Abstract
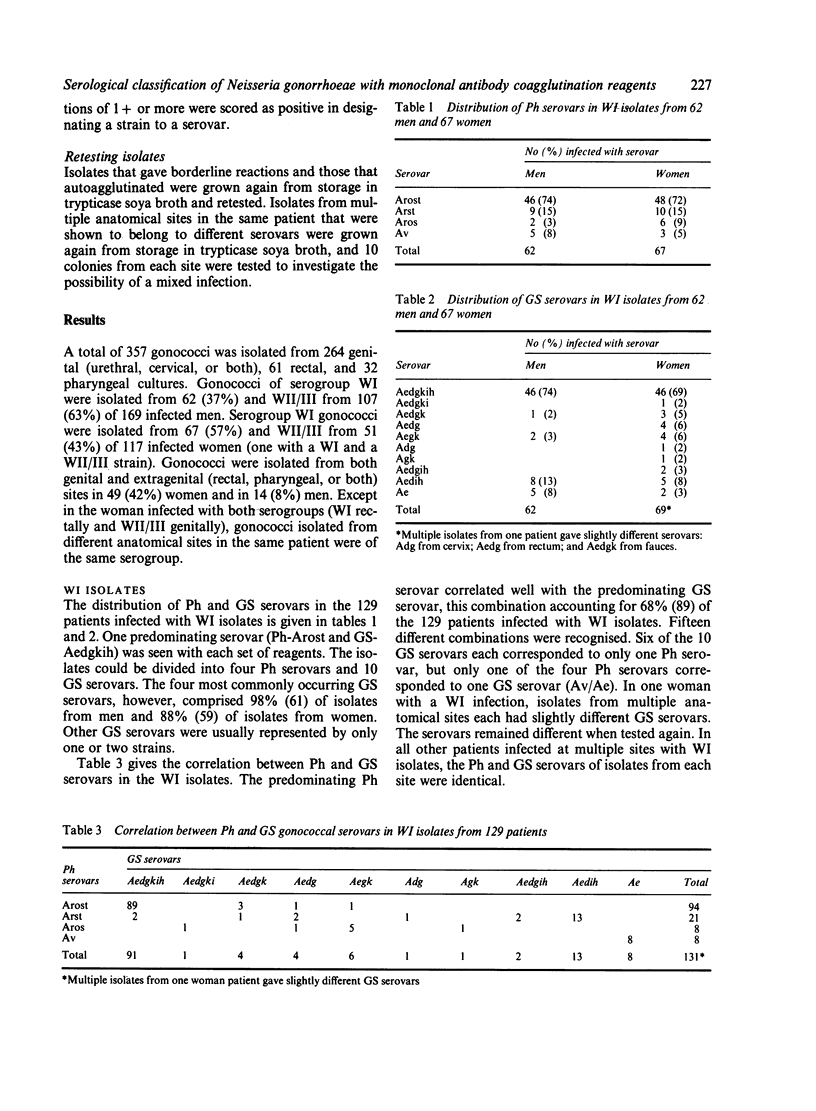
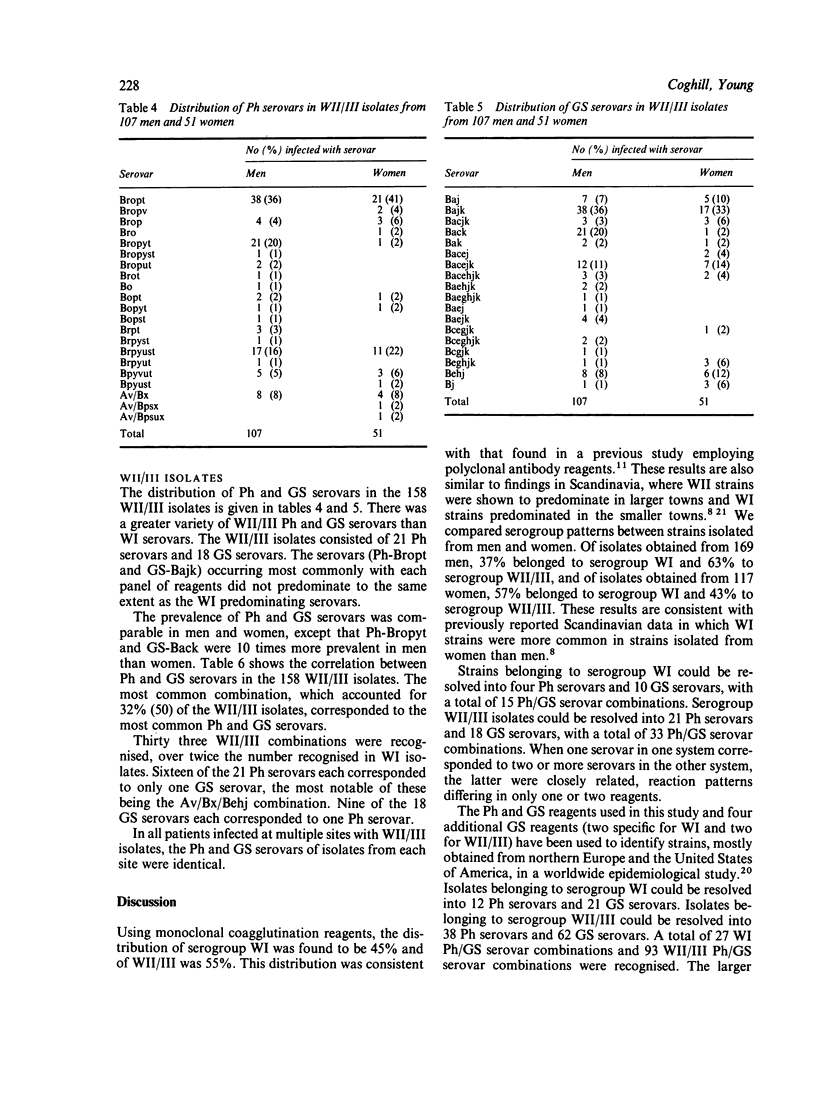
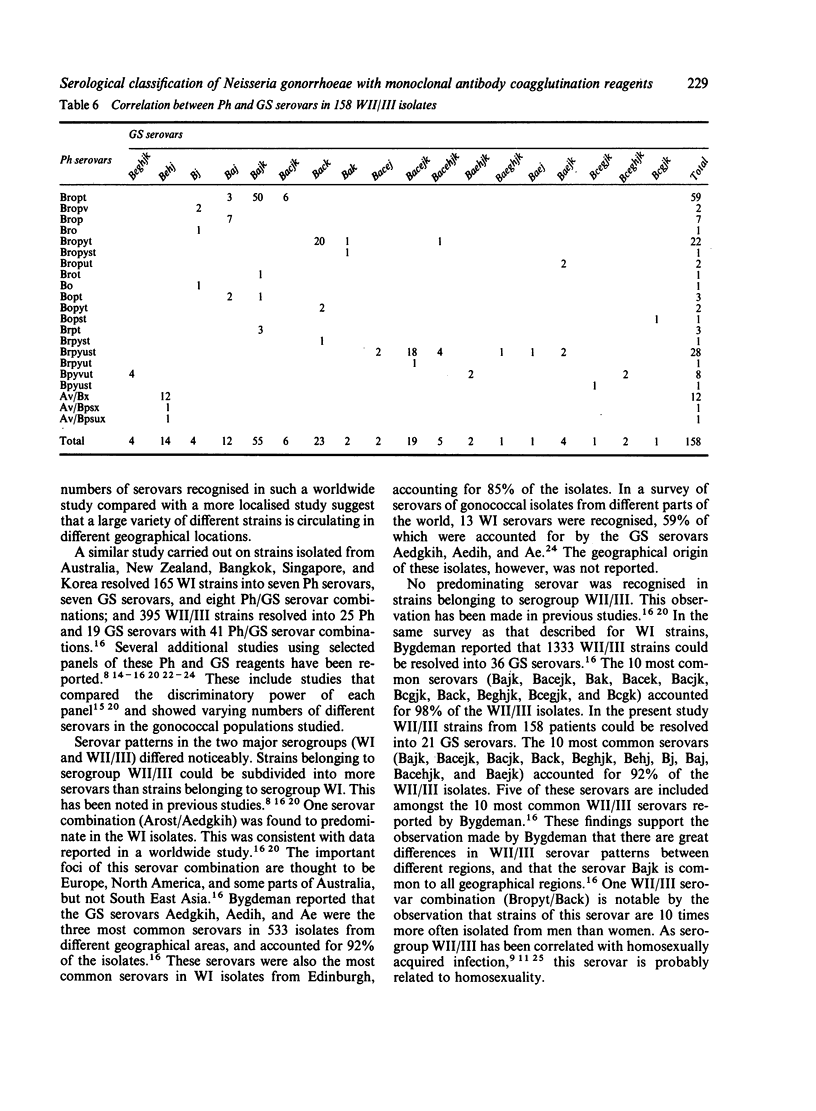
A total of 357 clinical isolates of Neisseria gonorrhoeae from 286 patients were classified serologically using two independently developed panels of monoclonal coagglutination reagents. The Pharmacia (Ph) Diagnostics panel comprised 14 reagents, five specific for serogroup WI strains and nine specific for serogroup WII/III strains, whereas the Genetic Systems (GS) panel comprised 14 reagents, seven specific for serogroup WI strains and seven specific for serogroup WII/III strains. Serogroup WI represented 45% and WII/III represented 55% of the patients. Using the monoclonal antibody reagents, the serogroups could be further subdivided into so-called serovars. The Ph reagents identified four WI serovars and 21 WII/III serovars, whereas the GS reagents identified 10 WI serovars and 18 WII/III serovars. By combining the results obtained with each panel, 15 Ph/GS WI serovars and 33 Ph/GS WII/III serovars were recognised. In the WI isolates, one predominating serovar was recognised, whereas in the WII/III isolates, no single serovar predominated and a much greater variety of serovars was identified. The serovar patterns for men and women patients were very similar, except for one WII/III serovar that was 10 times more common in isolates from men than from women. Most isolates from different anatomical sites in the same patient were of the same serogroup and serovar. Two double infections were found. One patient had a genital infection with serogroup WII/III and a rectal infection with serogroup WI. Another patient with genital, rectal, and throat infections with serogroup WI was found to have gonococci of different GS serovars at each site. It was concluded that the level of discrimination achieved with the monoclonal antibody reagents should prove to be valuable in studying the micro epidemiology of gonococcal infection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchanan T. M., Hildebrandt J. F. Antigen-specific serotyping of Neisseria gonorrhoeae: characterization based upon principal outer membrane protein. Infect Immun. 1981 Jun;32(3):985–994. doi: 10.1128/iai.32.3.985-994.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bygdeman S. M., Mårdh P. A., Sandström E. G. Susceptibility of Neisseria gonorrhoeae to rifampicin and thiamphenicol: correlation with protein I antigenic determinants. Sex Transm Dis. 1984 Oct-Dec;11(4 Suppl):366–370. doi: 10.1097/00007435-198410001-00012. [DOI] [PubMed] [Google Scholar]
- Bygdeman S. Antibiotic susceptibility of Neisseria gonorrhoeae in relation to serogroups. Acta Pathol Microbiol Scand B. 1981 Aug;89(4):227–237. doi: 10.1111/j.1699-0463.1981.tb00181_89b.x. [DOI] [PubMed] [Google Scholar]
- Bygdeman S., Danielsson D., Sandström E. Gonococcal W serogroups in Scandinavia. A study with polyclonal and monoclonal antibodies. Acta Pathol Microbiol Immunol Scand B. 1983 Oct;91(5):293–305. [PubMed] [Google Scholar]
- Bygdeman S., Danielsson D., Sandström E. Serological classification of Neisseria gonorrhoeae by co-agglutination: a study of serological patterns in two geographical areas of Sweden. Acta Derm Venereol. 1981;61(5):423–427. [PubMed] [Google Scholar]
- Bygdeman S. Gonorrhoea in men with homosexual contacts. Serogroups of isolated gonococcal strains related to antibiotic susceptibility, site of infection, and symptoms. Br J Vener Dis. 1981 Oct;57(5):320–324. doi: 10.1136/sti.57.5.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman M., Rudén A. K., Bygdeman S. M., Jonsson A., Ringertz O., Sandström E. G. Gonococcal serovar distribution in Stockholm, with special reference to multiple infections and infected partners. Acta Pathol Microbiol Immunol Scand B. 1985 Jun;93(3):225–232. doi: 10.1111/j.1699-0463.1985.tb02880.x. [DOI] [PubMed] [Google Scholar]
- Danielsson D., Bygdeman S., Kallings I. Epidemiology of gonorrhoea: serogroup, antibiotic susceptibility and auxotype patterns of consecutive gonococcal isolates from ten different areas of Sweden. Scand J Infect Dis. 1983;15(1):33–42. doi: 10.3109/inf.1983.15.issue-1.07. [DOI] [PubMed] [Google Scholar]
- Hildebrandt J. F., Mayer L. W., Wang S. P., Buchanan T. M. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect Immun. 1978 Apr;20(1):267–272. doi: 10.1128/iai.20.1.267-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S., Tam M. R., Nowinski R. C., Holmes K. K., Sandström E. G. Serological classification of Neisseria gonorrhoeae with use of monoclonal antibodies to gonococcal outer membrane protein I. J Infect Dis. 1984 Jul;150(1):44–48. doi: 10.1093/infdis/150.1.44. [DOI] [PubMed] [Google Scholar]
- Kohl P. K., Knapp J. S., Hofmann H., Gruender K., Petzoldt D., Tams M. R., Holmes K. K. Epidemiological analysis of Neisseria gonorrhoeae in the Federal Republic of Germany by auxotyping and serological classification using monoclonal antibodies. Genitourin Med. 1986 Jun;62(3):145–150. doi: 10.1136/sti.62.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Lysko P. G., McFarland L., Knapp J. S., Sandstrom E., Critchlow C., Holmes K. K. Gonococcal strains from homosexual men have outer membranes with reduced permeability to hydrophobic molecules. Infect Immun. 1982 Aug;37(2):432–438. doi: 10.1128/iai.37.2.432-438.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramstedt K. M., Hallhagen G. J., Bygdeman S. M., Lincoln K. A., Kallings I., Gillenius C., Sandström E. G. Serologic classification and contact-tracing in the control of microepidemics of beta-lactamase-producing Neisseria gonorrhoeae. Sex Transm Dis. 1985 Oct-Dec;12(4):209–214. doi: 10.1097/00007435-198510000-00008. [DOI] [PubMed] [Google Scholar]
- Reid K. G., Young H. Serogrouping Neisseria gonorrhoeae: correlation of coagglutination serogroup WII with homosexually acquired infection. Br J Vener Dis. 1984 Oct;60(5):302–305. doi: 10.1136/sti.60.5.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudén A. K., Werner Y. K., Ringertz O., Bygdeman S. M., Bäckman M., Sandström E. G. Use of gonococcal W serogrouping in the evaluation of a clinical trial of rosoxacin. Sex Transm Dis. 1985 Jan-Mar;12(1):19–24. doi: 10.1097/00007435-198501000-00005. [DOI] [PubMed] [Google Scholar]
- Sandstrom E. G., Chen K. C., Buchanan T. M. Serology of Neisseria gonorrhoeae: coagglutination serogroups WI and WII/III correspond to different outer membrane protein I molecules. Infect Immun. 1982 Nov;38(2):462–470. doi: 10.1128/iai.38.2.462-470.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom E. G., Knapp J. S., Buchanan T. B. Serology of Neisseria gonorrhoeae: W-antigen serogrouping by coagglutination and protein I serotyping by enzyme-linked immunosorbent assay both detect protein I antigens. Infect Immun. 1982 Jan;35(1):229–239. doi: 10.1128/iai.35.1.229-239.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandström E., Danielsson D. Serology of Neisseria gonorrhoeae. Classification by co-agglutination. Acta Pathol Microbiol Scand B. 1980 Feb;88(1):27–38. doi: 10.1111/j.1699-0463.1980.tb02600.x. [DOI] [PubMed] [Google Scholar]
- Tam M. R., Buchanan T. M., Sandström E. G., Holmes K. K., Knapp J. S., Siadak A. W., Nowinski R. C. Serological classification of Neisseria gonorrhoeae with monoclonal antibodies. Infect Immun. 1982 Jun;36(3):1042–1053. doi: 10.1128/iai.36.3.1042-1053.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Holmes K. K., Knapp J. S., Ott S., Kyzer D. D. Immunologic classification of Neisseria gonorrhoeae with micro-immunofluorescence. J Immunol. 1977 Sep;119(3):795–803. [PubMed] [Google Scholar]
- Young H., Paterson I. C., McDonald D. R. Rapid carbohydrate utilization test for the identification of Neisseria gonorrhoeae. Br J Vener Dis. 1976 Jun;52(3):172–175. doi: 10.1136/sti.52.3.172. [DOI] [PMC free article] [PubMed] [Google Scholar]



