Abstract
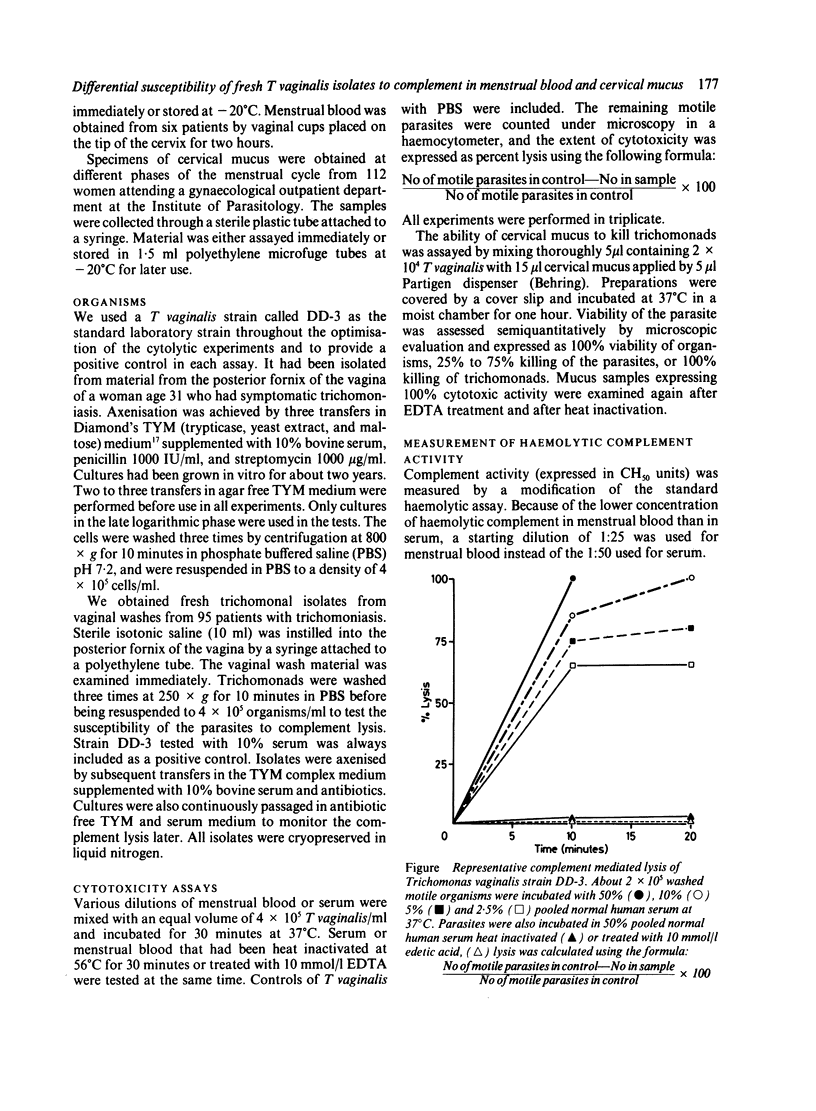
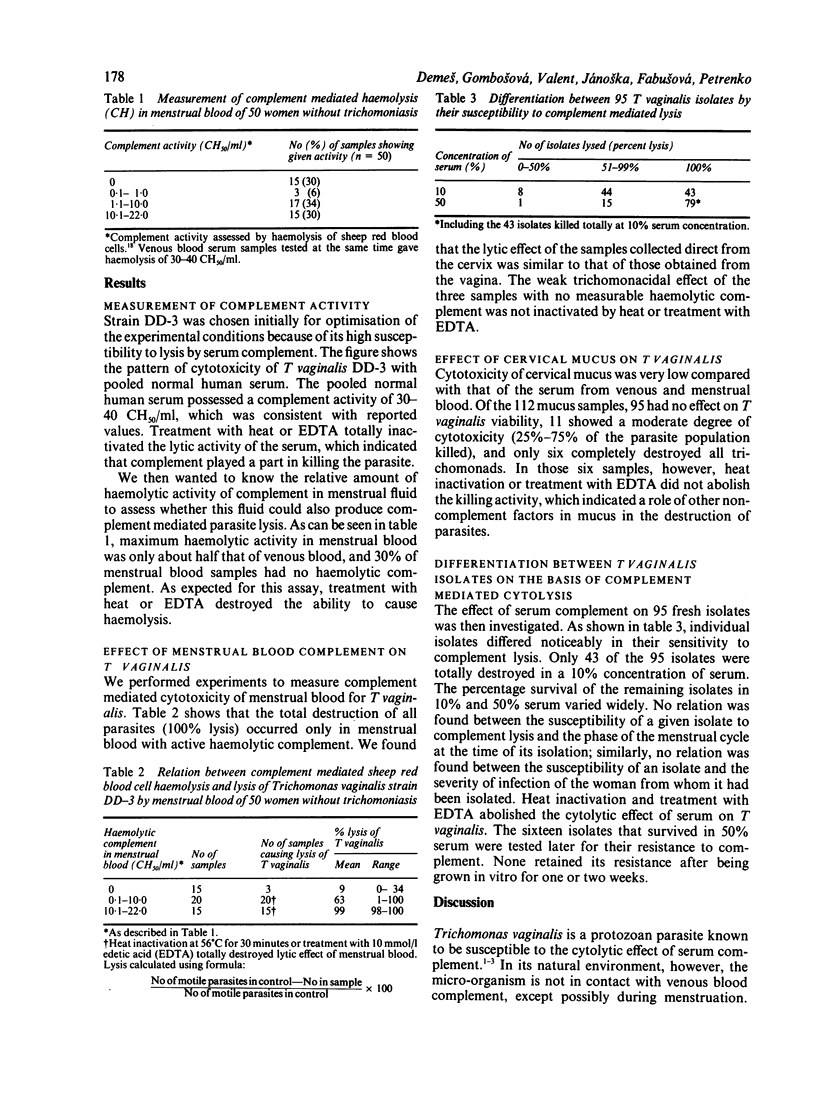
The ability of complement in human menstrual blood and cervical mucus to kill Trichomonas vaginalis was compared with that of complement in serum, and 95 fresh trichomonal isolates obtained from vaginal wash material were evaluated for susceptibility to complement immediately after isolation. Only serum and menstrual blood with haemolytic activity produced total cytolysis of T vaginalis. The cytolytic abilities of these fluids were totally inactivated by treatment with heat or edetic acid (EDTA), which confirms the role of complement in cytolysis. Most cervical mucus samples had no detectable trichomonal cytotoxic properties. The cytotoxic activity in the remaining samples was not due to complement, as it was heat stable. Fresh isolates of T vaginalis and subpopulations of fresh isolates differed in their susceptibility or resistance to complement mediated lysis in serum. Resistance to complement did not remain stable after trichomonads were grown in vitro.
Full text
PDF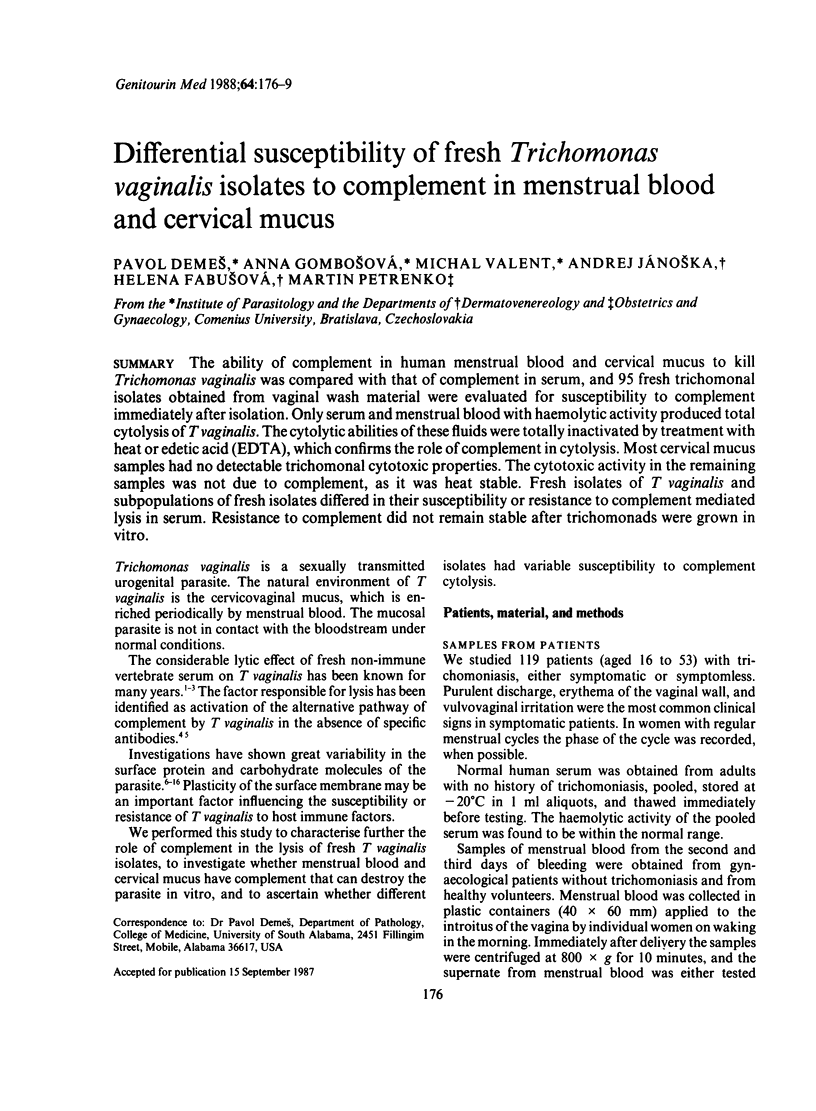

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F. Antigen analysis of several pathogenic strains of Trichomonas vaginalis. Infect Immun. 1983 Mar;39(3):1041–1047. doi: 10.1128/iai.39.3.1041-1047.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Garza G., Smith J., Spence M. Trichomonas vaginalis: electrophoretic analysis and heterogeneity among isolates due to high-molecular-weight trichomonad proteins. Exp Parasitol. 1986 Apr;61(2):244–251. doi: 10.1016/0014-4894(86)90158-x. [DOI] [PubMed] [Google Scholar]
- Alderete J. F. Identification of immunogenic and antibody-binding membrane proteins of pathogenic Trichomonas vaginalis. Infect Immun. 1983 Apr;40(1):284–291. doi: 10.1128/iai.40.1.284-291.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Kasmala L., Metcalfe E., Garza G. E. Phenotypic variation and diversity among Trichomonas vaginalis isolates and correlation of phenotype with trichomonal virulence determinants. Infect Immun. 1986 Aug;53(2):285–293. doi: 10.1128/iai.53.2.285-293.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Kasmala L. Monoclonal antibody to a major glycoprotein immunogen mediates differential complement-independent lysis of Trichomonas vaginalis. Infect Immun. 1986 Sep;53(3):697–699. doi: 10.1128/iai.53.3.697-699.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Suprun-Brown L., Kasmala L. Monoclonal antibody to a major surface glycoprotein immunogen differentiates isolates and subpopulations of Trichomonas vaginalis. Infect Immun. 1986 Apr;52(1):70–75. doi: 10.1128/iai.52.1.70-75.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Suprun-Brown L., Kasmala L., Smith J., Spence M. Heterogeneity of Trichomonas vaginalis and discrimination among trichomonal isolates and subpopulations with sera of patients and experimentally infected mice. Infect Immun. 1985 Sep;49(3):463–468. doi: 10.1128/iai.49.3.463-468.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly R. J., Torian B. E., Stibbs H. H. Identification of a surface antigen of Trichomonas vaginalis. Infect Immun. 1985 Aug;49(2):270–274. doi: 10.1128/iai.49.2.270-274.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND L. S. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol. 1957 Aug;43(4):488–490. [PubMed] [Google Scholar]
- Demes P., Gombosová A., Valent M., Fabusová H., Jánoska A. Fewer Trichomonas vaginalis organisms in vaginas of infected women during menstruation. Genitourin Med. 1988 Feb;64(1):22–24. doi: 10.1136/sti.64.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillin F. D., Sher A. Activation of the alternative complement pathway by Trichomonas vaginalis. Infect Immun. 1981 Oct;34(1):268–273. doi: 10.1128/iai.34.1.268-273.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook T. W., Boackle R. J., Vesely J., Parker B. W. Trichomonas vaginalis: alternative pathway activation of complement. Trans R Soc Trop Med Hyg. 1982;76(4):473–475. doi: 10.1016/0035-9203(82)90140-7. [DOI] [PubMed] [Google Scholar]
- Krieger J. N., Holmes K. K., Spence M. R., Rein M. F., McCormack W. M., Tam M. R. Geographic variation among isolates of Trichomonas vaginalis: demonstration of antigenic heterogeneity by using monoclonal antibodies and the indirect immunofluorescence technique. J Infect Dis. 1985 Nov;152(5):979–984. doi: 10.1093/infdis/152.5.979. [DOI] [PubMed] [Google Scholar]
- SAMUELS R., CHUN-HOON H. SEROLOGICAL INVESTIGATIONS OF TRICHOMONADS. I. COMPARISONS OF "NATURAL" AND IMMUNE ANTIBODIES. J Protozool. 1964 Feb;11:36–46. doi: 10.1111/j.1550-7408.1964.tb01718.x. [DOI] [PubMed] [Google Scholar]
- Su K. E. Antibody to Trichomonas vaginalis in human cervicovaginal secretions. Infect Immun. 1982 Sep;37(3):852–857. doi: 10.1128/iai.37.3.852-857.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torian B. E., Connelly R. J., Stephens R. S., Stibbs H. H. Specific and common antigens of Trichomonas vaginalis detected by monoclonal antibodies. Infect Immun. 1984 Jan;43(1):270–275. doi: 10.1128/iai.43.1.270-275.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELD J. T., KEAN B. H. A factor in serum of human beings and animals that destroys T. vaginalis. Proc Soc Exp Biol Med. 1958 Jul;98(3):494–496. doi: 10.3181/00379727-98-24086. [DOI] [PubMed] [Google Scholar]
- Wartoń A., Honigberg B. M. Analysis of surface saccharides in Trichomonas vaginalis strains with various pathogenicity levels by fluorescein-conjugated plant lectins. Z Parasitenkd. 1983;69(2):149–159. doi: 10.1007/BF00926951. [DOI] [PubMed] [Google Scholar]


