Abstract
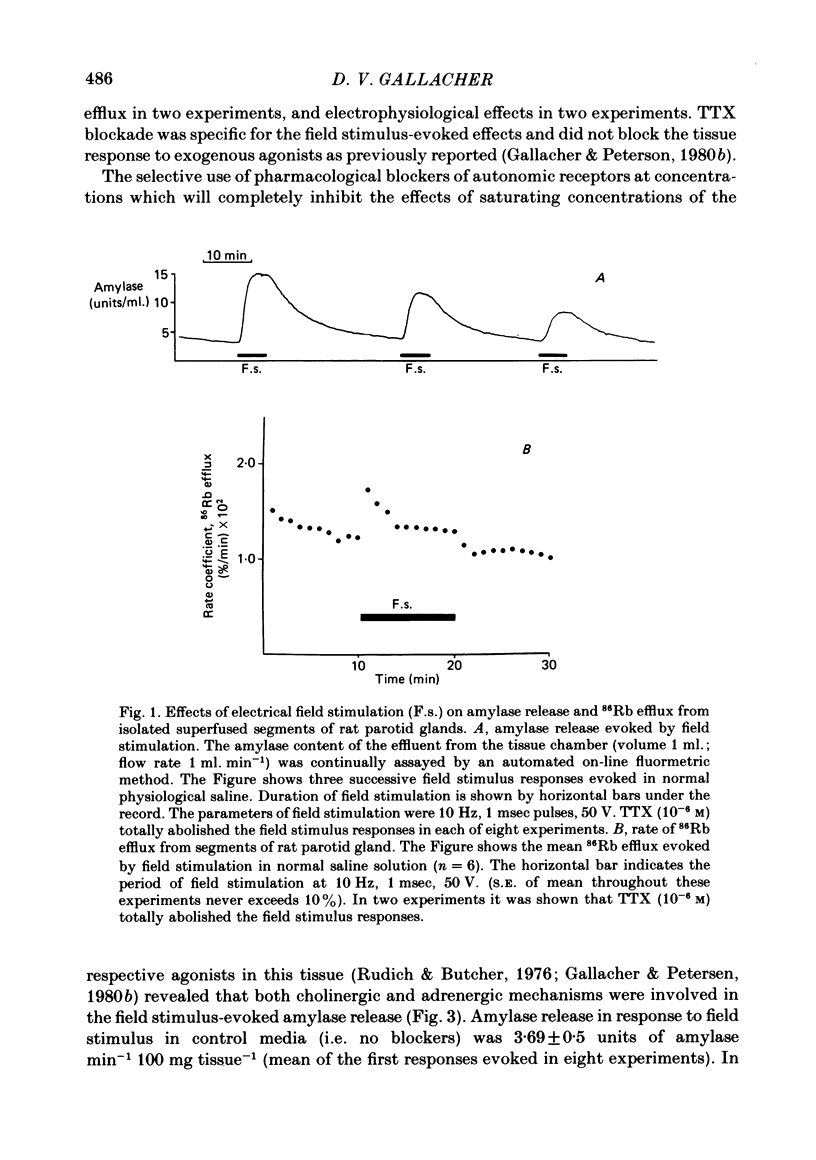
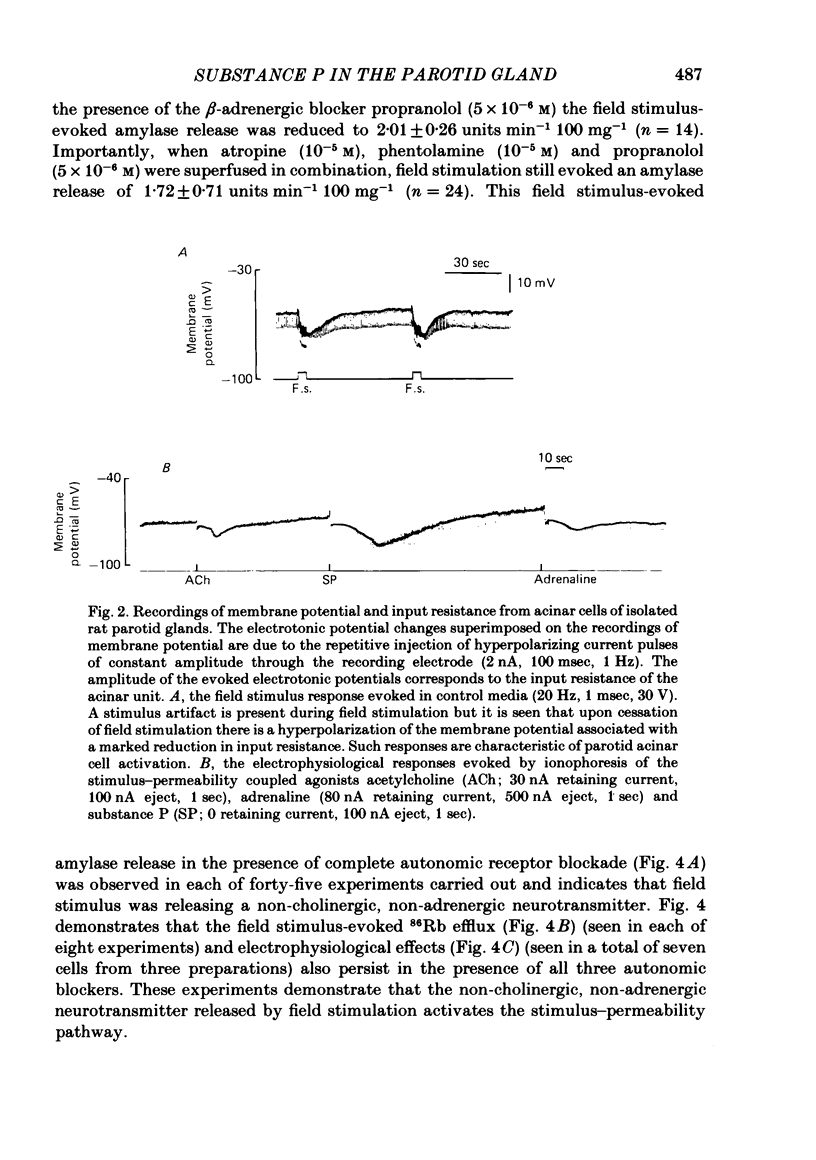
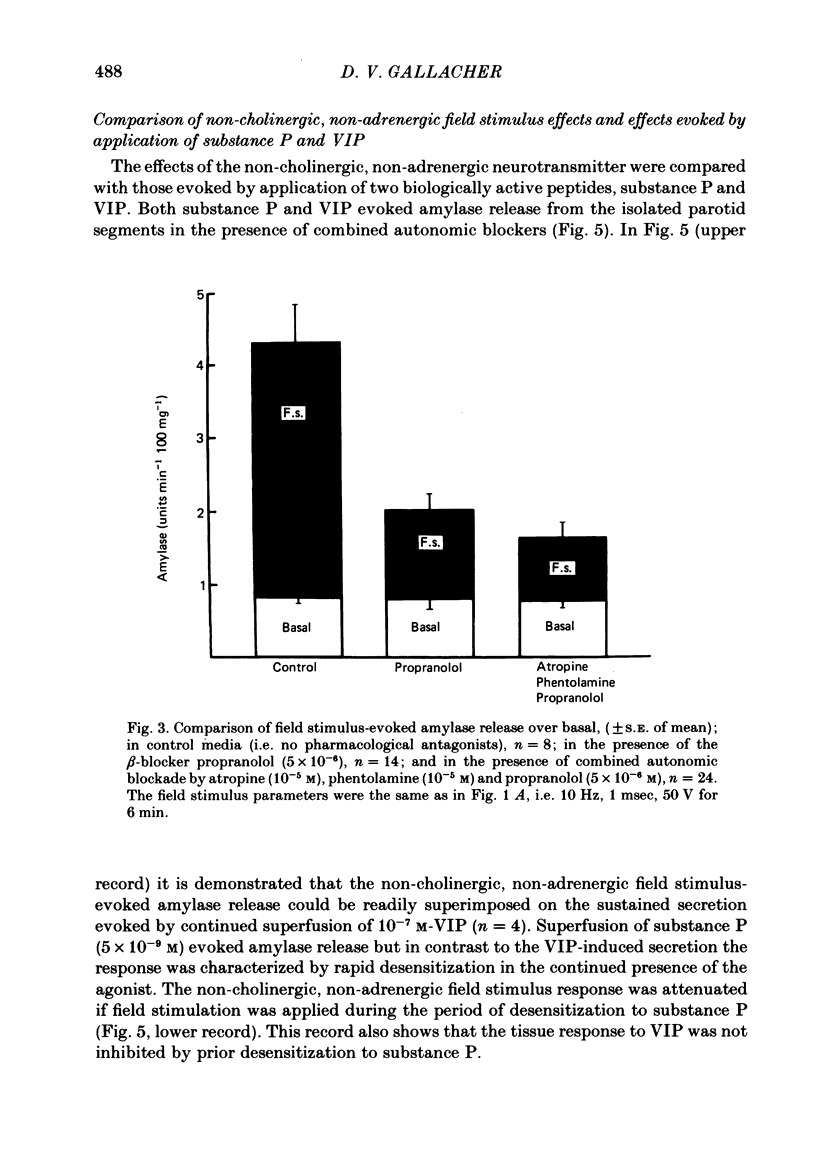
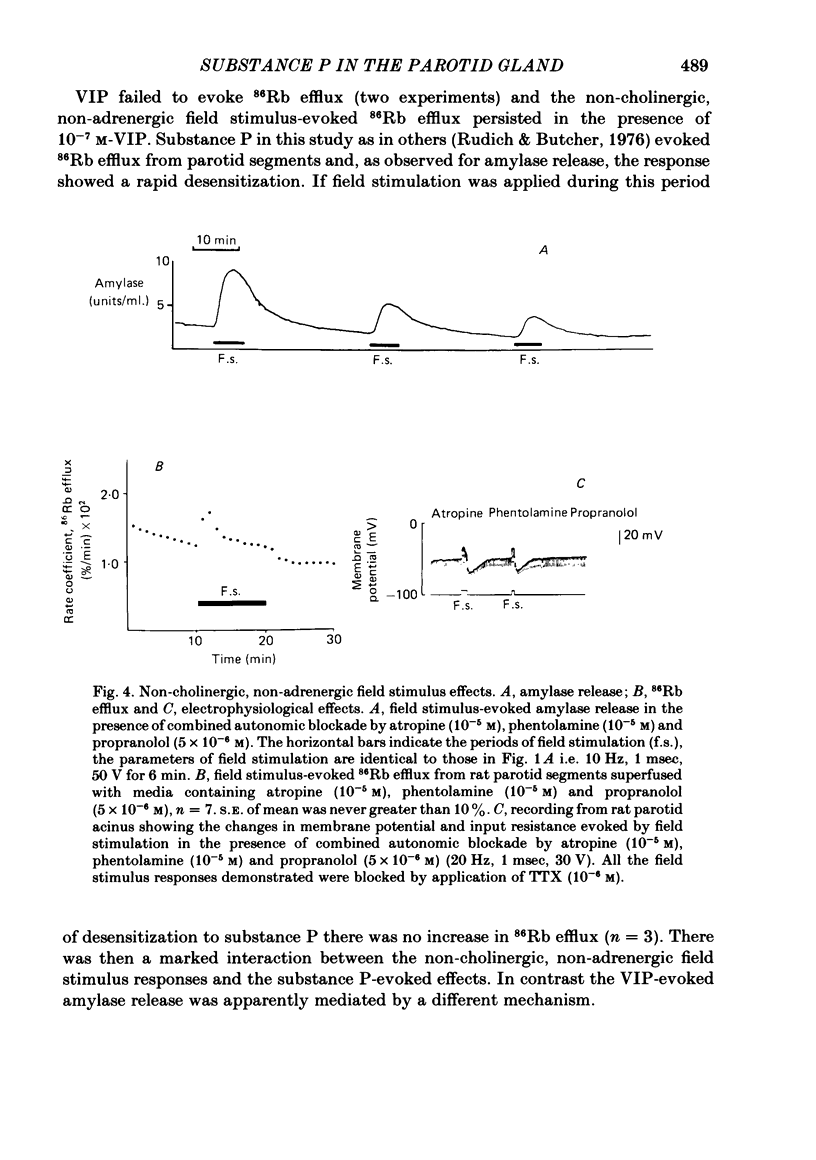
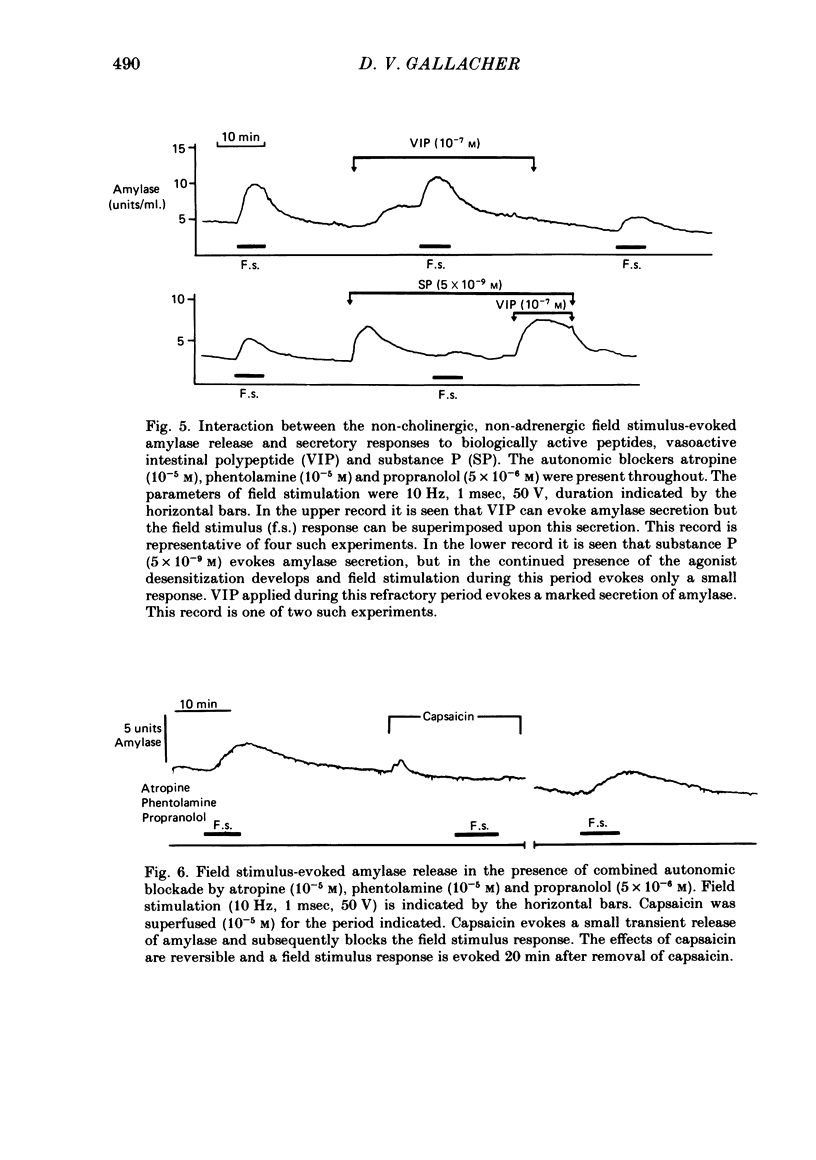
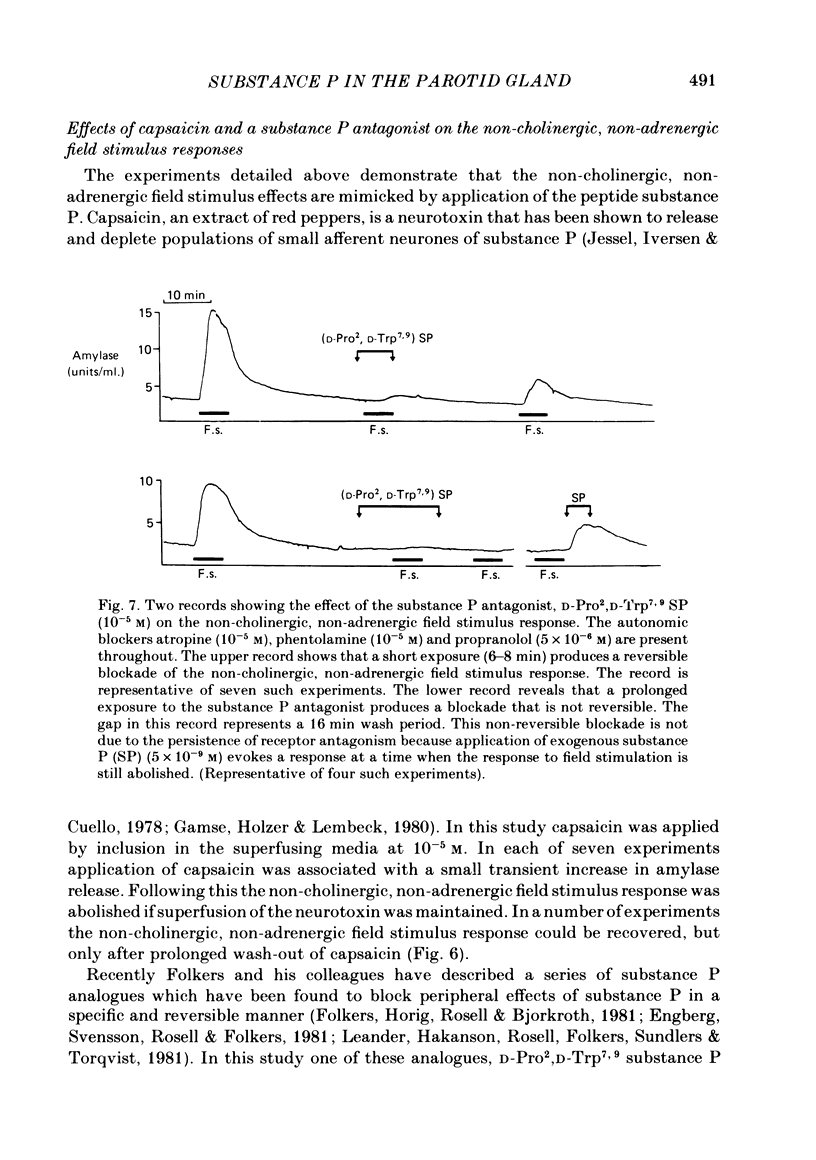
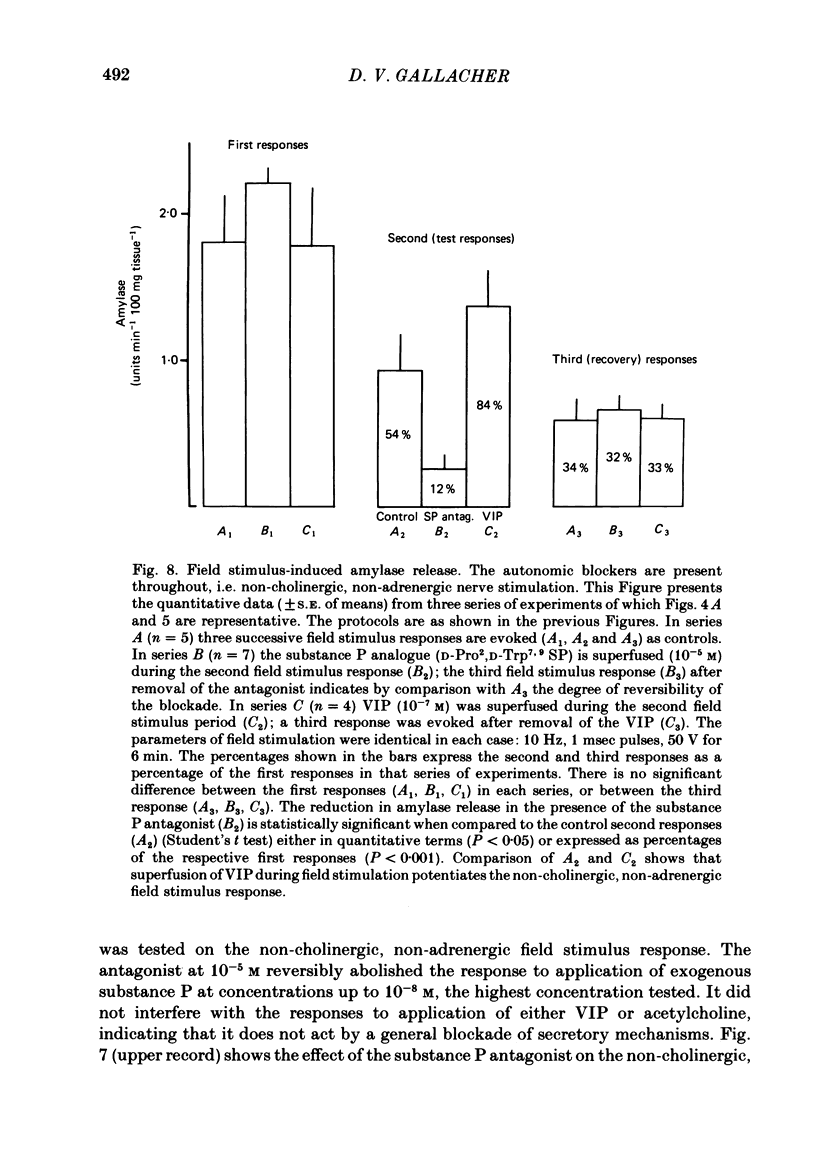
The technique of electrical field stimulation was employed to stimulate the intrinsic nerves of isolated rat parotid gland fragments. Responses to field stimulation were recorded as changes in enzyme secretion (amylase release), radiolabelled ion fluxes (86Rb efflux) and electrophysiological effects (changes in acinar cell membrane potential and input resistance). All effects of field stimulation were abolished by the neurotoxin, tetrodotoxin (TTX). Selective use of pharmacological antagonists revealed that both the sympathetic and parasympathetic nerves to this tissue were being excited by field stimulation. Importantly a significant component of the response to field stimulation persisted in the presence of combined autonomic receptor blockade by atropine, phentolamine and propranolol, i.e. due to release of a non-cholinergic, non-adrenergic neurotransmitter. The non-cholinergic, non-adrenergic neurotransmitter evoked amylase release, 86Rb efflux and electrophysiological effects seen as changes in acinar cell membrane potential and conductance, i.e. stimulus-permeability coupled. Two biologically active peptides, substance P (SP) and vasoactive intestinal polypeptide (VIP) were shown to evoke amylase release in the presence of combined autonomic blockade. VIP however did not evoke any increase in 86Rb efflux, i.e. not stimulus-permeability coupled. All the effects of the non-cholinergic, non-adrenergic transmitter were mimicked by substance P which evokes 86Rb efflux and electrophysiological effects in addition to amylase release. The non-cholinergic, non-adrenergic field stimulus effects on amylase release and 86Rb efflux were abolished or markedly attenuated in tissues which had been desensitized by prior exposure to exogenous substance P. In the presence of VIP, however, the non-cholinergic, non-adrenergic effects persisted and were apparently potentiated. Acute application of the neurotoxin capsaicin first stimulated a transient release of amylase and subsequently abolished the non-cholinergic, non-adrenergic field stimulus-evoked enzyme release. The putative substance P antagonist, D-Pro2, D-Trp7,9 substance P, reversibly blocked the response to both non-cholinergic, non-adrenergic nerve stimulation and exogenous substance P. It was demonstrated however that prolonged exposure to this antagonist is associated with non-reversible and, importantly, non-specific neurotoxic effects. It is concluded that substance P or a closely related peptide is a functional neurotransmitter in the rat parotid gland.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brodin E., Nilsson G. Concentration of substance P-like immunoreactivity (SPLI) in tissues of dog, rat and mouse. Acta Physiol Scand. 1981 Jul;112(3):305–312. doi: 10.1111/j.1748-1716.1981.tb06821.x. [DOI] [PubMed] [Google Scholar]
- Brown C. L., Hanley M. R. The effects of substance P and related peptides on alpha-amylase release from rat parotid gland slices. Br J Pharmacol. 1981 Jun;73(2):517–523. doi: 10.1111/j.1476-5381.1981.tb10451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher F. R., Putney J. W., Jr Regulation of parotid gland function by cyclic nucleotides and calcium. Adv Cyclic Nucleotide Res. 1980;13:215–249. [PubMed] [Google Scholar]
- Davis J. N., Olender E., Maury W., McDaniel R. Alpha-adrenergic regulation of cholinergic responses in rat parotid acinar cells. Mol Pharmacol. 1980 Nov;18(3):356–361. [PubMed] [Google Scholar]
- Engberg G., Svensson T. H., Rosell S., Folkders K. A synthetic peptide as an antagonist of substance P. Nature. 1981 Sep 17;293(5829):222–223. doi: 10.1038/293222a0. [DOI] [PubMed] [Google Scholar]
- Folkers K., Hörig J., Rosell S., Björkroth U. Chemical design of antagonists of substance P. Acta Physiol Scand. 1981 Apr;111(4):505–506. doi: 10.1111/j.1748-1716.1981.tb06771.x. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Lundberg J. M. VIP-induced cyclic AMP formation in the cat submandibular gland. Potentiation by carbacholine. Acta Physiol Scand. 1982 Jan;114(1):157–159. doi: 10.1111/j.1748-1716.1982.tb06966.x. [DOI] [PubMed] [Google Scholar]
- Gallacher D. V. Are there purinergic receptors on parotid acinar cells? Nature. 1982 Mar 4;296(5852):83–86. doi: 10.1038/296083a0. [DOI] [PubMed] [Google Scholar]
- Gallacher D. V., Petersen O. H. Electrophysiology of mouse parotid acini: effects of electrical field stimulation and ionophoresis of neurotransmitters. J Physiol. 1980 Aug;305:43–57. doi: 10.1113/jphysiol.1980.sp013348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallacher D. V., Petersen O. H. Substance P increases membrane conductance in parotid acinar cells. Nature. 1980 Jan 24;283(5745):393–395. doi: 10.1038/283393a0. [DOI] [PubMed] [Google Scholar]
- Gamse R., Holzer P., Lembeck F. Decrease of substance P in primary afferent neurones and impairment of neurogenic plasma extravasation by capsaicin. Br J Pharmacol. 1980 Feb;68(2):207–213. doi: 10.1111/j.1476-5381.1980.tb10409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamse R., Leeman S. E., Holzer P., Lembeck F. Differential effects of capsaicin on the content of somatostatin, substance P, and neurotensin in the nervous system of the rat. Naunyn Schmiedebergs Arch Pharmacol. 1981 Sep;317(2):140–148. doi: 10.1007/BF00500070. [DOI] [PubMed] [Google Scholar]
- Gamse R., Petsche U., Lembeck F., Jancsò G. Capsaicin applied to peripheral nerve inhibits axoplasmic transport of substance P and somatostatin. Brain Res. 1982 May 13;239(2):447–462. doi: 10.1016/0006-8993(82)90521-2. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Vincent S., Hellsten L., Rosell S., Folkers K., Markey K., Goldstein M., Cuello C. Immunohistochemical evidence for a "neurotoxic" action of (D-Pro2, D-Trp7,9)-substance P, an analogue with substance P antagonistic activity. Acta Physiol Scand. 1981 Dec;113(4):571–573. doi: 10.1111/j.1748-1716.1981.tb06943.x. [DOI] [PubMed] [Google Scholar]
- Jessell T. M., Iversen L. L., Cuello A. C. Capsaicin-induced depletion of substance P from primary sensory neurones. Brain Res. 1978 Aug 18;152(1):183–188. doi: 10.1016/0006-8993(78)90146-4. [DOI] [PubMed] [Google Scholar]
- Leander S., Håkanson R., Rosell S., Folkers K., Sundler F., Tornqvist K. A specific substance P antagonist blocks smooth muscle contractions induced by non-cholinergic, non-adrenergic nerve stimulation. Nature. 1981 Dec 3;294(5840):467–469. doi: 10.1038/294467a0. [DOI] [PubMed] [Google Scholar]
- Leeman S. E., Hammerschlag R. Stimulation of salivary secretion by a factor extracted from hypothalamic tissue. Endocrinology. 1967 Oct;81(4):803–810. doi: 10.1210/endo-81-4-803. [DOI] [PubMed] [Google Scholar]
- Liang T., Cascieri M. A. Specific binding of an immunoreactive and biologically active 125I-labeled N(1)acylated substance P derivative to parotid cells. Biochem Biophys Res Commun. 1980 Oct 31;96(4):1793–1799. doi: 10.1016/0006-291x(80)91382-0. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Anggård A., Fahrenkrug J., Hökfelt T., Mutt V. Vasoactive intestinal polypeptide in cholinergic neurons of exocrine glands: functional significance of coexisting transmitters for vasodilation and secretion. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1651–1655. doi: 10.1073/pnas.77.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Hedlund B., Bartfai T. Vasoactive intestinal polypeptide enhances muscarinic ligand binding in cat submandibular salivary gland. Nature. 1982 Jan 14;295(5845):147–149. doi: 10.1038/295147a0. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Petersen O. H., Williams J. A. Analysis of tissue amylase output by an automated method. Anal Biochem. 1974 Mar;58(1):155–160. doi: 10.1016/0003-2697(74)90452-7. [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Katoh K., Saitoh S., Wakui M. Effect of neural stimulation on acinar cell membrane potentials in isolated pancreas and salivary gland segments. Membr Biochem. 1980;3(1-2):49–66. doi: 10.3109/09687688009063878. [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Membrane potential and resistance measurement in acinar cells from salivary glands in vitro: effect of acetylcholine. J Physiol. 1974 Oct;242(1):173–188. doi: 10.1113/jphysiol.1974.sp010700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr Biphasic modulation of potassium release in rat parotid gland by carbachol and phenylephrine. J Pharmacol Exp Ther. 1976 Aug;198(2):375–384. [PubMed] [Google Scholar]
- Putney J. W., Jr Muscarinic, alpha-adrenergic and peptide receptors regulate the same calcium influx sites in the parotid gland. J Physiol. 1977 Jun;268(1):139–149. doi: 10.1113/jphysiol.1977.sp011851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudich L., Butcher F. R. Effect of substance P and eledoisin on K+ efflux, amylase release and cyclic nucleotide levels in slices of rat parotid gland. Biochim Biophys Acta. 1976 Oct 22;444(3):704–711. doi: 10.1016/0304-4165(76)90317-2. [DOI] [PubMed] [Google Scholar]
- Ueda N., Muramatsu I., Sakakibara Y., Fujiwara M. Noncholinergic, nonadrenergic contraction and substance P in rabbit iris sphincter muscle. Jpn J Pharmacol. 1981 Dec;31(6):1071–1079. doi: 10.1254/jjp.31.1071. [DOI] [PubMed] [Google Scholar]


