Abstract
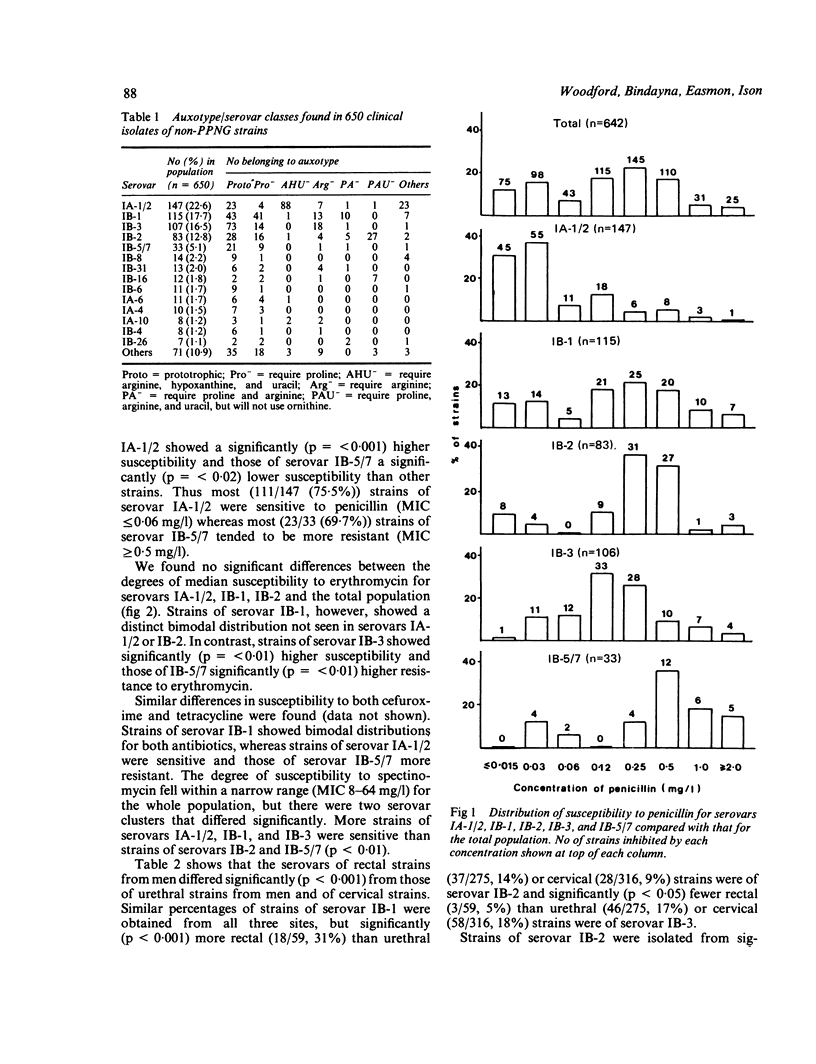
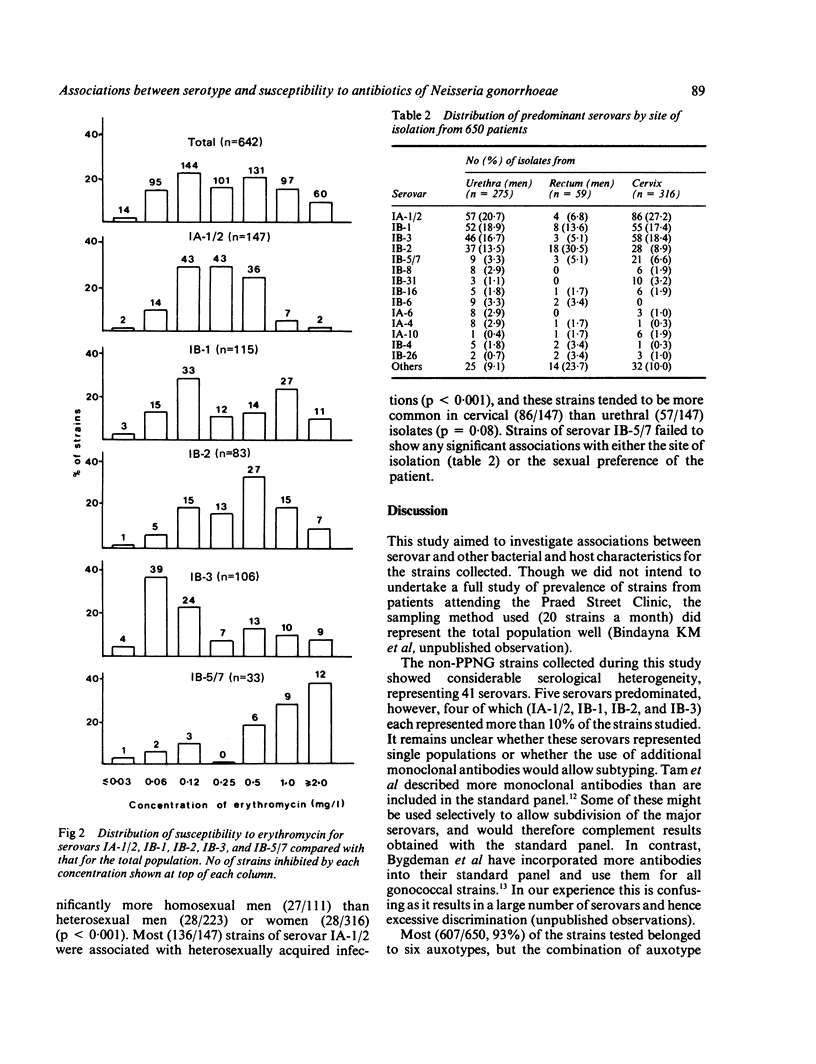
A serological classification scheme for Neisseria gonorrhoeae was used to investigate the epidemiological associations between gonococcal serotype and other bacterial and host characters. Six hundred and fifty clinical isolates of non-penicillinase producing N gonorrhoeae from the Praed Street Clinic, St Mary's Hospital, were included in this study. The strains collected represented 41 serovars, although 485 (75%) of the 650 strains belonged to five serovars. Strains of serovar IA-1/2 were commonly isolated from the cervix and tended to be sensitive to penicillin and moderately resistant to erythromycin. Strains of serovar IB-1 showed bimodal patterns of susceptibility to both penicillin and erythromycin and were obtained equally from all anatomical sites. Strains of serovar IB-2 were isolated more often from the rectum and were associated with homosexually acquired infections, whereas those of serovar IB-3 were sensitive to erythromycin and were rarely isolated from the rectum. Strains of IB-5/7 were more resistant to penicillin and erythromycin than strains of other serovars. The serological classification of N gonorrhoeae is thus a powerful tool that may be used to study biological characteristics of the gonococcus, such as susceptibility to antimicrobials and site tropism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. M. Cyanobacterial cell inclusions. Annu Rev Microbiol. 1984;38:1–25. doi: 10.1146/annurev.mi.38.100184.000245. [DOI] [PubMed] [Google Scholar]
- Ashall F., Bramwell M. E., Harris H. A new marker for human cancer cells. 1 The Ca antigen and the Ca1 antibody. Lancet. 1982 Jul 3;2(8288):1–6. doi: 10.1016/s0140-6736(82)91150-3. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Gotschlich E. C. Gonococcal membrane proteins: speculation on their role in pathogenesis. Prog Allergy. 1983;33:298–313. [PubMed] [Google Scholar]
- Bygdeman S. Gonorrhoea in men with homosexual contacts. Serogroups of isolated gonococcal strains related to antibiotic susceptibility, site of infection, and symptoms. Br J Vener Dis. 1981 Oct;57(5):320–324. doi: 10.1136/sti.57.5.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W. Genetic transformation of biosynthetically defective Neisseria gonorrhoeae clinical isolates. J Bacteriol. 1974 Oct;120(1):203–209. doi: 10.1128/jb.120.1.203-209.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copley C. G., Egglestone S. I. Auxotyping of Neisseria gonorrhoeae isolated in the United Kingdom. J Med Microbiol. 1983 Aug;16(3):295–302. doi: 10.1099/00222615-16-3-295. [DOI] [PubMed] [Google Scholar]
- Copley C. G. Neisseria gonorrhoeae: subdivision of auxogroups by genetic transformation. Genitourin Med. 1987 Jun;63(3):153–156. doi: 10.1136/sti.63.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowsett E. G. Penicillin-resistant gonococci. Lancet. 1980 Jul 26;2(8187):202–202. [PubMed] [Google Scholar]
- Faruki H., Kohmescher R. N., McKinney W. P., Sparling P. F. A community-based outbreak of infection with penicillin-resistant Neisseria gonorrhoeae not producing penicillinase (chromosomally mediated resistance). N Engl J Med. 1985 Sep 5;313(10):607–611. doi: 10.1056/NEJM198509053131004. [DOI] [PubMed] [Google Scholar]
- Ison C. A., Gedney J., Easmon C. S. Chromosomal resistance of gonococci to antibiotics. Genitourin Med. 1987 Aug;63(4):239–243. doi: 10.1136/sti.63.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James J. F., Swanson J. Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures. Infect Immun. 1978 Jan;19(1):332–340. doi: 10.1128/iai.19.1.332-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S., Holmes K. K., Bonin P., Hook E. W., 3rd Epidemiology of gonorrhea: distribution and temporal changes in auxotype/serovar classes of Neisseria gonorrhoeae. Sex Transm Dis. 1987 Jan-Mar;14(1):26–32. [PubMed] [Google Scholar]
- Knapp J. S., Tam M. R., Nowinski R. C., Holmes K. K., Sandström E. G. Serological classification of Neisseria gonorrhoeae with use of monoclonal antibodies to gonococcal outer membrane protein I. J Infect Dis. 1984 Jul;150(1):44–48. doi: 10.1093/infdis/150.1.44. [DOI] [PubMed] [Google Scholar]
- Knapp J. S., Thornsberry C., Schoolnik G. A., Wiesner P. J., Homes K. K. Phenotypic and epidemiologic correlates of auxotype in Neisseria gonorrhoeae. J Infect Dis. 1978 Aug;138(2):160–165. doi: 10.1093/infdis/138.2.160. [DOI] [PubMed] [Google Scholar]
- Lambden P. R., Heckels J. E., James L. T., Watt P. J. Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae. J Gen Microbiol. 1979 Oct;114(2):305–312. doi: 10.1099/00221287-114-2-305. [DOI] [PubMed] [Google Scholar]
- Lynch E. C., Blake M. S., Gotschlich E. C., Mauro A. Studies of Porins: Spontaneously Transferred from Whole Cells and Reconstituted from Purified Proteins of Neisseria gonorrhoeae and Neisseria meningitidis. Biophys J. 1984 Jan;45(1):104–107. doi: 10.1016/S0006-3495(84)84127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Johnson S. R., Biddle J. W., Roberts M. C. High-level tetracycline resistance in Neisseria gonorrhoeae is result of acquisition of streptococcal tetM determinant. Antimicrob Agents Chemother. 1986 Nov;30(5):664–670. doi: 10.1128/aac.30.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Lysko P. G., McFarland L., Knapp J. S., Sandstrom E., Critchlow C., Holmes K. K. Gonococcal strains from homosexual men have outer membranes with reduced permeability to hydrophobic molecules. Infect Immun. 1982 Aug;37(2):432–438. doi: 10.1128/iai.37.2.432-438.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYN A., KORNER B., BENTZON M. W. Effects of penicillin, streptomycin, and tetracycline on N. gonorrhoeae isolated in 1944 and in 1957. Br J Vener Dis. 1958 Dec;34(4):227–239. doi: 10.1136/sti.34.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. G., Young H. Serogrouping Neisseria gonorrhoeae: correlation of coagglutination serogroup WII with homosexually acquired infection. Br J Vener Dis. 1984 Oct;60(5):302–305. doi: 10.1136/sti.60.5.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R. J., Biddle J. W., JeanLouis Y. A., DeWitt W. E., Blount J. H., Morse S. A. Chromosomally mediated resistance in Neisseria gonorrhoeae in the United States: results of surveillance and reporting, 1983-1984. J Infect Dis. 1986 Feb;153(2):340–345. doi: 10.1093/infdis/153.2.340. [DOI] [PubMed] [Google Scholar]
- Tam M. R., Buchanan T. M., Sandström E. G., Holmes K. K., Knapp J. S., Siadak A. W., Nowinski R. C. Serological classification of Neisseria gonorrhoeae with monoclonal antibodies. Infect Immun. 1982 Jun;36(3):1042–1053. doi: 10.1128/iai.36.3.1042-1053.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji M., Everson J. S. Comparative virulence of opacity variants of Neisseria gonorrhoeae strain P9. Infect Immun. 1981 Mar;31(3):965–970. doi: 10.1128/iai.31.3.965-970.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


