Abstract
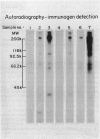
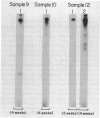
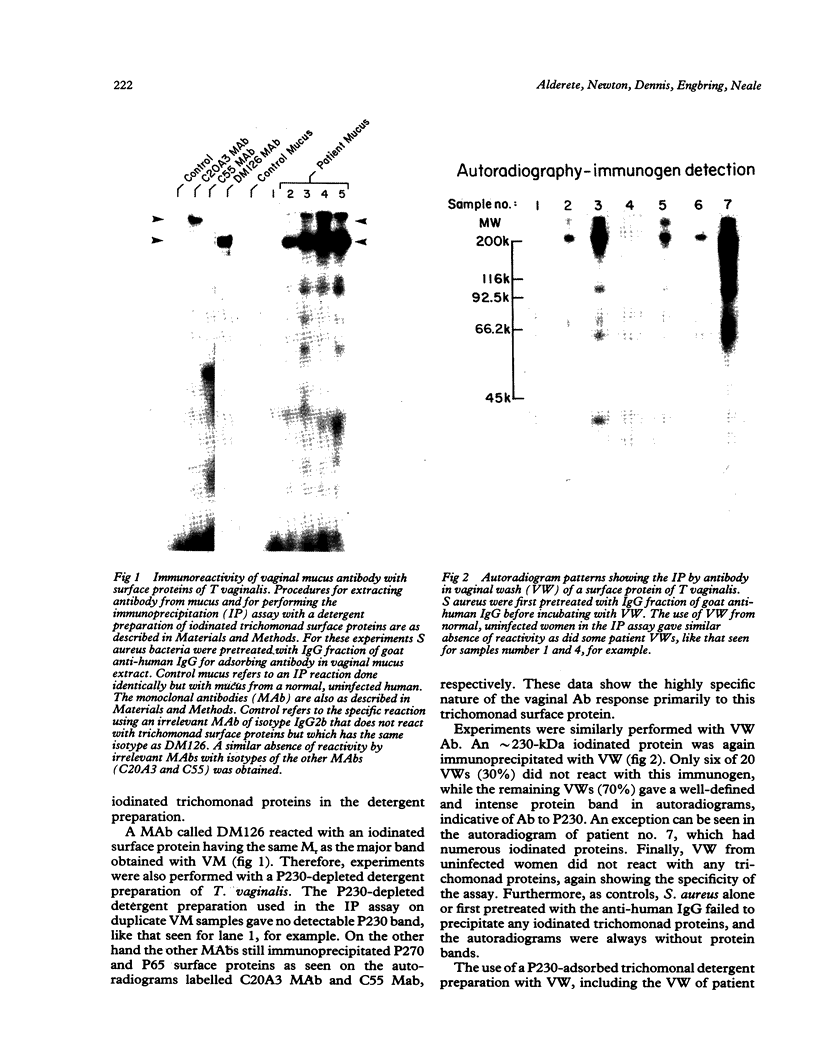
Twenty vaginal washes (VWs) and ten vaginal mucus (VM) samples from patients with trichomoniasis were examined for the presence of antibody to surface protein immunogens of Trichomonas vaginalis. Fourteen of 20 VWs (70%) and 8 of 10 VM (80%) had immunoglobulin G (IgG) antibody (Ab) that reacted in an immunoprecipitation (IP) assay with one iodinated Trichomonas vaginalis surface protein immunogen with a relative molecular mass of 230,000 daltons (230-kDa) (P230). No similar IP of any iodinated protein was observed when detergent extract was first depleted of P230 with monoclonal antibody (MAb), indicating a highly specific VW IgG response of patients to P230. VWs were also obtained from 10 patients from one to four weeks after treatment. These VWs had the same, or in one case a greater, level of IgG to P230. Under no circumstances was Ab to P230 or any other trichomonad protein detected in VWs or VM from normal, uninfected women. Flow cytofluorometry with VW Ab yielded heterogeneous fluorescent and non-fluorescent populations of trichomonads, reaffirming the restricted Ab response to one or a few epitopes on P230 in the vagina of patients. Under identical conditions, the MAb gave totally fluorescent parasite populations of some isolates, and the MAb again demonstrated variable epitope accessibility to Ab binding (Infect Immun 1987;55:1037). Finally, the MAb or VW Ab was never cytolytic for immunoreactive (fluorescent) parasites, even in the presence of complement. This study identifies the most important trichomonad surface immunogen on the basis of the vaginal Ab response, and data underscore the significance of immune evasion strategies of this sexually transmitted disease agent.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Demes P., Gombosová A., Valent M., Yánoska A., Fabusová H., Kasmala L., Garza G. E., Metcalfe E. C. Phenotypes and protein-epitope phenotypic variation among fresh isolates of Trichomonas vaginalis. Infect Immun. 1987 May;55(5):1037–1041. doi: 10.1128/iai.55.5.1037-1041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Demeś P., Gombosova A., Valent M., Fabusová M., Jánoska A., Stefanovic J., Arroyo R. Specific parasitism of purified vaginal epithelial cells by Trichomonas vaginalis. Infect Immun. 1988 Oct;56(10):2558–2562. doi: 10.1128/iai.56.10.2558-2562.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F. Enzyme linked immunosorbent assay for detecting antibody to Trichomonas vaginalis: use of whole cells and aqueous extract as antigen. Br J Vener Dis. 1984 Jun;60(3):164–170. doi: 10.1136/sti.60.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Kasmala L. Monoclonal antibody to a major glycoprotein immunogen mediates differential complement-independent lysis of Trichomonas vaginalis. Infect Immun. 1986 Sep;53(3):697–699. doi: 10.1128/iai.53.3.697-699.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Neale K. A. Relatedness of structures of a major immunogen in Trichomonas vaginalis isolates. Infect Immun. 1989 Jun;57(6):1849–1853. doi: 10.1128/iai.57.6.1849-1853.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Suprun-Brown L., Kasmala L. Monoclonal antibody to a major surface glycoprotein immunogen differentiates isolates and subpopulations of Trichomonas vaginalis. Infect Immun. 1986 Apr;52(1):70–75. doi: 10.1128/iai.52.1.70-75.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND L. S. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol. 1957 Aug;43(4):488–490. [PubMed] [Google Scholar]
- Lockwood B. C., North M. J., Coombs G. H. The release of hydrolases from Trichomonas vaginalis and Tritrichomonas foetus. Mol Biochem Parasitol. 1988 Aug;30(2):135–142. doi: 10.1016/0166-6851(88)90106-5. [DOI] [PubMed] [Google Scholar]
- Neale K. A., Alderete J. F. Analysis of the proteinases of representative Trichomonas vaginalis isolates. Infect Immun. 1990 Jan;58(1):157–162. doi: 10.1128/iai.58.1.157-162.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein M. F., Chapel T. A. Trichomoniasis, candidiasis, and the minor venereal diseases. Clin Obstet Gynecol. 1975 Mar;18(1):73–88. doi: 10.1097/00003081-197503000-00008. [DOI] [PubMed] [Google Scholar]
- Street D. A., Taylor-Robinson D., Ackers J. P., Hanna N. F., McMillan A. Evaluation of an enzyme-linked immunosorbent assay for the detection of antibody to Trichomonas vaginalis in sera and vaginal secretions. Br J Vener Dis. 1982 Oct;58(5):330–333. doi: 10.1136/sti.58.5.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su K. E. Antibody to Trichomonas vaginalis in human cervicovaginal secretions. Infect Immun. 1982 Sep;37(3):852–857. doi: 10.1128/iai.37.3.852-857.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]