Abstract
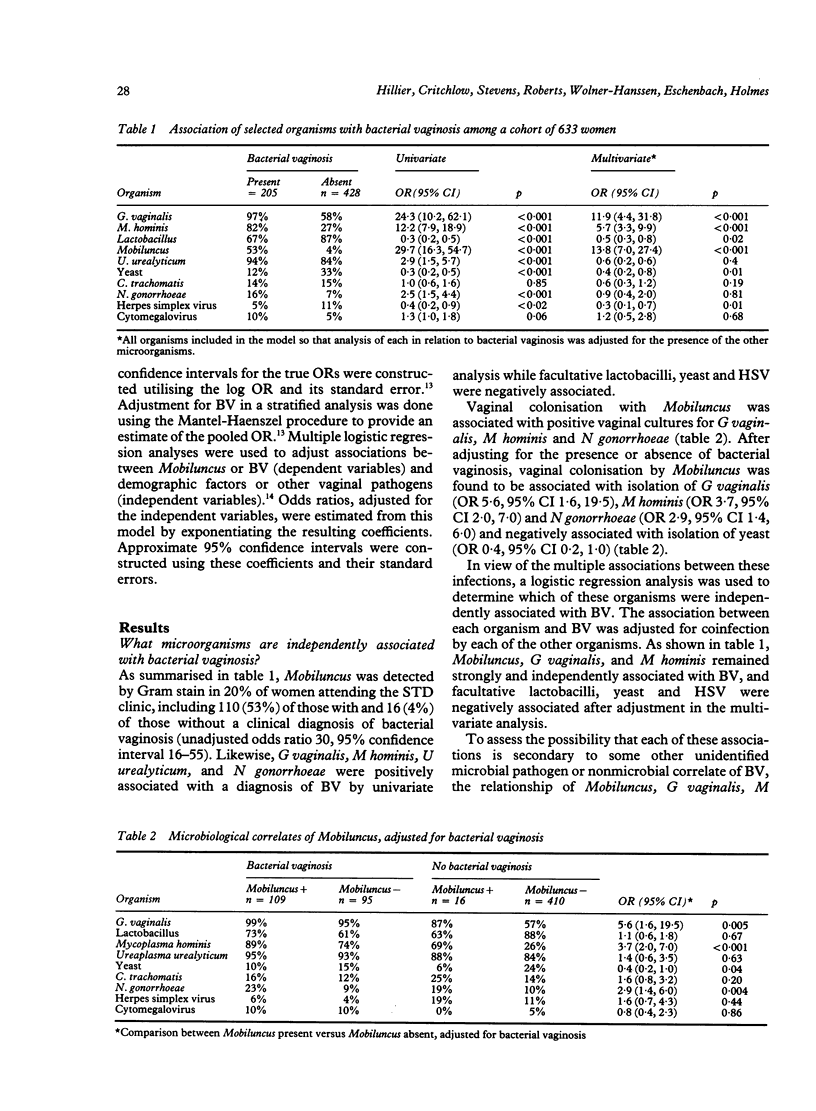
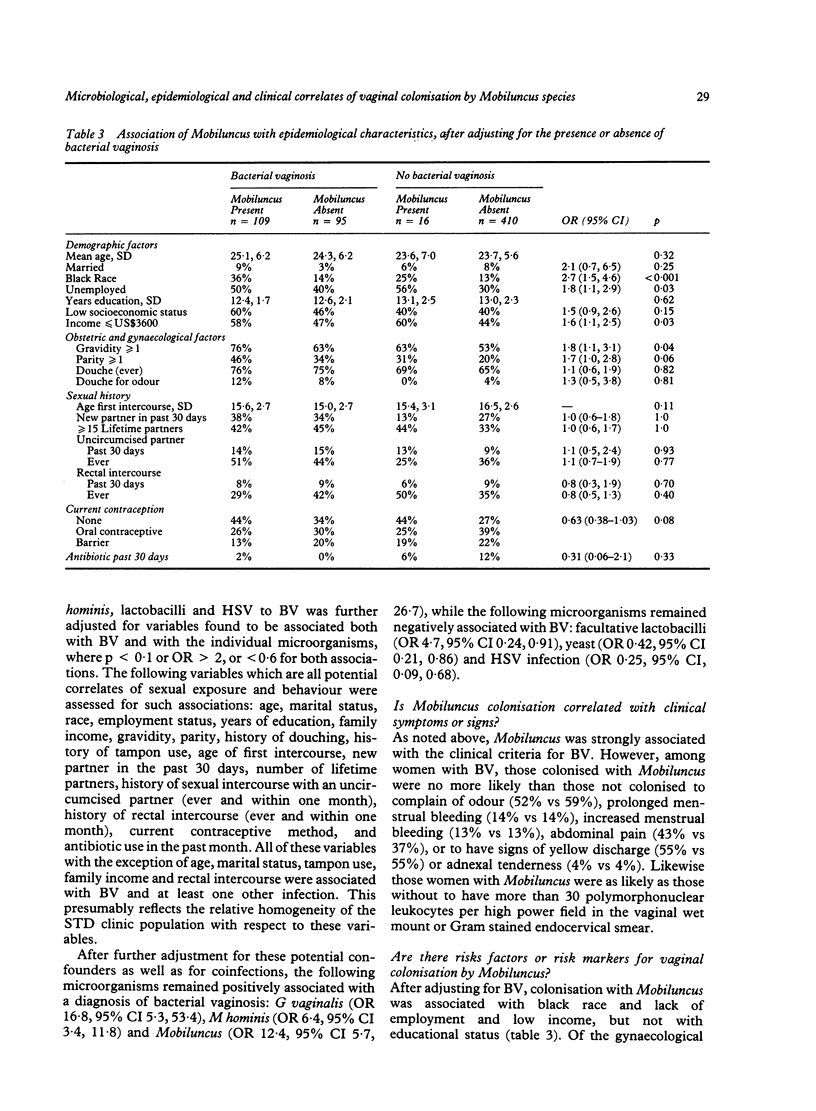
The microbiological and epidemiological correlates of vaginal colonisation by Mobiluncus species were examined among randomly selected women attending a sexually transmitted disease (STD) clinic. Women positive for Trichomonas vaginalis were excluded. Mobiluncus spp. were detected by Gram stained vaginal smear in 21% of 633 STD clinic patients, including 53% of those with and 4% of those without bacterial vaginosis (BV), as diagnosed by clinical criteria. Gardnerella vaginalis and Mycoplasma hominis detected by vaginal culture and Mobiluncus detected by vaginal Gram stain were each independently associated with BV after adjusting by logistic regression for the presence of sexually transmitted disease pathogens, gravidity, parity and number of lifetime sexual partners (p less than 0.001 for each organism). Bacterial vaginosis was negatively correlated with isolation of lactobacilli, yeast and herpes simplex virus. After adjusting for presence or absence of BV, women with Mobiluncus were more likely to harbour G vaginalis (odds ratio 5.6, 95% confidence interval 1.6-19.5), M hominis (OR 3.7, 95% CI 2.0-7.0) and Neisseria gonorrhoeae (OR 2.9, 95% CI 1.4-6.0) and less likely to harbour vaginal yeast (OR 0.4, 95% CI 0.2-1.0); were more likely to be black (OR 2.7, 95% CI 1.5-4.6), and to have been pregnant (OR 1.8, 95% CI 1.1-3.1); but after the adjustment for BV, vaginal colonisation by Mobiluncus was not associated with symptoms of odour, abdominal pain, menstrual irregularities, or with adnexal tenderness. In summary, Mobiluncus, Gardnerella vaginalis and Mycoplasma hominis were independently associated with a clinical diagnosis of bacterial vaginosis, and Mobiluncus was further associated with the presence of BV-associated microorganisms (M hominis and G vaginalis), N gonorrhoeae, black race, and gravidity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsel R., Totten P. A., Spiegel C. A., Chen K. C., Eschenbach D., Holmes K. K. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983 Jan;74(1):14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- Cristiano L., Coffetti N., Dalvai G., Lorusso L., Lorenzi M. Bacterial vaginosis: prevalence in outpatients, association with some micro-organisms and laboratory indices. Genitourin Med. 1989 Dec;65(6):382–387. doi: 10.1136/sti.65.6.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenbach D. A., Hillier S., Critchlow C., Stevens C., DeRouen T., Holmes K. K. Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol. 1988 Apr;158(4):819–828. doi: 10.1016/0002-9378(88)90078-6. [DOI] [PubMed] [Google Scholar]
- Hallén A., Påhlson C., Forsum U. Bacterial vaginosis in women attending STD clinic: diagnostic criteria and prevalence of Mobiluncus spp. Genitourin Med. 1987 Dec;63(6):386–389. doi: 10.1136/sti.63.6.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallén A., Påhlson C., Forsum U. Rectal occurrence of Mobiluncus species. Genitourin Med. 1988 Aug;64(4):273–275. doi: 10.1136/sti.64.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst E., Wathne B., Hovelius B., Mårdh P. A. Bacterial vaginosis: microbiological and clinical findings. Eur J Clin Microbiol. 1987 Oct;6(5):536–541. doi: 10.1007/BF02014242. [DOI] [PubMed] [Google Scholar]
- Krohn M. A., Hillier S. L., Eschenbach D. A. Comparison of methods for diagnosing bacterial vaginosis among pregnant women. J Clin Microbiol. 1989 Jun;27(6):1266–1271. doi: 10.1128/jcm.27.6.1266-1271.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson P. G., Bergman B. B. Is there a causal connection between motile curved rods, Mobiluncus species, and bleeding complications? Am J Obstet Gynecol. 1986 Jan;154(1):107–108. doi: 10.1016/0002-9378(86)90403-5. [DOI] [PubMed] [Google Scholar]
- Martius J., Krohn M. A., Hillier S. L., Stamm W. E., Holmes K. K., Eschenbach D. A. Relationships of vaginal Lactobacillus species, cervical Chlamydia trachomatis, and bacterial vaginosis to preterm birth. Obstet Gynecol. 1988 Jan;71(1):89–95. [PubMed] [Google Scholar]
- Paavonen J., Miettinen A., Stevens C. E., Chen K. C., Holmes K. K. Mycoplasma hominis in nonspecific vaginitis. Sex Transm Dis. 1983 Oct-Dec;10(4 Suppl):271–275. [PubMed] [Google Scholar]
- Piot P., Van Dyck E., Godts P., Vanderheyden J. The vaginal microbial flora in non-specific vaginitis. Eur J Clin Microbiol. 1982 Oct;1(5):301–306. doi: 10.1007/BF02019976. [DOI] [PubMed] [Google Scholar]
- Påhlson C., Hallén A., Forsum U. Curved rods related to Mobiluncus--phenotypes as defined by monoclonal antibodies. Acta Pathol Microbiol Immunol Scand B. 1986 Jun;94(3):117–125. doi: 10.1111/j.1699-0463.1986.tb03030.x. [DOI] [PubMed] [Google Scholar]
- Påhlson C., Hallén A., Forsum U. Improved yield of Mobiluncus species from clinical specimens after alkaline treatment. Acta Pathol Microbiol Immunol Scand B. 1986 Jun;94(3):113–116. doi: 10.1111/j.1699-0463.1986.tb03029.x. [DOI] [PubMed] [Google Scholar]
- Roberts M. C., Hillier S. L., Schoenknecht F. D., Holmes K. K. Comparison of gram stain, DNA probe, and culture for the identification of species of Mobiluncus in female genital specimens. J Infect Dis. 1985 Jul;152(1):74–77. doi: 10.1093/infdis/152.1.74. [DOI] [PubMed] [Google Scholar]
- Smith H. J., Moore H. B. Isolation of Mobiluncus species from clinical specimens by using cold enrichment and selective media. J Clin Microbiol. 1988 Jun;26(6):1134–1137. doi: 10.1128/jcm.26.6.1134-1137.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel C. A., Amsel R., Eschenbach D., Schoenknecht F., Holmes K. K. Anaerobic bacteria in nonspecific vaginitis. N Engl J Med. 1980 Sep 11;303(11):601–607. doi: 10.1056/NEJM198009113031102. [DOI] [PubMed] [Google Scholar]
- Spiegel C. A., Amsel R., Holmes K. K. Diagnosis of bacterial vaginosis by direct gram stain of vaginal fluid. J Clin Microbiol. 1983 Jul;18(1):170–177. doi: 10.1128/jcm.18.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel C. A., Eschenbach D. A., Amsel R., Holmes K. K. Curved anaerobic bacteria in bacterial (nonspecific) vaginosis and their response to antimicrobial therapy. J Infect Dis. 1983 Nov;148(5):817–822. doi: 10.1093/infdis/148.5.817. [DOI] [PubMed] [Google Scholar]
- Thomason J. L., Schreckenberger P. C., Spellacy W. N., Riff L. J., LeBeau L. J. Clinical and microbiological characterization of patients with nonspecific vaginosis associated with motile, curved anaerobic rods. J Infect Dis. 1984 May;149(5):801–809. doi: 10.1093/infdis/149.5.801. [DOI] [PubMed] [Google Scholar]


