Abstract
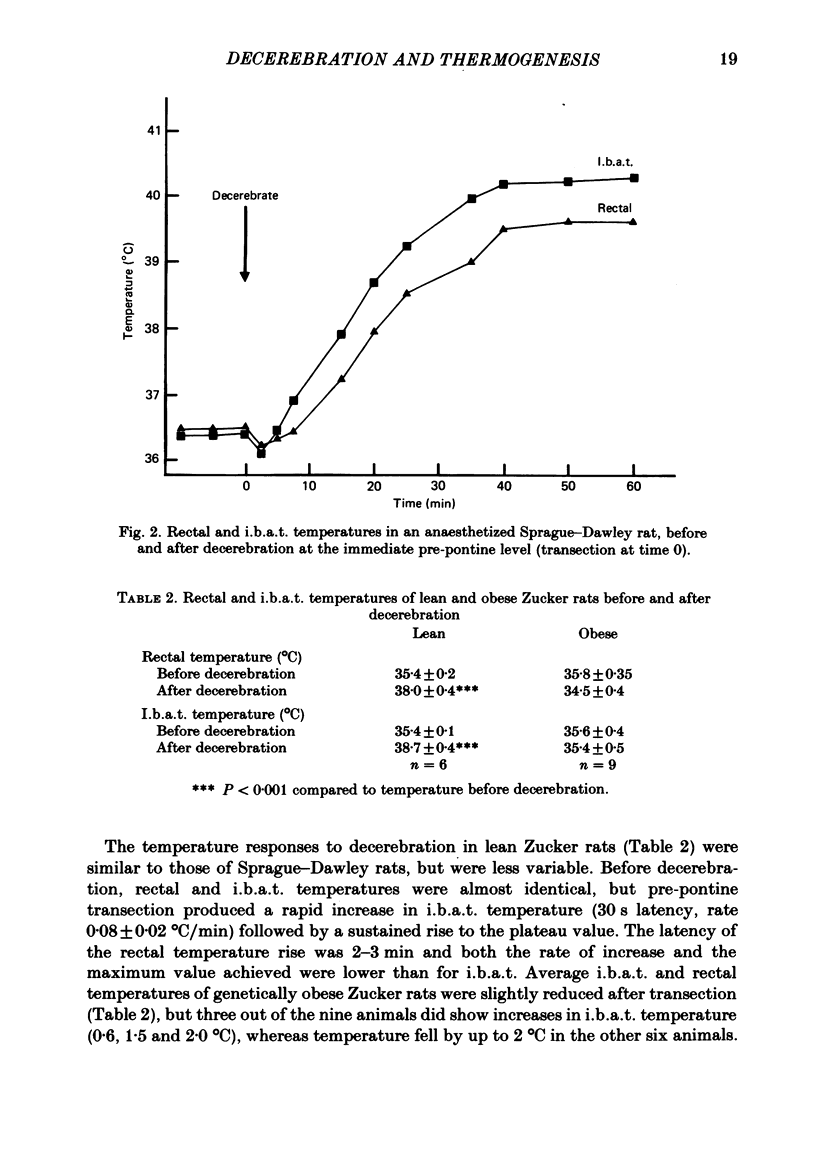
Under fluothane anaesthesia, suction decerebration was performed at the immediate pre-pontine level in adult, male, Sprague-Dawley rats; this resulted in a large and sustained rise in rectal temperature from 35.6 +/- 0.2 (control) to 38.8 +/- 0.5 degrees C (decerebrate) following recovery from anaesthesia. Propranolol inhibited this rise. In a separate group of continuously (urethane) anaesthetized rats, brain transection at the immediate pre-pontine level produced marked increases in rectal temperature and oxygen consumption, both of which were inhibited by injection of the beta-adrenergic antagonist propranolol (10 mg/kg). The rise in rectal temperature (2.8 +/- 0.4 degrees C) after transection was preceded by a greater increase (3.6 +/- 0.3 degrees C) in the temperature of the interscapular brown adipose tissue (i.b.a.t.). Skin temperature on the tail showed no immediate response. In anaesthetized lean (+/?) male Zucker rats, rectal and i.b.a.t. temperatures showed similar responses to Sprague-Dawley rats after decerebration, but in the genetically obese (fa/fa) Zucker rat, temperatures were not significantly altered by decerebration. The above results, together with macroscopic examination of the transected brains, suggest that descending pathways (possibly arising in the mid-brain tegmentum) normally inhibit a sustained thermogenic drive from areas in the lower brain stem. Decerebration can release this inhibition and cause a large rise in body temperature and in metabolic rate, which apparently result from sympathetic activation of i.b.a.t. The genetically obese Zucker rat exhibits an impaired thermogenic response to decerebration.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bignall K. E., Heggeness F. W., Palmer J. E. Effect of neonatal decerebration on thermogenesis during starvation and cold exposure in the rat. Exp Neurol. 1975 Oct;49(1 Pt 1):174–188. doi: 10.1016/0014-4886(75)90203-4. [DOI] [PubMed] [Google Scholar]
- Flaim K. E., Horowitz J. M., Horwitz B. A. Functional and anatomical characteristics of the nerve-brown adipose interaction in the rat. Pflugers Arch. 1976 Sep 3;365(1):9–14. doi: 10.1007/BF00583622. [DOI] [PubMed] [Google Scholar]
- Foster D. O., Frydman M. L. Nonshivering thermogenesis in the rat. II. Measurements of blood flow with microspheres point to brown adipose tissue as the dominant site of the calorigenesis induced by noradrenaline. Can J Physiol Pharmacol. 1978 Feb;56(1):110–122. doi: 10.1139/y78-015. [DOI] [PubMed] [Google Scholar]
- Gale C. C. Neuroendocrine aspects of thermoregulation. Annu Rev Physiol. 1973;35:391–430. doi: 10.1146/annurev.ph.35.030173.002135. [DOI] [PubMed] [Google Scholar]
- Goldman J. K., Bernardis L. L., Frohman L. A. Food intake in hypothalamic obesity. Am J Physiol. 1974 Jul;227(1):88–91. doi: 10.1152/ajplegacy.1974.227.1.88. [DOI] [PubMed] [Google Scholar]
- Han P. W. Hypothalamic obesity in rats without hyperphagia. Trans N Y Acad Sci. 1967 Dec;30(2):229–243. doi: 10.1111/j.2164-0947.1967.tb02460.x. [DOI] [PubMed] [Google Scholar]
- Kissileff H. R., Van Itallie T. B. Physiology of the control of food intake. Annu Rev Nutr. 1982;2:371–418. doi: 10.1146/annurev.nu.02.070182.002103. [DOI] [PubMed] [Google Scholar]
- Perkins M. N., Rothwell N. J., Stock M. J., Stone T. W. Activation of brown adipose tissue thermogenesis by the ventromedial hypothalamus. Nature. 1981 Jan 29;289(5796):401–402. doi: 10.1038/289401a0. [DOI] [PubMed] [Google Scholar]
- Rothwell N. J., Saville M. E., Stock M. J. Acute effects of food, 2-deoxy-D-glucose and noradrenaline on metabolic rate and brown adipose tissue in normal and atropinised lean and obese (fa/fa) Zucker rats. Pflugers Arch. 1981 Dec;392(2):172–177. doi: 10.1007/BF00581268. [DOI] [PubMed] [Google Scholar]
- Rothwell N. J., Stock M. J. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979 Sep 6;281(5726):31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- Rothwell N. J., Stock M. J. Influence of noradrenaline on blood flow to brown adipose tissue in rats exhibiting diet-induced thermogenesis. Pflugers Arch. 1981 Mar;389(3):237–242. doi: 10.1007/BF00584784. [DOI] [PubMed] [Google Scholar]
- Seydoux J., Rohner-Jeanrenaud F., Assimacopoulos-Jeannet F., Jeanrenaud B., Girardier L. Functional disconnection of brown adipose tissue in hypothalamic obesity in rats. Pflugers Arch. 1981 Apr;390(1):1–4. doi: 10.1007/BF00582702. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Horwitz B. A. Brown fat and thermogenesis. Physiol Rev. 1969 Apr;49(2):330–425. doi: 10.1152/physrev.1969.49.2.330. [DOI] [PubMed] [Google Scholar]
- Stock M. J. An automatic, closed-circuit oxygen consumption apparatus for small animals. J Appl Physiol. 1975 Nov;39(5):849–850. doi: 10.1152/jappl.1975.39.5.849. [DOI] [PubMed] [Google Scholar]
- Young R. A., Tulp O. L., Horton E. S. Thyroid and growth responses of young Zucker obese and lean rats to a low protein-high carbohydrate diet. J Nutr. 1980 Jul;110(7):1421–1431. doi: 10.1093/jn/110.7.1421. [DOI] [PubMed] [Google Scholar]


