Abstract
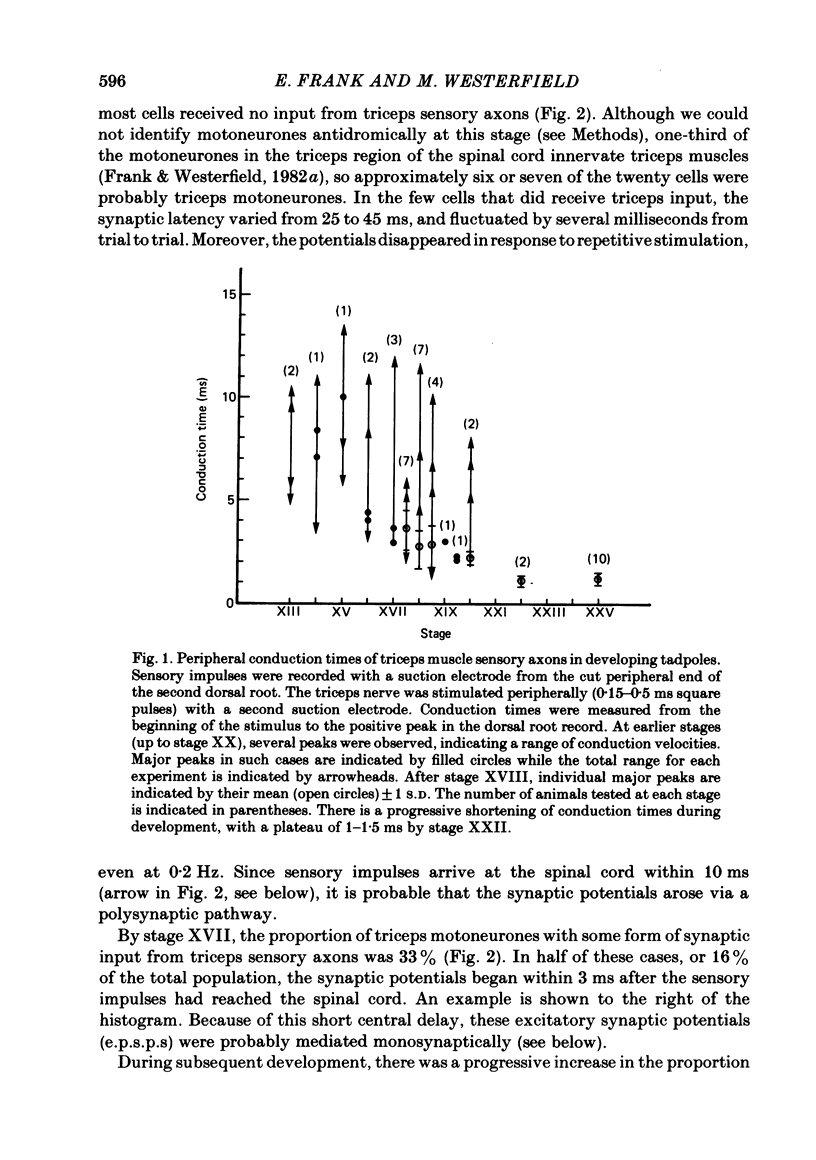
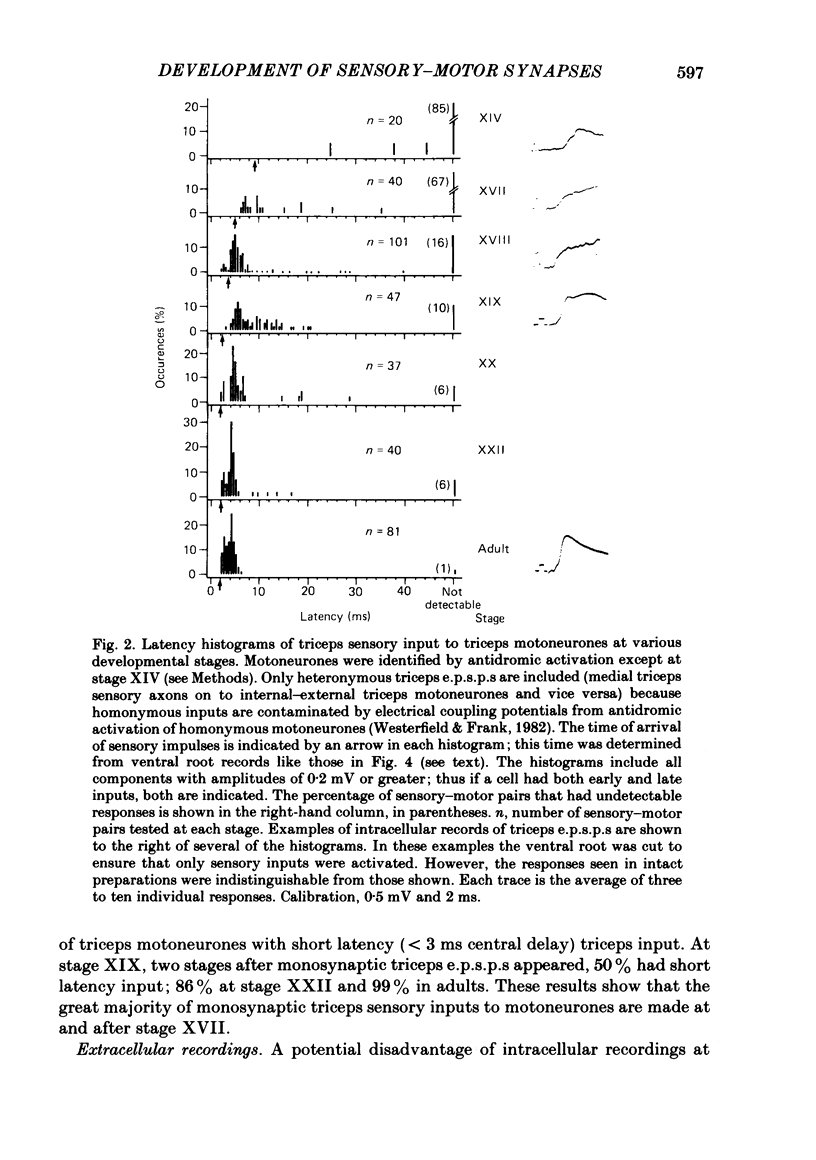
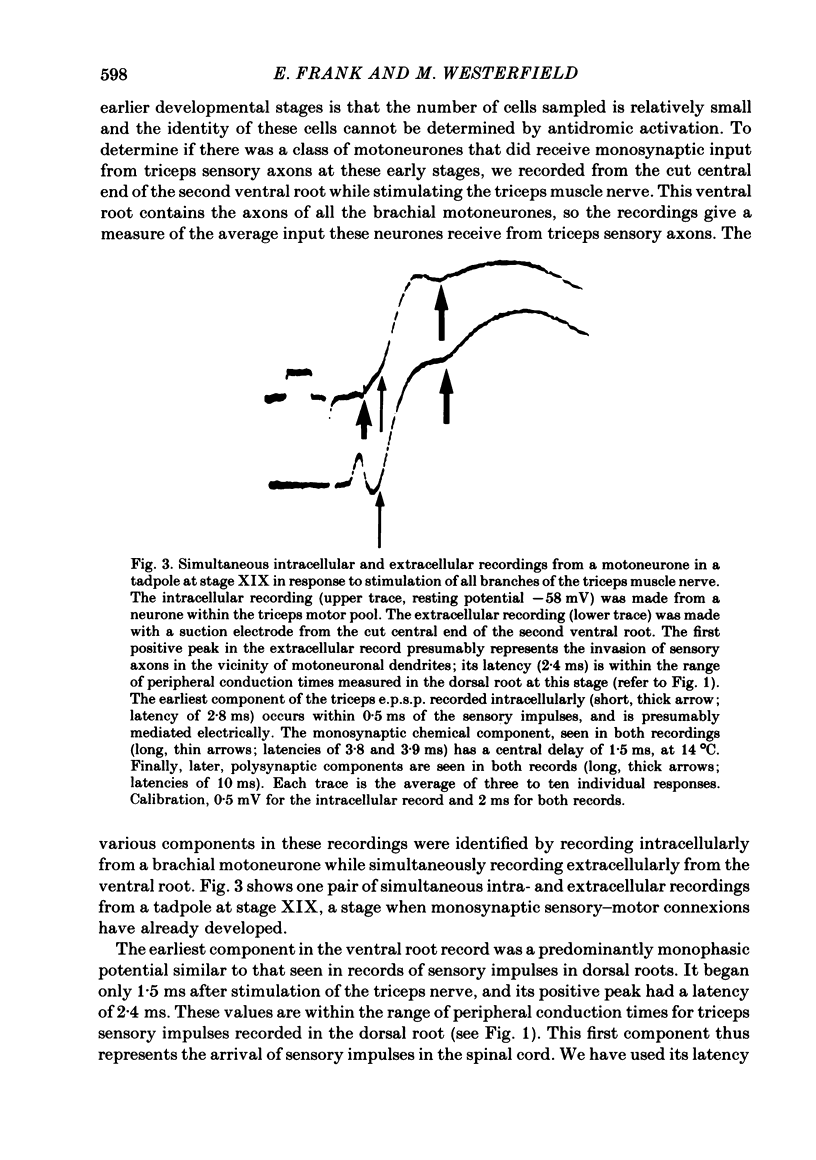
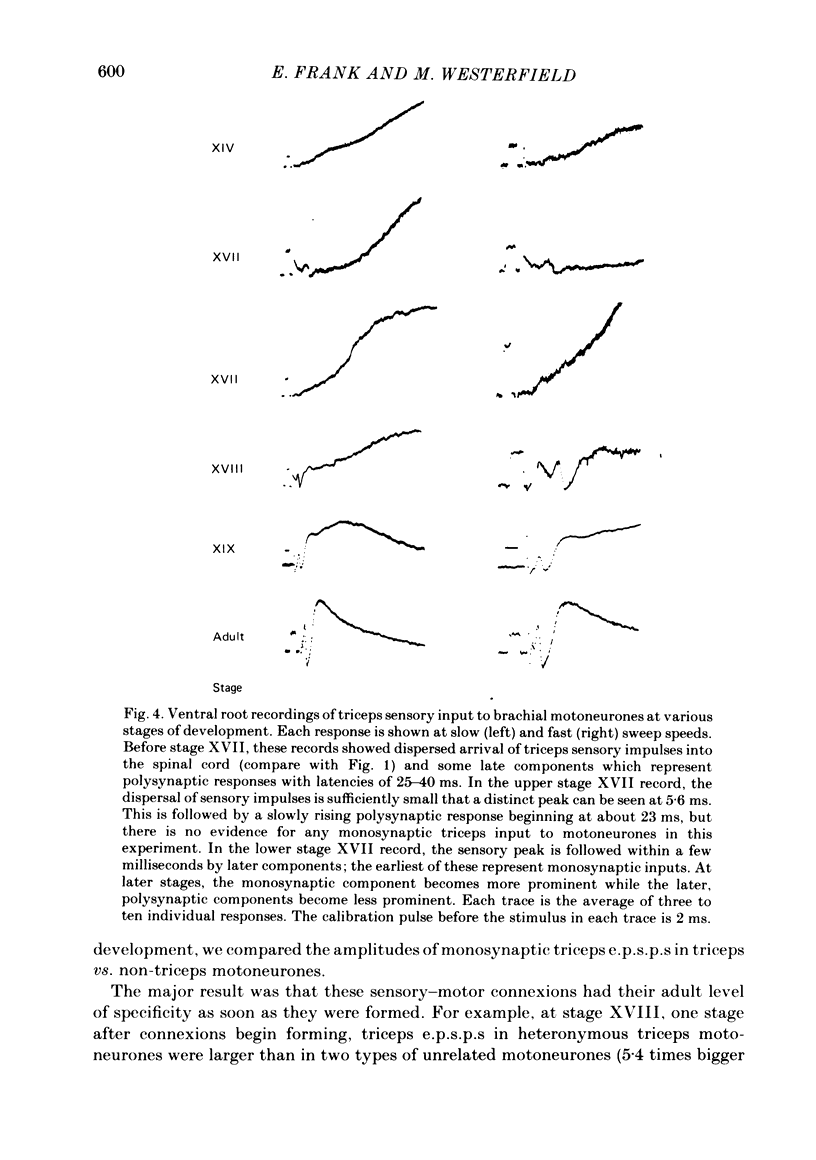
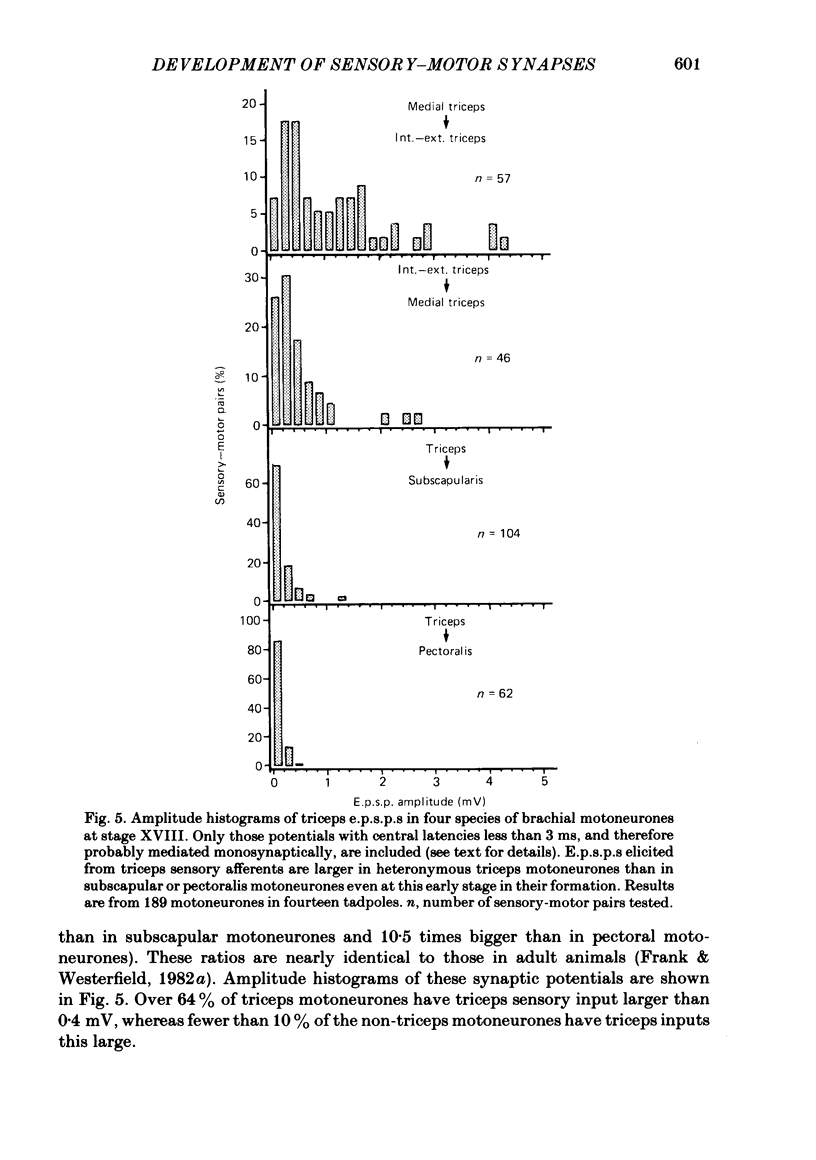
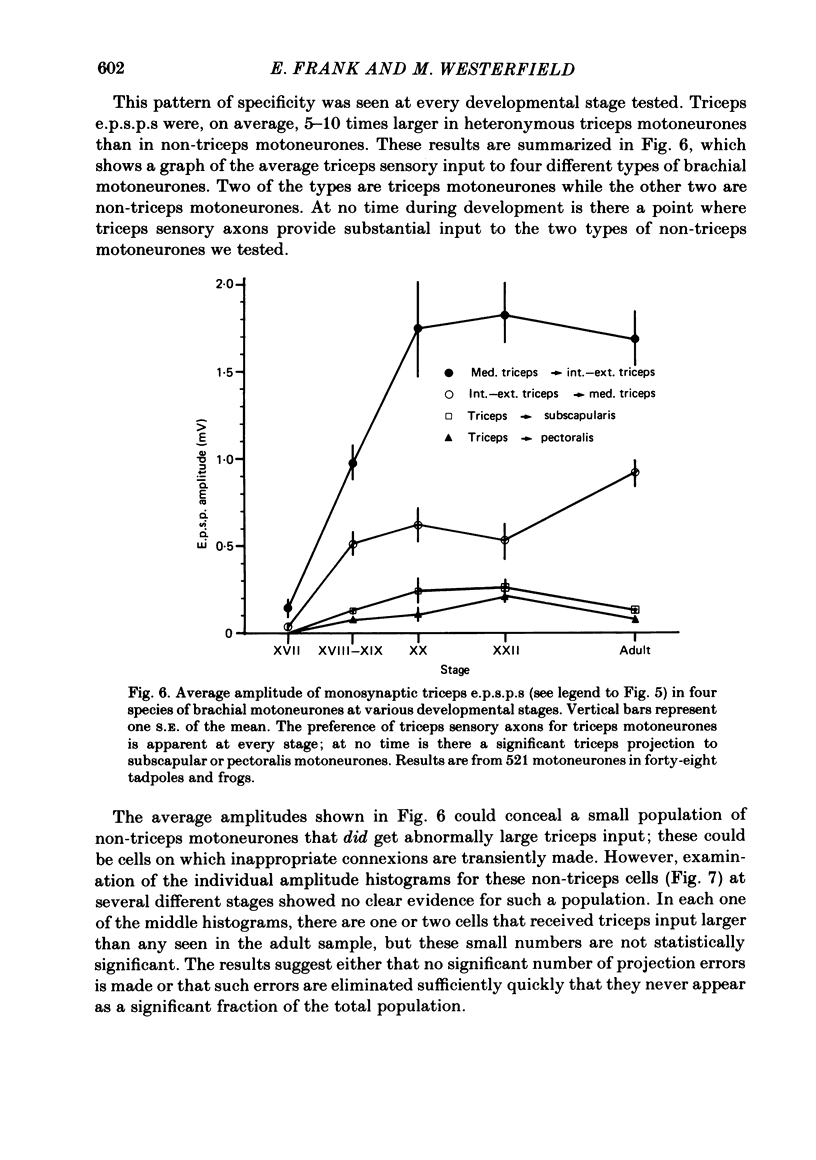
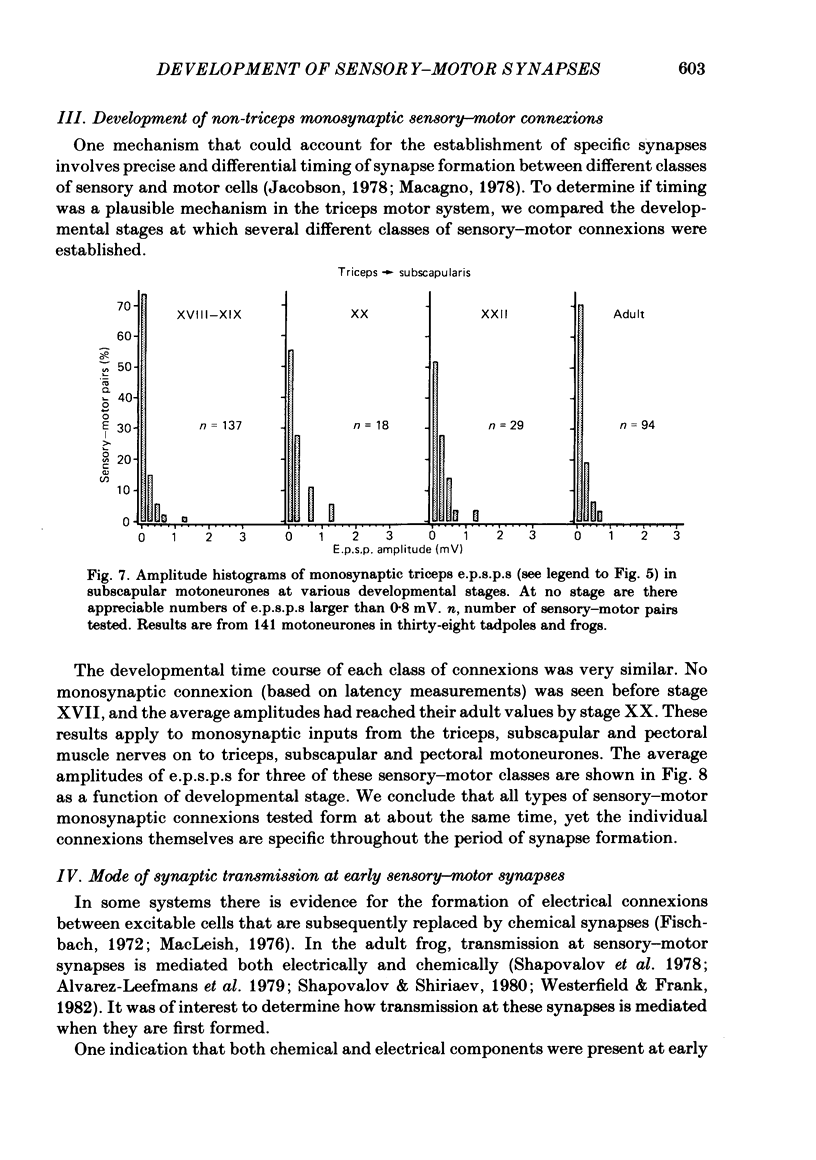
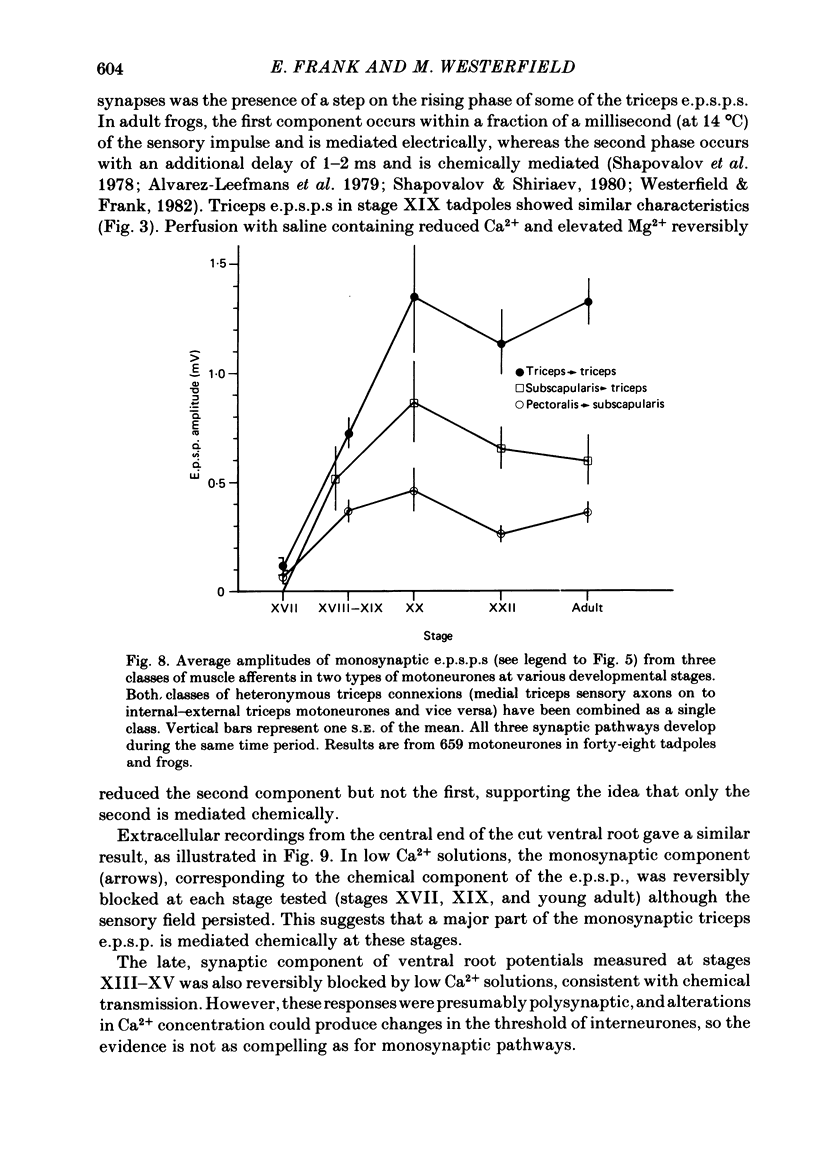
The development and specificity of monosynaptic sensory-motor synapses were studied in the brachial spinal cord of bullfrog tadpoles. Intracellular and extracellular recordings were made from motoneurones innervating several different muscles of the forelimb. Excitatory synaptic potentials (e.p.s.p.s) were elicited by stimulation of various peripheral muscle nerves. Sensory and motor axons in the triceps brachii muscle nerves were electrically excitable at stage XIII, the earliest stage studied. Their conduction velocities were 0.2-0.4 m/s. These velocities increased during subsequent development so that by stage XXII they were approximately 5 m/s. Before stage XVII, synaptic potentials evoked in motoneurones by stimulation of the triceps sensory fibres had a long central latency and fatigued easily. These potentials were probably mediated polysynaptically. At stage XVII, the first short-latency triceps synaptic potentials appeared. They had central latencies of less than 3 ms and represented the direct, monosynaptic input from muscle sensory cells on to motoneurones. During subsequent development the percentage of triceps motoneurones innervated by triceps sensory fibres increased, while the number of long-latency polysynaptic inputs decreased. Both the electrical and chemical components, characteristic of these monosynaptic e.p.s.p.s in adult frogs, were prominent from the time the e.p.s.p.s first appeared. The pattern of innervation of brachial motoneurones by triceps sensory afferents was specific from the beginning. Triceps sensory fibres innervated most triceps motoneurones but very few subscapular or pectoralis motoneurones, just as in adult frogs. At no time were there appreciable numbers of 'aberrant' connexions. The developmental time course of several different classes of sensory-motor connexions was similar. Thus the synaptic specificity of this system cannot be explained by a differential timing of synaptogenesis.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Leefmans F. J., De Santis A., Miledi R. Effects of some divalent cations on synaptic transmission in frog spinal neurones. J Physiol. 1979 Sep;294:387–406. doi: 10.1113/jphysiol.1979.sp012936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R. E., Jacobson M. Development of reflexes from skin grafts in Rana pipiens: influence of size and position of grafts. Dev Biol. 1970 Jul;22(3):476–494. doi: 10.1016/0012-1606(70)90164-8. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., WILLIS W. D. THE EFFECT OF REPETITIVE STIMULATION UPON MONOSYNAPTIC TRANSMISSION IN KITTENS. J Physiol. 1965 Jan;176:311–321. doi: 10.1113/jphysiol.1965.sp007552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles R. M., Shealy C. N., Willis W. D. Patterns of innervation of kitten motoneurones. J Physiol. 1963 Mar;165(3):392–402. doi: 10.1113/jphysiol.1963.sp007065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach G. D. Synapse formation between dissociated nerve and muscle cells in low density cell cultures. Dev Biol. 1972 Jun;28(2):407–429. doi: 10.1016/0012-1606(72)90023-1. [DOI] [PubMed] [Google Scholar]
- Frank E., Westerfield M. Synaptic organization of sensory and motor neurones innervating triceps brachii muscles in the bullfrog. J Physiol. 1982 Mar;324:479–494. doi: 10.1113/jphysiol.1982.sp014125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E., Westerfield M. The formation of appropriate central and peripheral connexions by foreign sensory neurones of the bullfrog. J Physiol. 1982 Mar;324:495–505. doi: 10.1113/jphysiol.1982.sp014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito F. Muscle spindle responses during contractions of extrafusal muscle fibers in the frog. Jpn J Physiol. 1968 Oct 15;18(5):601–608. doi: 10.2170/jjphysiol.18.601. [DOI] [PubMed] [Google Scholar]
- Kellerth J. O., Mellström A., Skoglund S. Postnatal excitability changes of kitten motoneurones. Acta Physiol Scand. 1971 Sep;83(1):31–41. doi: 10.1111/j.1748-1716.1971.tb05048.x. [DOI] [PubMed] [Google Scholar]
- Landmesser L. T. The generation of neuromuscular specificity. Annu Rev Neurosci. 1980;3:279–302. doi: 10.1146/annurev.ne.03.030180.001431. [DOI] [PubMed] [Google Scholar]
- Letinsky M. S. The development of nerve-muscle junctions in Rana catesbeiana tadpoles. Dev Biol. 1974 Sep;40(1):129–153. doi: 10.1016/0012-1606(74)90114-6. [DOI] [PubMed] [Google Scholar]
- Lichtman J. W., Purves D. The elimination of redundant preganglionic innervation to hamster sympathetic ganglion cells in early post-natal life. J Physiol. 1980 Apr;301:213–228. doi: 10.1113/jphysiol.1980.sp013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W., Purves D., Yip J. W. On the purpose of selective innervation of guinea-pig superior cervical ganglion cells. J Physiol. 1979 Jul;292:69–84. doi: 10.1113/jphysiol.1979.sp012839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINER N. Integumental specification of sensory fibers in the development of cutaneous local sign. J Comp Neurol. 1956 Aug;105(1):161–170. doi: 10.1002/cne.901050109. [DOI] [PubMed] [Google Scholar]
- Macagno E. R. Mechanism for the formation of synaptic projections in the arthropod visual system. Nature. 1978 Sep 28;275(5678):318–320. doi: 10.1038/275318a0. [DOI] [PubMed] [Google Scholar]
- Mellström A. Postnatal excitability changes of the ankle monosynaptic reflexes in the cat. Acta Physiol Scand. 1971 Aug;82(4):477–489. doi: 10.1111/j.1748-1716.1971.tb04993.x. [DOI] [PubMed] [Google Scholar]
- Mellström A. Recurrent and antidromic effects on the monosynaptic reflex during postnatal development in the cat. Acta Physiol Scand. 1971 Aug;82(4):490–499. doi: 10.1111/j.1748-1716.1971.tb04994.x. [DOI] [PubMed] [Google Scholar]
- Mendell L. M., Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol. 1971 Jan;34(1):171–187. doi: 10.1152/jn.1971.34.1.171. [DOI] [PubMed] [Google Scholar]
- NAKA K. I. ELECTROPHYSIOLOGY OF THE FETAL SPINAL CORD. I. ACTION POTENTIALS OF THE MOTONEURON. J Gen Physiol. 1964 May;47:1003–1022. doi: 10.1085/jgp.47.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKA K. I. ELECTROPHYSIOLOGY OF THE FETAL SPINAL CORD. II. INTERACTION AMONG PERIPHERAL INPUTS AND RECURRENT INHIBITION. J Gen Physiol. 1964 May;47:1023–1038. doi: 10.1085/jgp.47.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provine R. R., Rogers L. Development of spinal cord bioelectric activity in spinal chick embryos and its behavioral implications. J Neurobiol. 1977 May;8(3):217–228. doi: 10.1002/neu.480080305. [DOI] [PubMed] [Google Scholar]
- Provine R. R., Sharma S. C., Sandel T. T., Hamburger V. Electrical activity in the spinal cord of the chick embryo, in situ. Proc Natl Acad Sci U S A. 1970 Mar;65(3):508–515. doi: 10.1073/pnas.65.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Lichtman J. W. Elimination of synapses in the developing nervous system. Science. 1980 Oct 10;210(4466):153–157. doi: 10.1126/science.7414326. [DOI] [PubMed] [Google Scholar]
- SPERRY R. W. CHEMOAFFINITY IN THE ORDERLY GROWTH OF NERVE FIBER PATTERNS AND CONNECTIONS. Proc Natl Acad Sci U S A. 1963 Oct;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K. Development of spinal reflexes in the rat fetus studied in vitro. J Physiol. 1979 Sep;294:581–594. doi: 10.1113/jphysiol.1979.sp012947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapovalov A. I., Shiriaev B. I. Dual mode of junctional transmission at synapses between single primary afferent fibres and motoneurones in the amphibian. J Physiol. 1980 Sep;306:1–15. doi: 10.1113/jphysiol.1980.sp013381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapovalov A. I., Shiriaev B. I. Two types of electronic EPSP evoked in amphibian motoneurons by ventral root stimulation. Exp Brain Res. 1978 Nov 15;33(3-4):313–323. doi: 10.1007/BF00235556. [DOI] [PubMed] [Google Scholar]
- Shapovalov A. I., Shiriaev B. I., Velumian A. A. Mechanisms of post-synaptic excitation in amphibian motoneurones. J Physiol. 1978 Jun;279:437–455. doi: 10.1113/jphysiol.1978.sp012355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S. C., Provine R. R., Hamburger V., Sandel T. T. Unit activity in the isolated spinal cord of chick embryo, in situ. Proc Natl Acad Sci U S A. 1970 May;66(1):40–47. doi: 10.1073/pnas.66.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamarova Z. A. Vozbuzhdaiushchie postsinapticheskie potentsialy v poiasnichnykh motoneironakh liagushki, vyzvannye razdrazheniem myshechnykh i kozhnykh nervov. Fiziol Zh SSSR Im I M Sechenova. 1977 Jun;63(6):806–813. [PubMed] [Google Scholar]
- WILSON V. J. Reflex transmission in the kitten. J Neurophysiol. 1962 Mar;25:263–276. doi: 10.1152/jn.1962.25.2.263. [DOI] [PubMed] [Google Scholar]
- Westerfield M., Frank E. Specificity of electrical coupling among neurons innervating forelimb muscles of the adult bullfrog. J Neurophysiol. 1982 Oct;48(4):904–913. doi: 10.1152/jn.1982.48.4.904. [DOI] [PubMed] [Google Scholar]


