Abstract
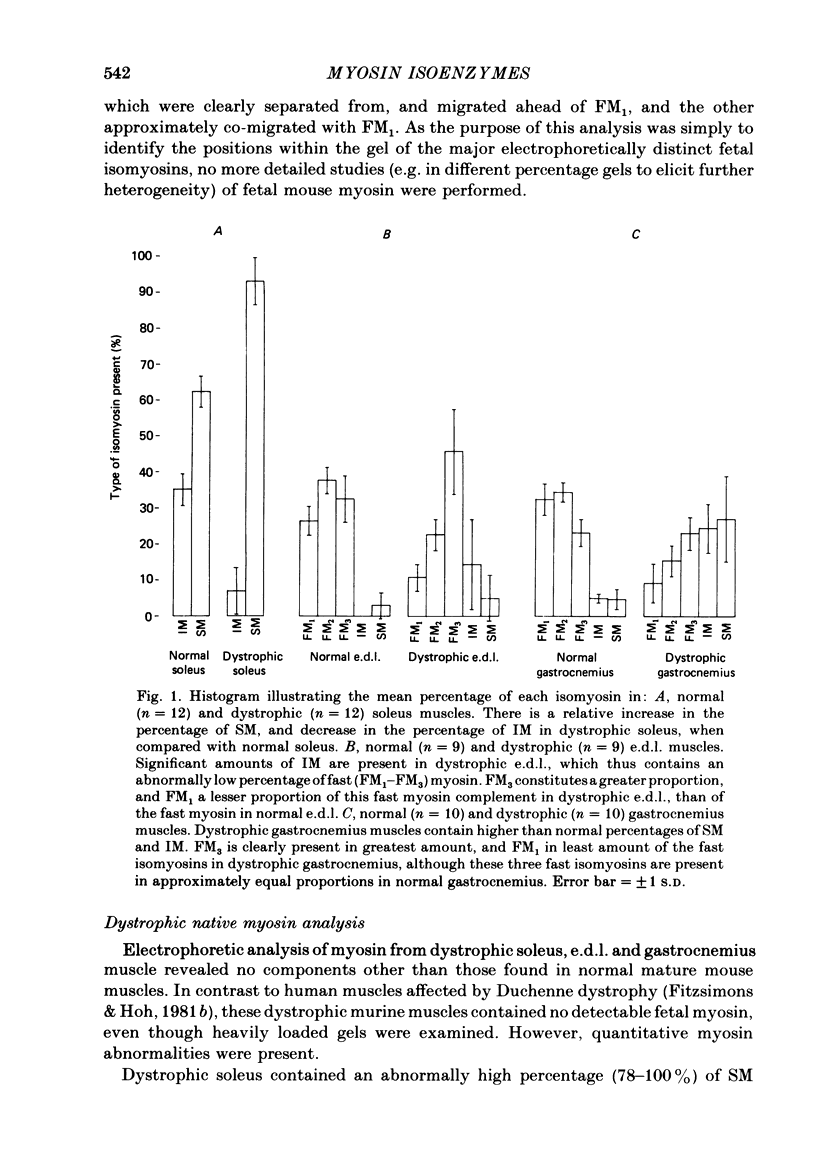
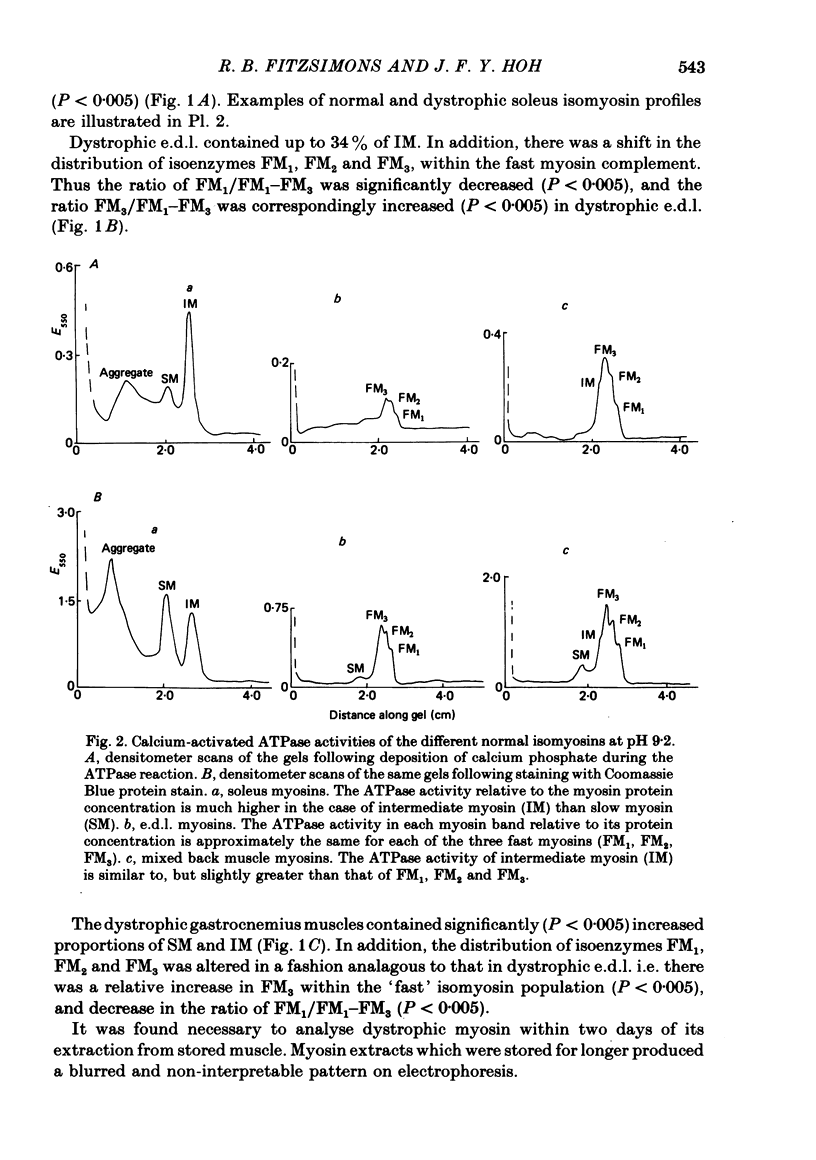
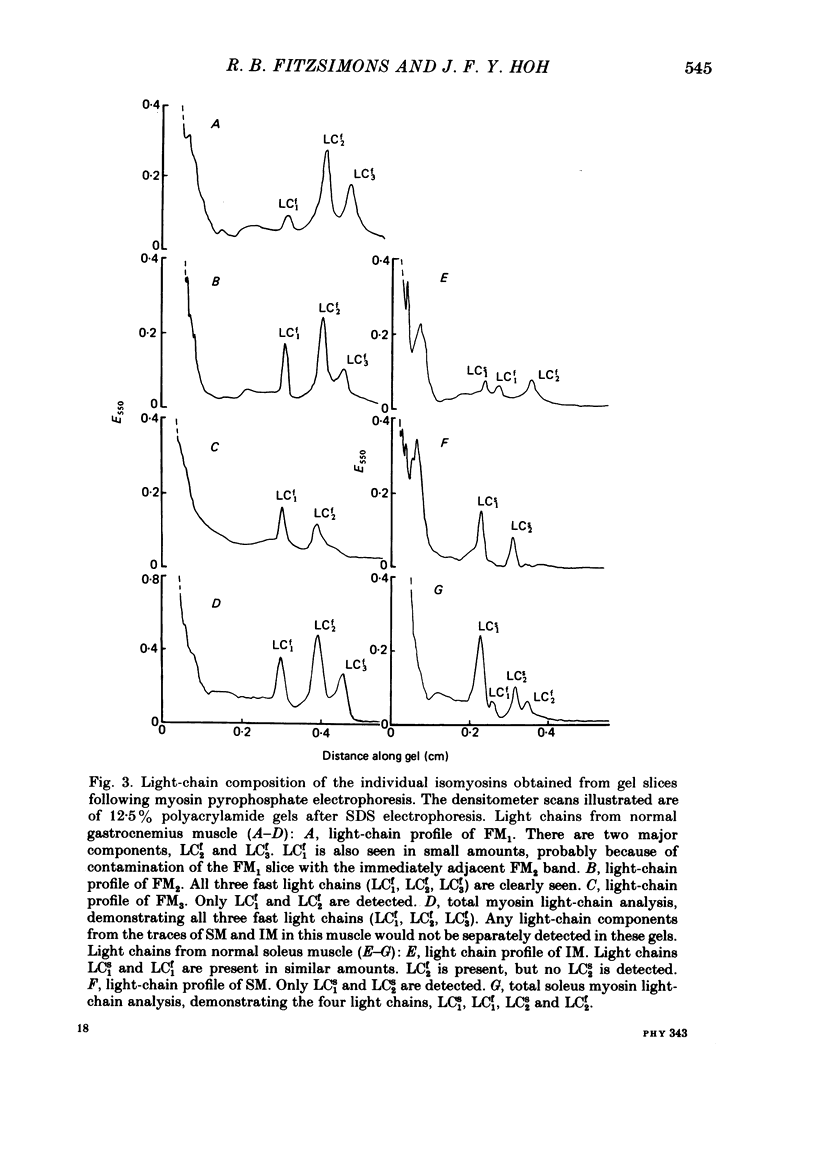
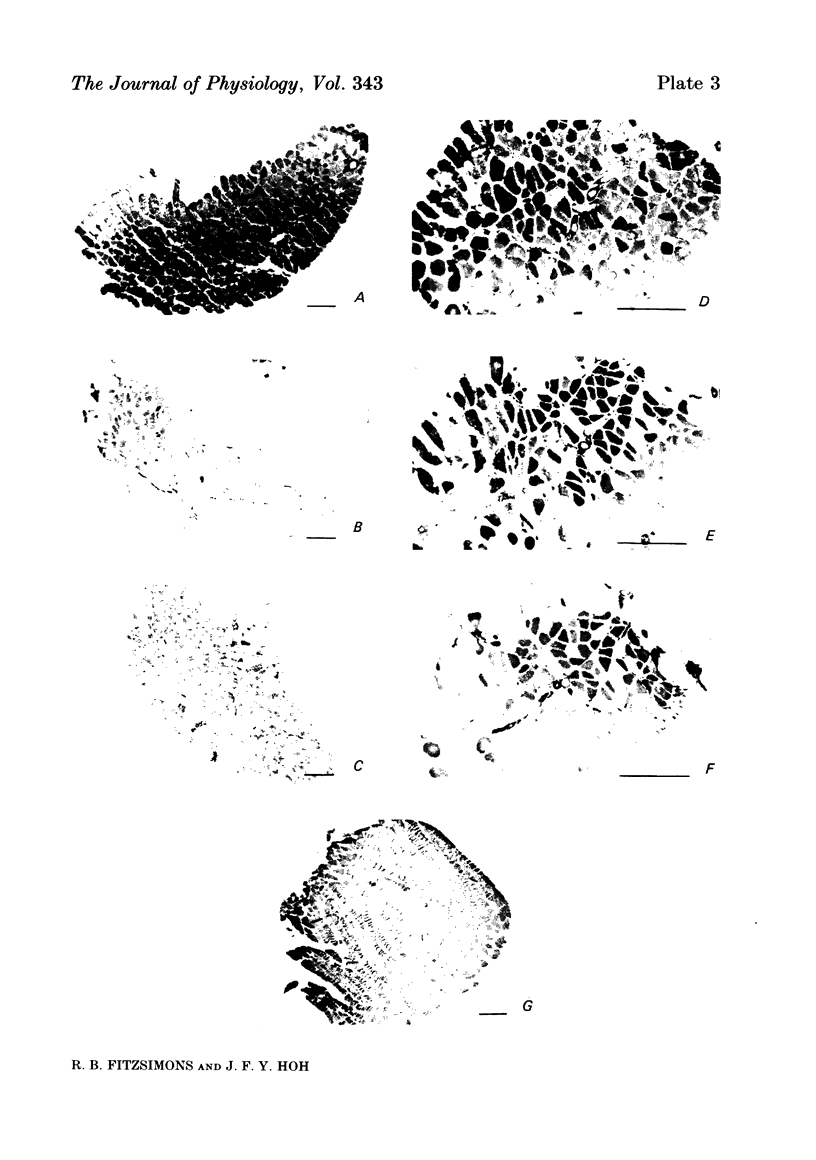
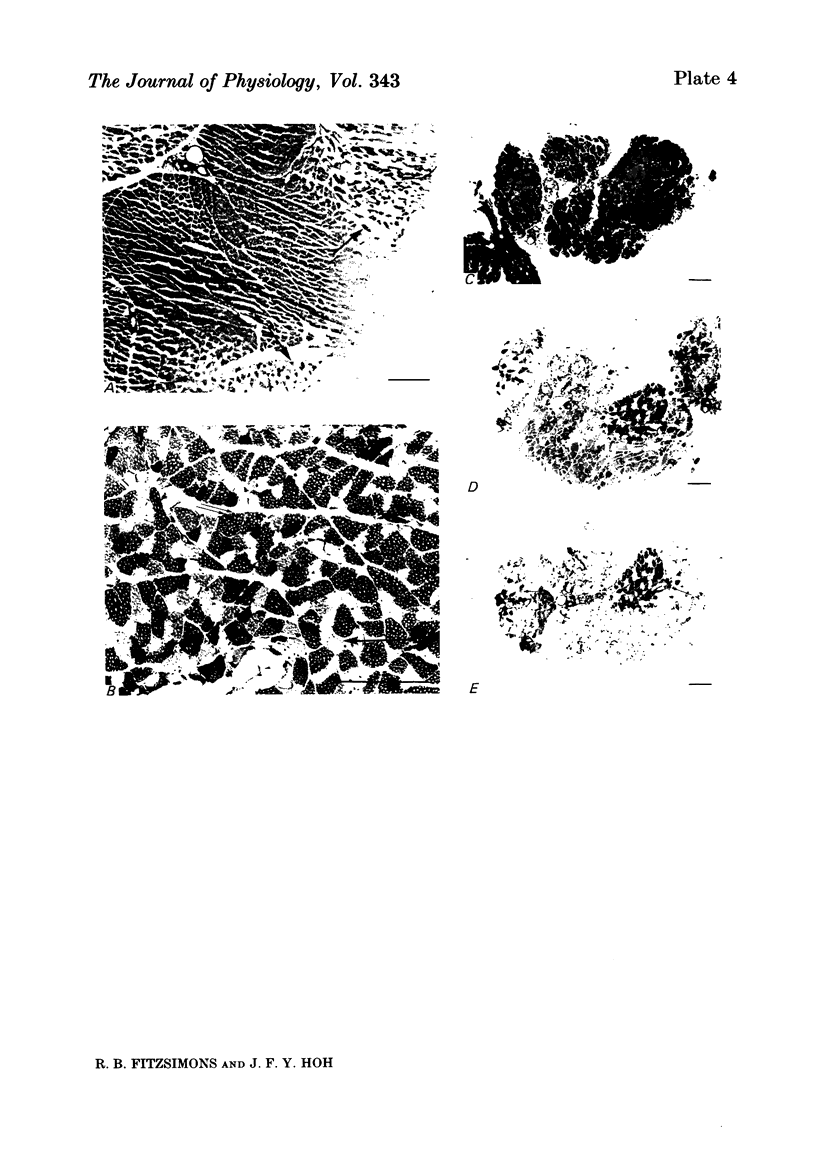
An analysis of the native myosin isoenzyme composition, myosin light-chain distribution and histochemical profile of fast-twitch and slow-twitch muscles of normal and dystrophic (129 REJ dy/dy) mice has been performed, and the results correlated with the known contractile abnormalities of murine dystrophic muscles. Normal mouse slow-twitch soleus contained two isomyosins (slow myosin, SM and intermediate myosin, IM) which were electrophoretically distinct from the three major isomyosins (FM1, FM2, FM3) of fast-twitch extensor digitorum longus (e.d.l.) muscle. The calcium-activated ATPase activities of FM1, FM2, FM3 and IM at pH 9.2 were each much higher than that of SM, and this difference is reflected in the histochemical profile of muscle, as demonstrated with the myofibrillar ATPase reaction at alkaline pH. E.d.l. Type II fibres retained myofibrillar ATPase activity following pre-incubation of histochemical sections at pH 4.6, and were therefore classified Type IIB, whereas soleus Type II fibres did not, and were classified Type IIA. It was concluded that Type I (slow) fibres contain SM, Type IIA (intermediate) fibres contain IM, and Type IIB (fast) fibres contain FM1-FM3. Each electrophoretically distinct myosin contained a different combination of the five skeletal myosin light chains (LCs). Thus different normal muscles, which differed in their isomyosin profiles, differed also in their light-chain composition. Analysis of the distribution of native myosins (FM1, FM2, FM3, IM, SM, in order of decreasing gel migration rate) in dystrophic muscles revealed increased proportions of the slower-migrating forms, when compared with the distribution in the corresponding normal muscles. The shift in isomyosin distribution would explain the known decrease in the proportion of myosin light chain (LCf3) in murine dystrophic muscle. The abnormal isomyosin distribution in the dystrophic muscle is correlated with its altered histochemical characteristics, and with well-established abnormalities in its isometric and isotonic properties. It is concluded that the altered isomyosin distribution in murine dystrophic muscle would result in decreased power output per unit muscle mass when compared with normal muscle. The possibility is considered that defective myelination of the innervating nerve may contribute to these abnormalities by preventing higher frequency impulses from reaching muscle.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley W. G., Jenkison M. Abnormalities of peripheral nerves in murine muscular dystrophy. J Neurol Sci. 1973 Feb;18(2):227–247. doi: 10.1016/0022-510x(73)90009-9. [DOI] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Muscle fiber types: how many and what kind? Arch Neurol. 1970 Oct;23(4):369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Close R. Properties of motor units in fast and slow skeletal muscles of the rat. J Physiol. 1967 Nov;193(1):45–55. doi: 10.1113/jphysiol.1967.sp008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. The relation between intrinsic speed of shortening and duration of the active state of muscle. J Physiol. 1965 Oct;180(3):542–559. doi: 10.1113/jphysiol.1965.sp007716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla Libera L., Sartore S., Pierobon-Bormioli S., Schiaffino S. Fast-white and fast-red isomyosins in guinea pig muscles. Biochem Biophys Res Commun. 1980 Oct 31;96(4):1662–1670. doi: 10.1016/0006-291x(80)91365-0. [DOI] [PubMed] [Google Scholar]
- Douglas W. B., Jr, Baskin R. J. Contractile properties of developing mouse dystrophic muscle. Am J Physiol. 1971 May;220(5):1344–1354. doi: 10.1152/ajplegacy.1971.220.5.1344. [DOI] [PubMed] [Google Scholar]
- Fitzsimons R. B., Hoh J. F. Embryonic and foetal myosins in human skeletal muscle. The presence of foetal myosins in duchenne muscular dystrophy and infantile spinal muscular atrophy. J Neurol Sci. 1981 Nov-Dec;52(2-3):367–384. doi: 10.1016/0022-510x(81)90018-6. [DOI] [PubMed] [Google Scholar]
- Fitzsimons R. B., Hoh J. F. Isomyosins in human type 1 and type 2 skeletal muscle fibres. Biochem J. 1981 Jan 1;193(1):229–233. doi: 10.1042/bj1930229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons R. B., Hoh J. F., McLeod J. G. Myosins in murine muscular dystrophy. Clin Exp Neurol. 1977;14:260–263. [PubMed] [Google Scholar]
- Harris J. B., Wilson P. Mechanical properties of dystrophic mouse muscle. J Neurol Neurosurg Psychiatry. 1971 Oct;34(5):512–520. doi: 10.1136/jnnp.34.5.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinterbuchner L. P., Angyan A., Hirsch M. Effects of series of tetani on dystrophic and normal muscles of mouse. Am J Physiol. 1966 Oct;211(4):915–918. doi: 10.1152/ajplegacy.1966.211.4.915. [DOI] [PubMed] [Google Scholar]
- Hoh J. F. Light chain distribution of chicken skeletal muscle myosin isoenzymes. FEBS Lett. 1978 Jun 15;90(2):297–300. doi: 10.1016/0014-5793(78)80390-1. [DOI] [PubMed] [Google Scholar]
- Hoh J. F., Yeoh G. P. Rabbit skeletal myosin isoenzymes from fetal, fast-twitch and slow-twitch muscles. Nature. 1979 Jul 26;280(5720):321–323. doi: 10.1038/280321a0. [DOI] [PubMed] [Google Scholar]
- Hoh J. Y., McGrath P. A., White R. I. Electrophoretic analysis of multiple forms of myosin in fast-twitch and slow-twitch muscles of the chick. Biochem J. 1976 Jul 1;157(1):87–95. doi: 10.1042/bj1570087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F. Muscular contraction. J Physiol. 1974 Nov;243(1):1–43. [PMC free article] [PubMed] [Google Scholar]
- John H. A. The myosin of developing and dystrophic skeletal muscle. FEBS Lett. 1974 Mar 1;39(3):278–282. doi: 10.1016/0014-5793(74)80130-4. [DOI] [PubMed] [Google Scholar]
- Kugelberg E. Histochemical composition, contraction speed and fatiguability of rat soleus motor units. J Neurol Sci. 1973 Oct;20(2):177–198. doi: 10.1016/0022-510x(73)90029-4. [DOI] [PubMed] [Google Scholar]
- Lewis D. M., Parry D. J., Rowlerson A. Isometric contractions of motor units and immunohistochemistry of mouse soleus muscle. J Physiol. 1982 Apr;325:393–401. doi: 10.1113/jphysiol.1982.sp014157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomo T., Westgaard R. H., Dahl H. A. Contractile properties of muscle: control by pattern of muscle activity in the rat. Proc R Soc Lond B Biol Sci. 1974 Aug 27;187(1086):99–103. doi: 10.1098/rspb.1974.0064. [DOI] [PubMed] [Google Scholar]
- Luff A. R. Dynamic properties of the inferior rectus, extensor digitorum longus, diaphragm and soleus muscles of the mouse. J Physiol. 1981;313:161–171. doi: 10.1113/jphysiol.1981.sp013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald W. I., Sears T. A. The effects of experimental demyelination on conduction in the central nervous system. Brain. 1970;93(3):583–598. doi: 10.1093/brain/93.3.583. [DOI] [PubMed] [Google Scholar]
- Michelson A. M., Russell E. S., Harman P. J. Dystrophia Muscularis: A HEREDITARY PRIMARY MYOPATHY IN THE HOUSE MOUSE. Proc Natl Acad Sci U S A. 1955 Dec 15;41(12):1079–1084. doi: 10.1073/pnas.41.12.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J. R., Shafiq S. A. Muscle regeneration in the muscular dystrophies. Ann N Y Acad Sci. 1979;317:478–493. doi: 10.1111/j.1749-6632.1979.tb56564.x. [DOI] [PubMed] [Google Scholar]
- Pierobon-Bormioli S., Sartore S., Libera L. D., Vitadello M., Schiaffino S. "Fast" isomyosins and fiber types in mammalian skeletal muscle. J Histochem Cytochem. 1981 Oct;29(10):1179–1188. doi: 10.1177/29.10.7028858. [DOI] [PubMed] [Google Scholar]
- SANDOW A., BRUST M. Contractility of dystrophic mouse muscle. Am J Physiol. 1958 Sep;194(3):557–563. doi: 10.1152/ajplegacy.1958.194.3.557. [DOI] [PubMed] [Google Scholar]
- Whalen R. G., Sell S. M., Butler-Browne G. S., Schwartz K., Bouveret P., Pinset-Härstöm I. Three myosin heavy-chain isozymes appear sequentially in rat muscle development. Nature. 1981 Aug 27;292(5826):805–809. doi: 10.1038/292805a0. [DOI] [PubMed] [Google Scholar]
- d'Albis A., Pantaloni C., Bechet J. J. An electrophoretic study of native myosin isozymes and of their subunit content. Eur J Biochem. 1979 Sep;99(2):261–272. doi: 10.1111/j.1432-1033.1979.tb13253.x. [DOI] [PubMed] [Google Scholar]