Abstract
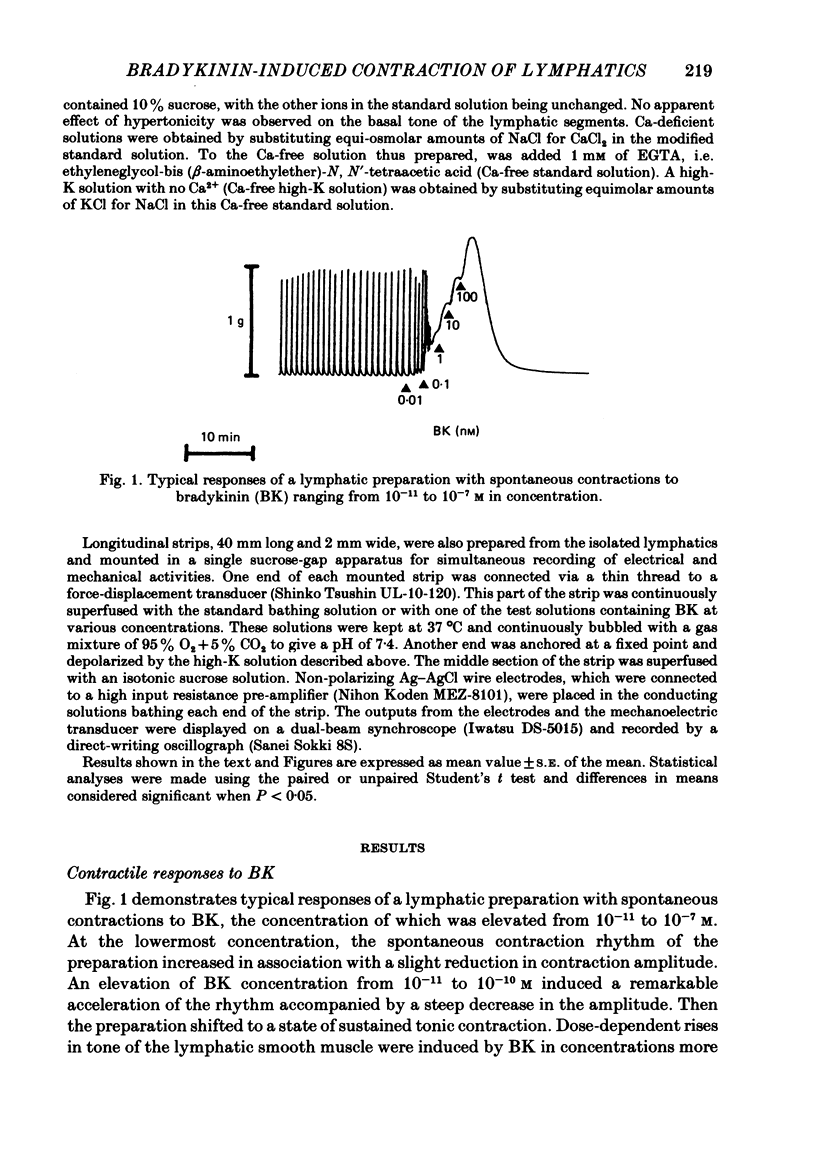
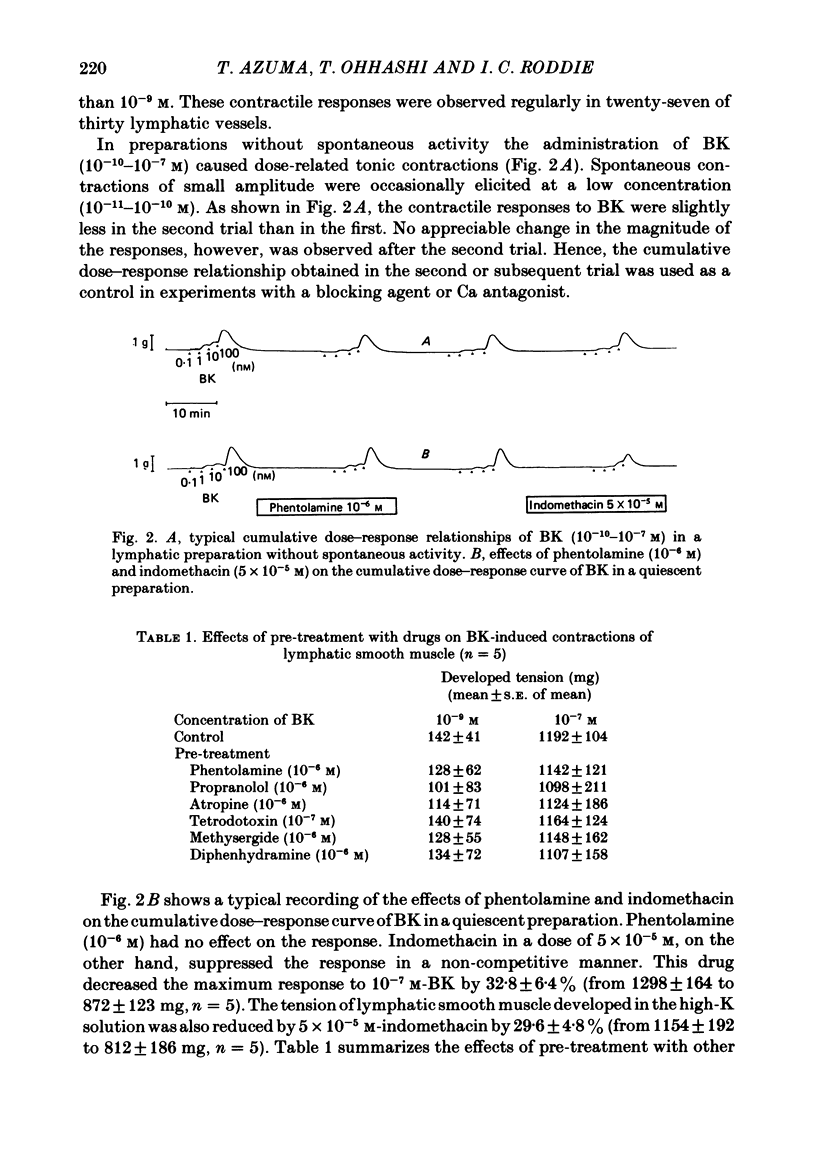
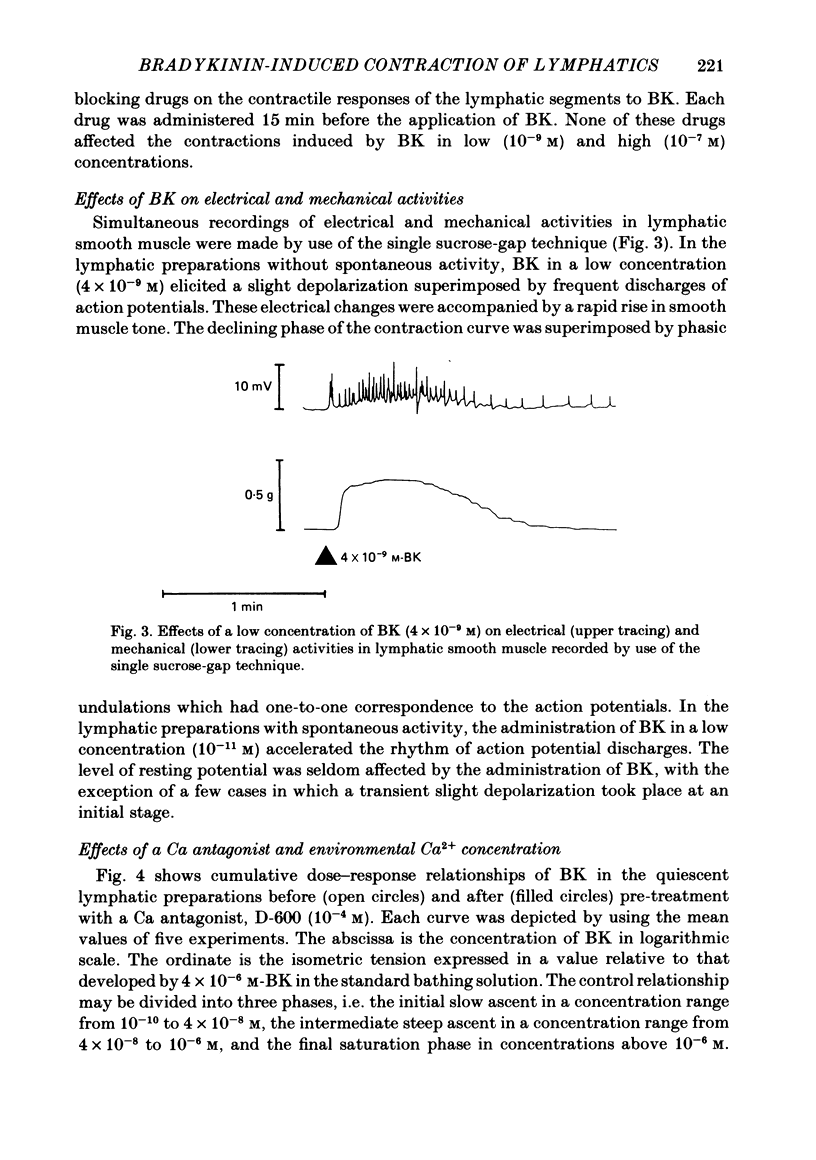
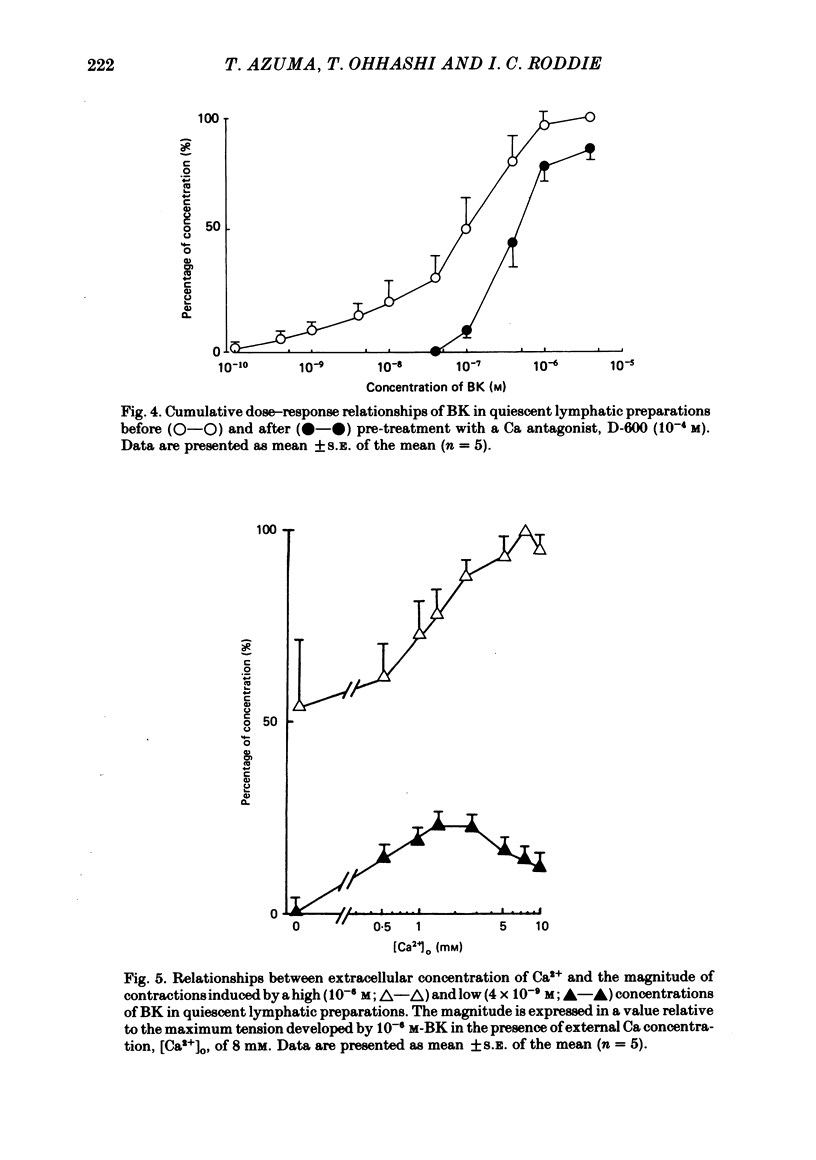
The mode of action of bradykinin (BK) on bovine mesenteric lymphatics was investigated by recording isometric tensions and action potentials in the isolated longitudinal segments. Addition of BK in concentrations from 10(-10) to 4 X 10(-6) M caused dose-related tonic contractions. BK in a low concentration accelerated the rhythm of action potential discharges in the spontaneously beating preparations and elicited frequent discharges of action potentials and a rapid rise in smooth muscle tone associated with phasic contractions. BK in high concentrations (more than 10(-7) M) caused a further rise of tension in the preparations which had already been depolarized in a high-K solution. The contraction induced by 4 X 10(-9) M-BK in the standard solution was abolished in a Ca-free environment or in the presence of a Ca-antagonist, 10(-4) M-D-600, though more than 50% of the contraction caused by 10(-6) M-BK still remained in both circumstances. In a Ca-free solution containing 1 mM-EGTA (Ca-free standard solution), 10(-6) M-BK caused a slight contraction even after high-K-induced contractions were completely blocked. The contractile response to 10(-6) M-BK in the Ca-free standard solution was augmented after activation of beta-receptors. It is concluded that the BK-induced contractions may be closely related to an increased Ca influx through the membrane and release of membrane-bound and intracellular Ca. The increased uptake of Ca into the BK-sensitive intracellular store may contribute to the relaxing effect of beta-agonist.
Full text
PDF

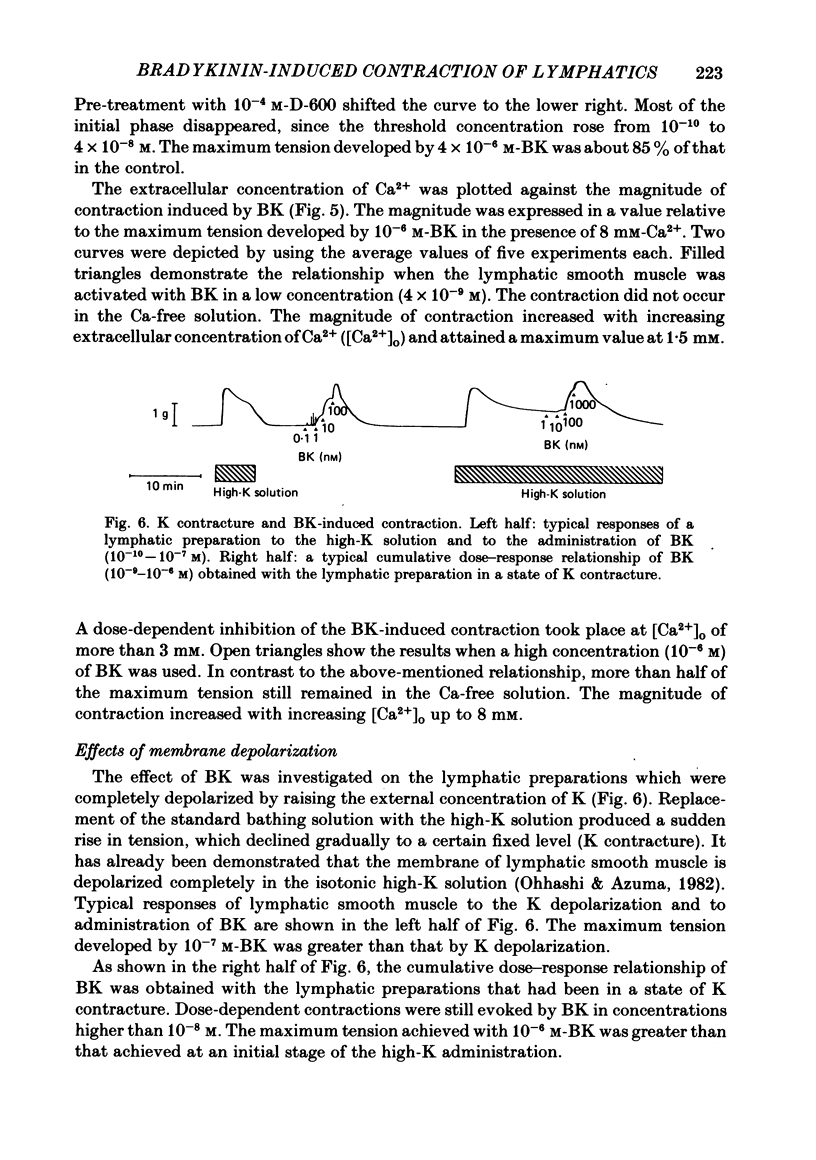
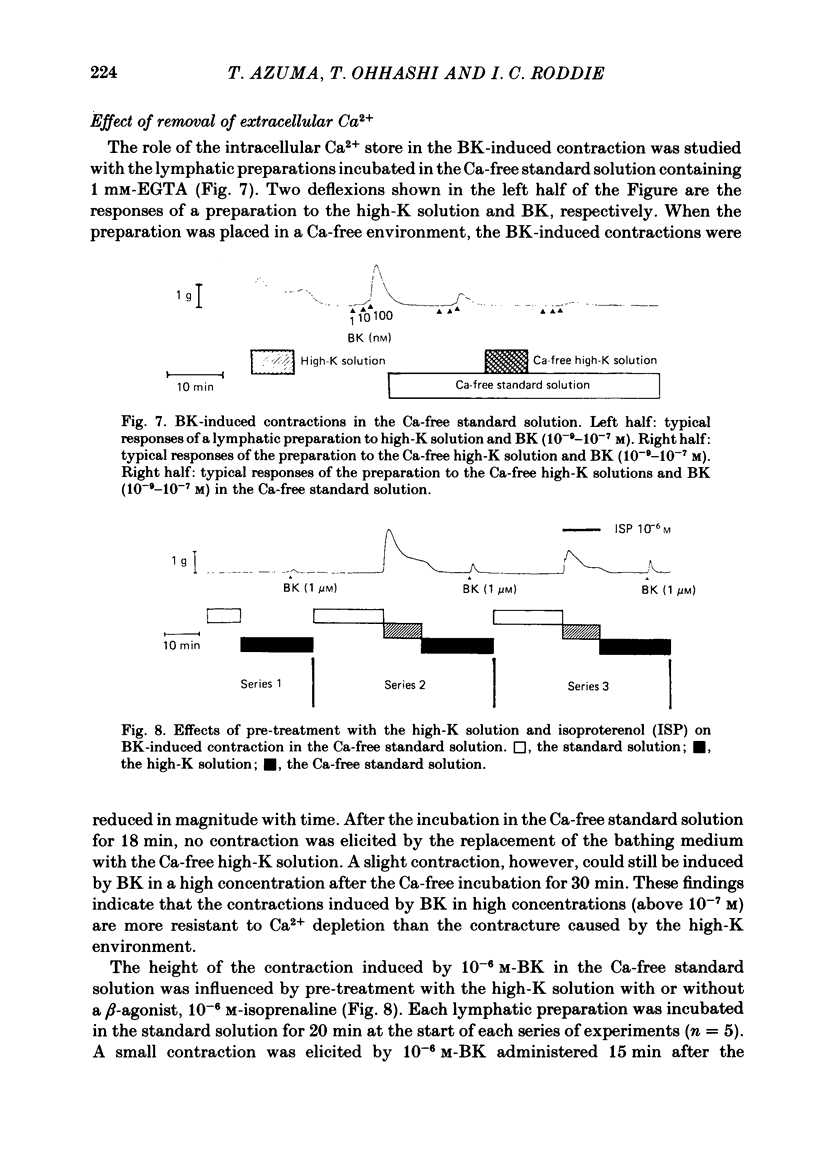



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma T., Ohhashi T., Sakaguchi M. Electrical activity of lymphatic smooth muscles. Proc Soc Exp Biol Med. 1977 Jun;155(2):270–273. doi: 10.3181/00379727-155-39787. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Casteels R., Raeymaekers L. The action of acetylcholine and catecholamines on an intracellular calcium store in the smooth muscle cells of the guinea-pig taenia coli. J Physiol. 1979 Sep;294:51–68. doi: 10.1113/jphysiol.1979.sp012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. N., Richardson P. D., Taylor A. E. The effects of isoprenaline and bradykinin on capillary filtration in the cat small intestine. Br J Pharmacol. 1979 Nov;67(3):361–366. doi: 10.1111/j.1476-5381.1979.tb08688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northover B. J. Mechanism of the inhibitory action of indomethacin on smooth muscle. Br J Pharmacol. 1971 Mar;41(3):540–551. doi: 10.1111/j.1476-5381.1971.tb08052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohhashi T., Azuma T. Effect of potassium on membrane potential and tension development in bovine mesenteric lymphatics. Microvasc Res. 1982 Jan;23(1):93–98. doi: 10.1016/0026-2862(82)90034-6. [DOI] [PubMed] [Google Scholar]
- Ohhashi T., Azuma T., Sakaguchi M. Active and passive mechanical characteristics of bovine mesenteric lymphatics. Am J Physiol. 1980 Jul;239(1):H88–H95. doi: 10.1152/ajpheart.1980.239.1.H88. [DOI] [PubMed] [Google Scholar]
- Ohhashi T., Kawai Y., Azuma T. The response of lymphatic smooth muscles to vasoactive substances. Pflugers Arch. 1978 Jul 18;375(2):183–188. doi: 10.1007/BF00584242. [DOI] [PubMed] [Google Scholar]
- Ohhashi T., Roddie I. C. Relaxation of bovine mesenteric lymphatics in response to transmural stimulation. Am J Physiol. 1981 Apr;240(4):H498–H504. doi: 10.1152/ajpheart.1981.240.4.H498. [DOI] [PubMed] [Google Scholar]
- Starr M. S., West G. B. The effect of bradykinin and anti-inflammatory agents on isolated arteries. J Pharm Pharmacol. 1966 Dec;18(12):838–840. doi: 10.1111/j.2042-7158.1966.tb07827.x. [DOI] [PubMed] [Google Scholar]
- Svensjö E., Persson C. G., Rutili G. Inhibition of bradykinin induced macromolecular leakage from post-capillary venules by a beta2-adrenoreceptor stimulant, terbutaline. Acta Physiol Scand. 1977 Dec;101(4):504–506. doi: 10.1111/j.1748-1716.1977.tb06038.x. [DOI] [PubMed] [Google Scholar]
- Toda N. Actions of bradykinin on isolated cerebral and peripheral arteries. Am J Physiol. 1977 Mar;232(3):H267–H274. doi: 10.1152/ajpheart.1977.232.3.H267. [DOI] [PubMed] [Google Scholar]
- Tsuru H., Ishikawa N., Shigei T. Responsiveness of isolated dog veins to bradykinin: distribution and a possible correlation with genesis of the venous system. Jpn J Pharmacol. 1974 Dec;24(6):931–934. doi: 10.1254/jjp.24.931. [DOI] [PubMed] [Google Scholar]


