Abstract
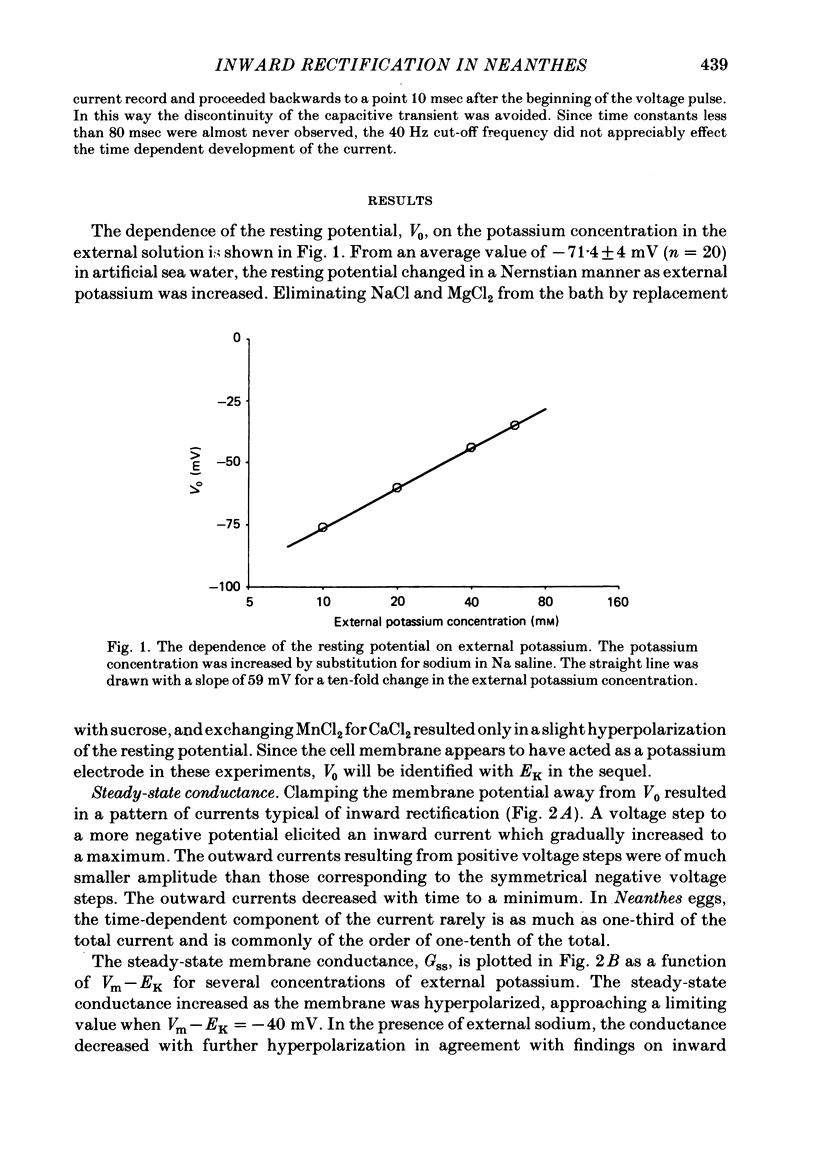
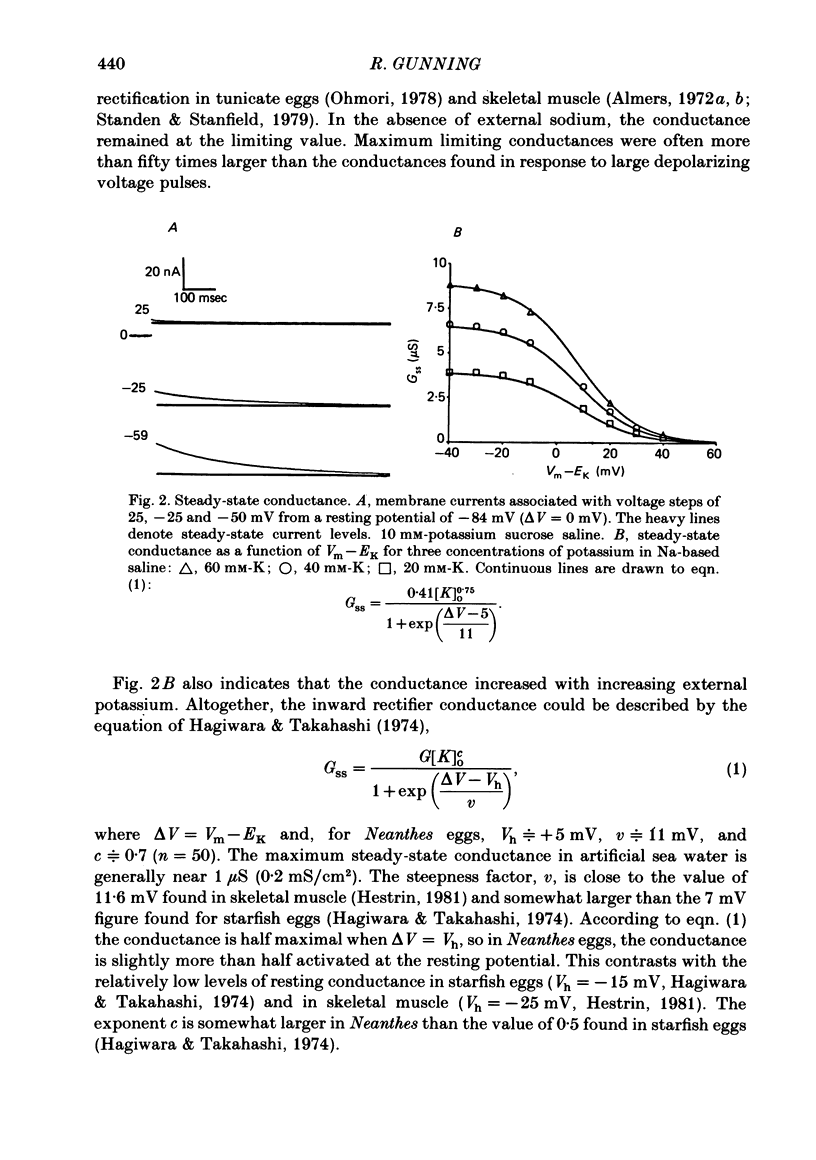
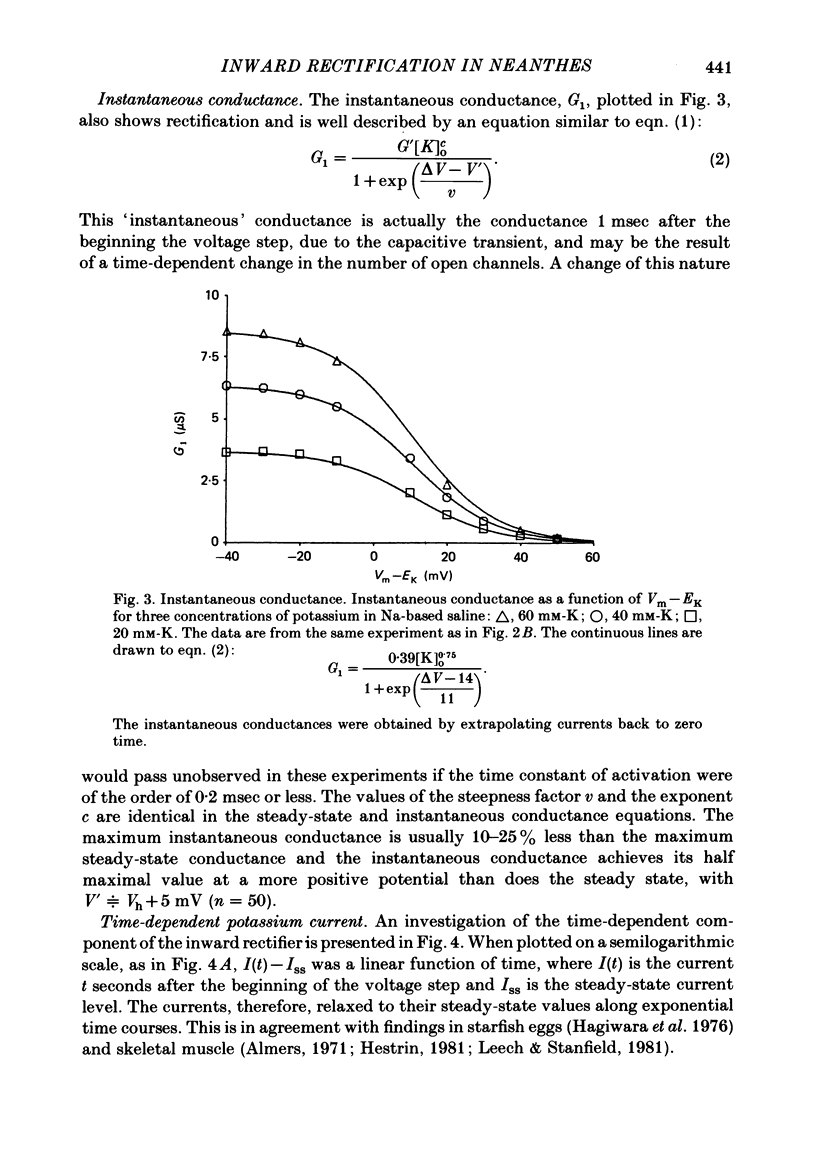
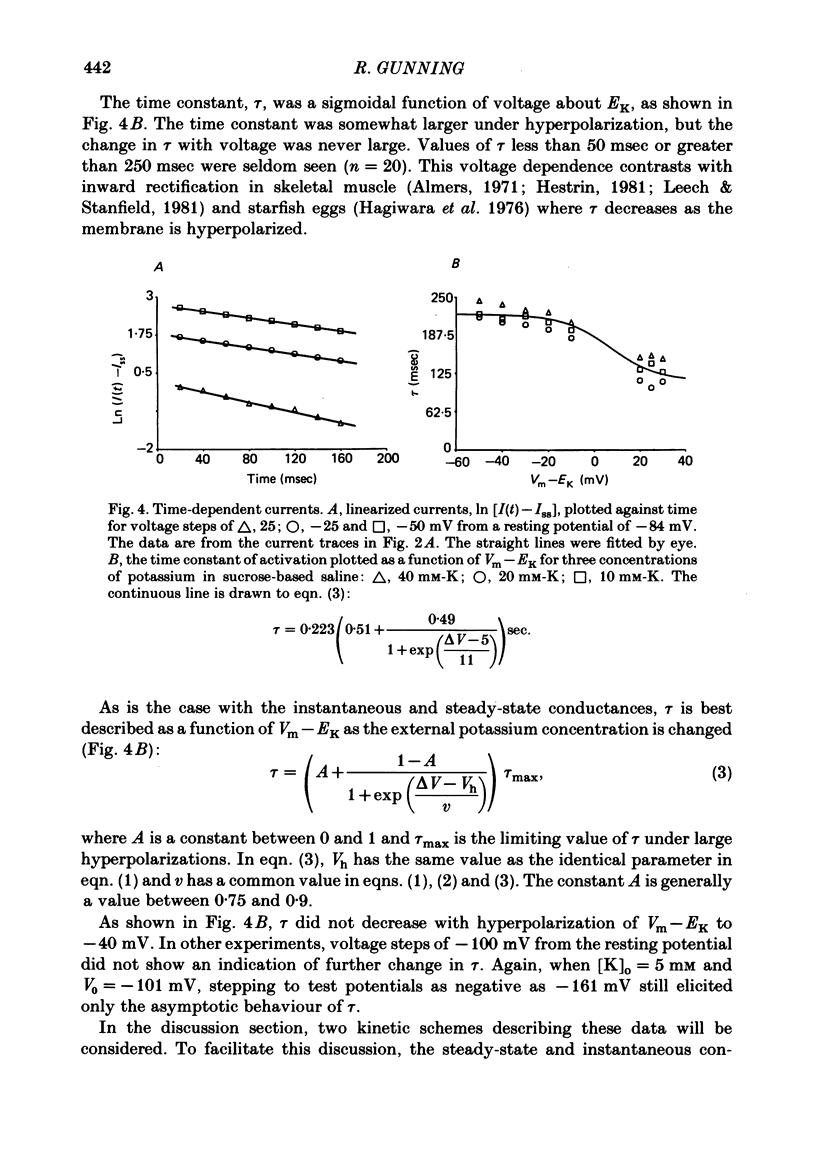
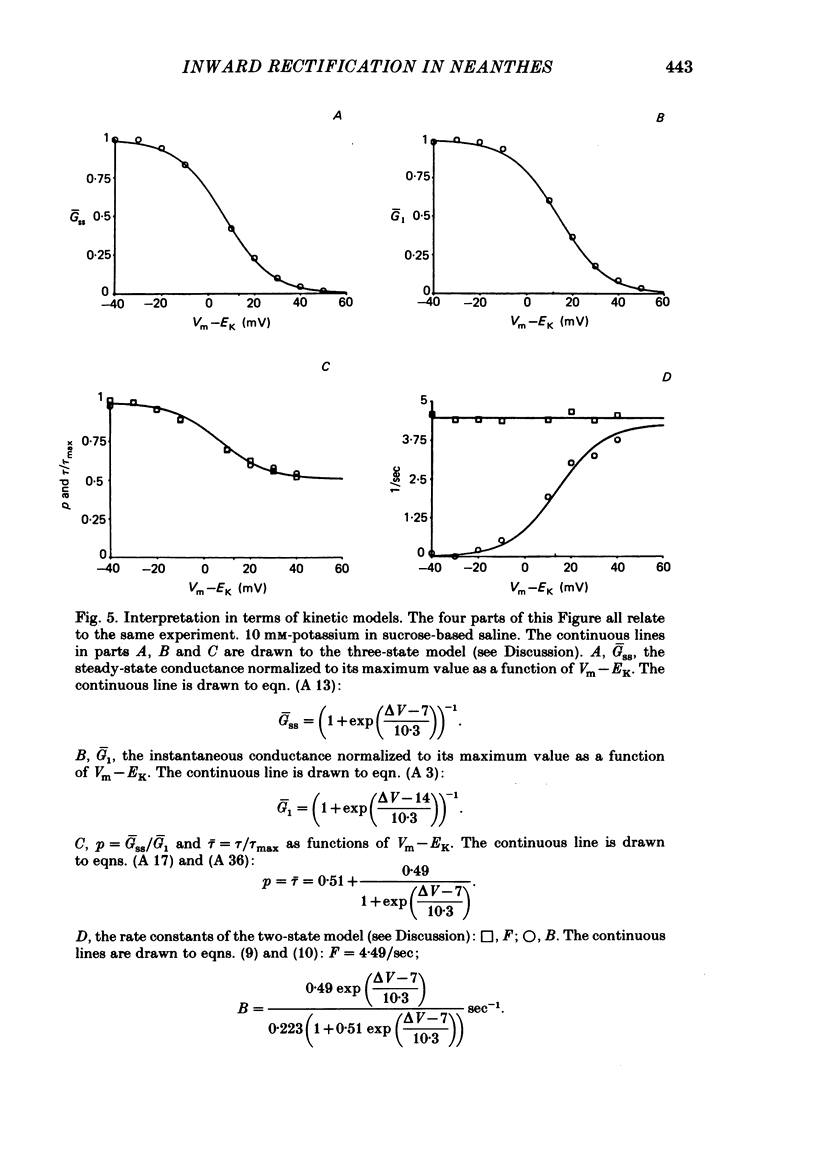
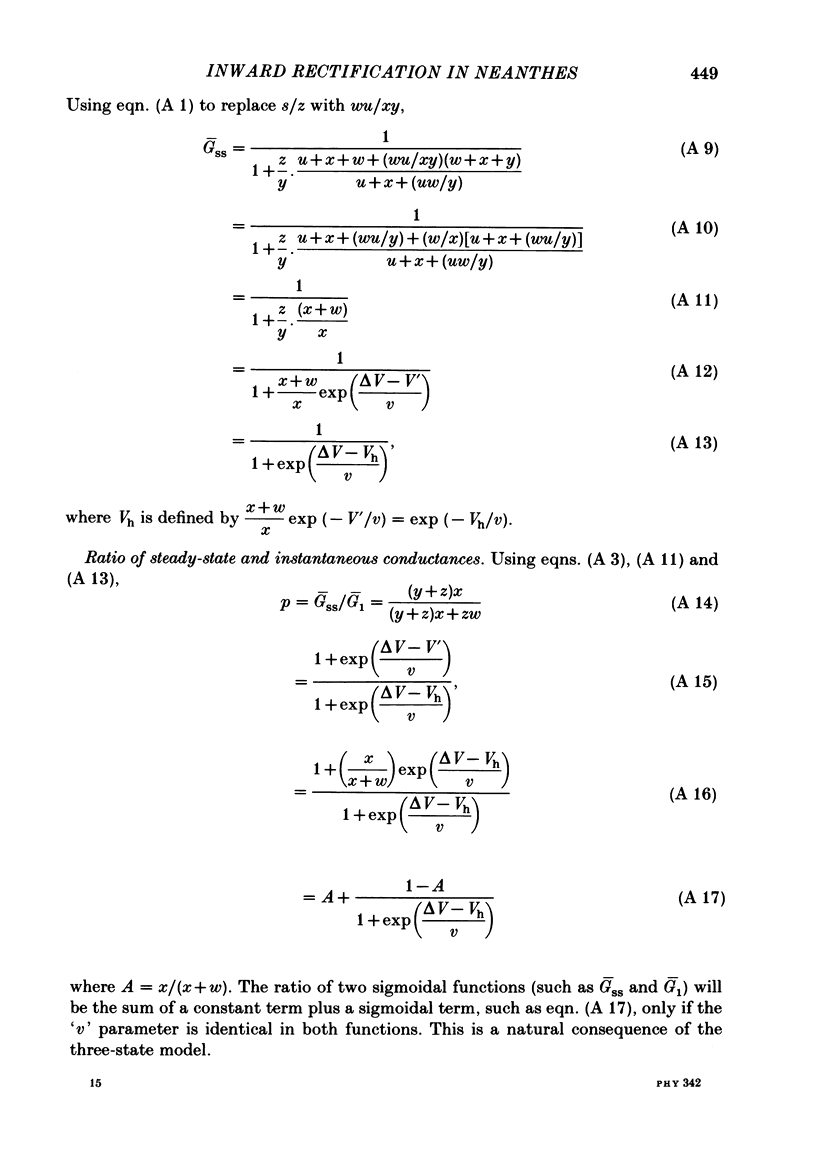
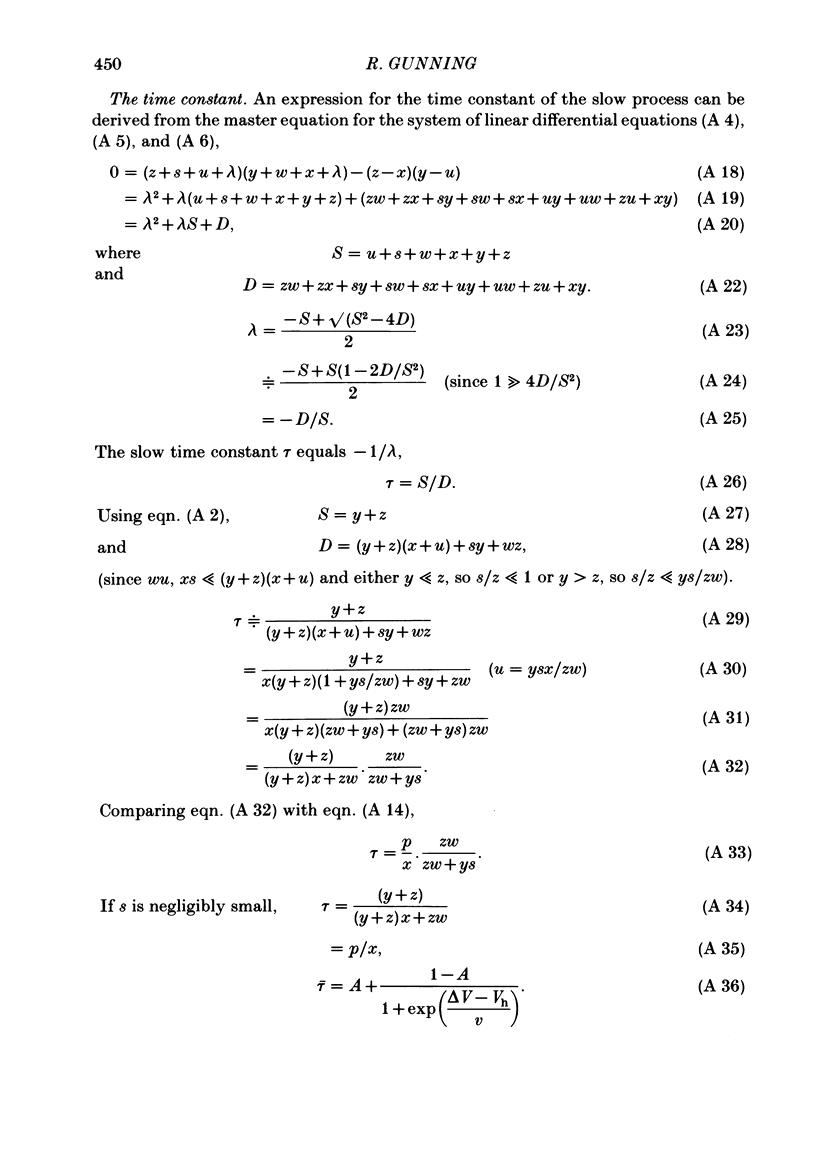
Potassium current through the inward rectifier of Neanthes arenaceodentata eggs was studied using a voltage-clamp technique. The instantaneous conductance, steady-state conductance and the time constant of current relaxation were analysed as functions of the membrane potential and the external potassium concentration ([K]o). Both the instantaneous and the steady-state conductances increased sigmoidally with hyperpolarization, reaching saturation values at potentials 40 mV more negative than the potassium equilibrium potential (EK). The time-dependent change in conductance followed first-order kinetics throughout an 80 mV potential range centred at EK. The conductances increased with time during hyperpolarizations and decreased with time during depolarizations. The time constant decreased sigmoidally about EK as the membrane potential (Vm) was made more positive. The results are interpreted in terms of two models. The first model assumes single-channel rectification. Forcing the data to conform with this assumption necessitates that the single-channel conductance, the open-channel probability, and the time constant of current relaxation all be nearly identical functions of Vm--EK. This coincidence is considered implausible. The second model ascribes the apparent instantaneous rectification to a fast kinetic process. This model correctly predicts the steady-state conductance, the time-constant of current relaxation, and the unexpected proportionality between the ratio of the steady-state and 'instantaneous' conductances and the time constant of current relaxation. This agreement between data and model is obtained even though neither of the rate constants of the slow gating process has the flexibility of voltage or potassium dependence. The results are considered to imply the existence of a fast gating mechanism in the kinetics of inward rectification.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H., FREYGANG W. H. Potassium conductance of frog muscle membrane under controlled voltage. J Physiol. 1962 Aug;163:104–114. doi: 10.1113/jphysiol.1962.sp006960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Freygang W. H. The potassium and chloride conductance of frog muscle membrane. J Physiol. 1962 Aug;163(1):61–103. doi: 10.1113/jphysiol.1962.sp006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W. Potassium conductance changes in skeletal muscle and the potassium concentration in the transverse tubules. J Physiol. 1972 Aug;225(1):33–56. doi: 10.1113/jphysiol.1972.sp009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W. The decline of potassium permeability during extreme hyperpolarization in frog skeletal muscle. J Physiol. 1972 Aug;225(1):57–83. doi: 10.1113/jphysiol.1972.sp009929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Hestrin S. The interaction of potassium with the activation of anomalous rectification in frog muscle membrane. J Physiol. 1981 Aug;317:497–508. doi: 10.1113/jphysiol.1981.sp013839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech C. A., Stanfield P. R. Inward rectification in frog skeletal muscle fibres and its dependence on membrane potential and external potassium. J Physiol. 1981;319:295–309. doi: 10.1113/jphysiol.1981.sp013909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H. Inactivation kinetics and steady-state current noise in the anomalous rectifier of tunicate egg cell membranes. J Physiol. 1978 Aug;281:77–99. doi: 10.1113/jphysiol.1978.sp012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. Potassium depletion and sodium block of potassium currents under hyperpolarization in frog sartorius muscle. J Physiol. 1979 Sep;294:497–520. doi: 10.1113/jphysiol.1979.sp012943. [DOI] [PMC free article] [PubMed] [Google Scholar]


