of spontaneously hypertensive and Wistar-Kyoto rats. Am J Physiol. 1980 Apr;238(4):H539–H544. doi: 10.1152/ajpheart.1980.238.4.H539. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., Billewicz W. Z., Hytten F. E. The weight of the placenta in relation to birthweight. J Obstet Gynaecol Br Commonw. 1969 Oct;76(10):865–872. doi: 10.1111/j.1471-0528.1969.tb15722.x. [DOI] [PubMed] [Google Scholar]
- Van den Berg B. J., Yerushalmy J. The relationship of the rate of intrauterine growth of infants of low birth weight to mortality, morbidity, and congenital anomalies. J Pediatr. 1966 Oct;69(4):531–545. doi: 10.1016/s0022-3476(66)80038-0. [DOI] [PubMed] [Google Scholar]
- WIGGLESWORTH J. S. EXPERIMENTAL GROWTH RETARDATION IN THE FOETAL RAT. J Pathol Bacteriol. 1964 Jul;88:1–13. [PubMed] [Google Scholar]
- Wootton R., McFadyen I. R., Cooper J. E. Measurement of placental blood flow in the pig and its relation to placental and fetal weight. Biol Neonate. 1977;31(5-6):333–339. doi: 10.1159/000240984. [DOI] [PubMed] [Google Scholar]


