Abstract
Objective:
To examine the effects of treatment sequence of parent stimulant medication (MED) and behavioral parent training (BPT) on child, maternal, and parenting outcomes among multiplex attention-deficit/hyperactivity disorder (ADHD) families using a pilot Sequential Multiple Assignment Randomized Trial (SMART) design.
Methods:
To be eligible, mothers had to meet DSM-IV diagnostic criteria for ADHD, and their children had to have elevated ADHD symptoms. Thirty-five mother-child dyads were randomized at baseline and again at week 8. The resulting 4 sequences were MED-MED, BPT-BPT, MED-BPT, and BPT-MED. Outcomes included child ADHD symptoms, child impairment, maternal ADHD symptoms, and parenting at week 16. Data were collected from September 2012 to December 2016.
Results:
The BPT-MED sequence demonstrated the most favorable outcomes for child ADHD symptoms (effect size = −0.36) and child impairment (effect sizes −0.33 to −0.51). All 3 sequences involving medication demonstrated similar impact on maternal ADHD symptoms (effect sizes ranged from −0.32 to −0.48). The BPT-MED (effect sizes ranged from 0.30 to 0.35) had the most favorable effects on positive parenting outcomes. For negative parenting outcomes, BPT-MED (effect size = −0.50 for selfreport) and BPT-BPT (effect size = −0.08 for observation) had the most favorable outcomes.
Conclusions:
Overall, based on this pilot SMART, combination treatment may be helpful for most multiplex ADHD families, and sequencing treatments with BPT first followed by stimulant medication for the mother may be the most promising approach to improve child ADHD symptoms, impairment, and parenting. These results require replication with a fully powered SMART design. We conclude with considerations for implementing a model of care for multiplex ADHD families.
Trial Registration:
ClinicalTrials.gov identifier: NCT01816074.
Attention-deficit/hyperactivity disorder (ADHD) is highly familial,1 with heritability estimates exceeding 0.75.2 In clinical practice, multiplex ADHD families, where both a parent and a child have ADHD, are quite common. Estimates suggest that 25%–50% of parents of children with ADHD have ADHD themselves,3 although often the child with ADHD is diagnosed first. ADHD in both parents and children contributes to reciprocal negative family interactions, including family discord and parenting difficulties.4 Despite the genetic and neurobiological underpinnings of ADHD, parental psychopathology and parenting behavior have been identified as robust predictors of developmental outcomes for children with ADHD, even when accounting for genetic influences.5 Thus, efforts to improve developmental outcomes for young children with, or at risk for, ADHD should encompass treatment of parental ADHD and associated parenting difficulties, when present.
Evidence-based treatments for child ADHD include pharmacologic and psychosocial interventions such as behavioral parent training (BPT).6 The American Academy of Pediatrics recommends BPT as first-line treatment for preschool children (ages 4–5 years) with ADHD prior to treatment with stimulant medications.7 Regardless of the type or modality of treatment, treatment for young children with ADHD relies on parents to obtain and consistently deliver the treatment. Because parent ADHD can impair a parent’s ability to access, structure, organize, plan, and follow through with child treatment(s), it is unsurprising that parental ADHD is associated with a diminished child treatment response.4 Challenges associated with following through with treatment may be especially notable for behavioral interventions such as BPT, which require parents to consistently and proactively incorporate skills at home.
Treatment of adult ADHD with stimulants has been well-established through numerous randomized controlled trials and meta-analyses, and these medications are FDA approved (in the U.S.) for adult ADHD. Theoretically, a positive response to pharmacotherapy in parents with ADHD could improve family functioning. A handful of studies have examined this possibility, with the majority showing no or modest effects of parent stimulant on parenting or child symptoms.8,9 Only 1 study to date has demonstrated small improvements in observed parenting and child behavior resulting from treating parents (mostly mothers) with stimulants.10 However, in Waxmonsky and colleagues’ study,10 despite improvements in parenting and child behavior, parent ADHD symptoms were still related to higher absolute frequencies of negative parenting behaviors at posttreatment. Thus, to improve parenting and family interactions in which the parent has ADHD, combining adult ADHD treatment with BPT interventions is likely necessary.4,11
What remains unknown is how best to sequence and personalize treatments for families where a parent has ADHD to improve the developmental trajectories of young children at high risk for persistent ADHD. It is likely that not all families will require both adult ADHD and BPT treatments and both treatments may not always be accessible or acceptable to families. Sequential Multiple Assignment Randomized Trials (SMARTs) allow for an examination of treatment selection, sequencing, and combinations and are designed to inform clinical decision-making.12 The SMART design is ideally suited to answer questions regarding how best to treat families in which a parent has ADHD, and the young child has, or is at risk for developing, ADHD. The current study aimed to assess the longitudinal trajectories (baseline, 8 weeks, and 16 weeks) of child ADHD, child impairment, maternal ADHD, and parenting outcomes in a pilot SMART involving parent medication and BPT among mothers with ADHD and their children. Findings on the feasibility of the pilot SMART and mothers’ reported acceptability of the treatment sequences have been reported elsewhere.13 We hypothesize that combined maternal medication and BPT will be associated with the best treatment outcomes at week 16 relative to maternal medication or BPT alone and that initiating maternal medication prior to BPT will be associated with better treatment outcomes relative to the other treatment sequences. Because maternal ADHD impacts BPT outcomes, when mothers are first treated with stimulants, thereby improving their attention and executive functioning, they may more maximally benefit from subsequent BPT and be more able to consistently implement the behavioral strategies learned, thus leading to more positive treatment outcomes.
METHOD
Participants
Full details of study design and participants can be found in the protocol paper.14 Briefly, mother-child dyads were recruited from clinics and community support groups. Mothers completed prescreening on the phone and those who met criteria (n = 67) were invited for a baseline assessment to determine eligibility for the SMART. Mothers had to meet DSM-IV diagnostic criteria for ADHD, and their children had to present with primary concerns of elevated ADHD symptoms (Conners T score >60). Children had to be between ages 4 and 8 years and be stimulant naïve, in line with our goal of intervening early and delaying the need for stimulant medication in young children. Comorbidities in mothers were assessed with the Structured Clinical Interview for DSM-IV.15 Mothers were excluded if they had medical conditions for which stimulants were contraindicated, were allergic to study medications, were pregnant or breastfeeding, had current bipolar disorder, schizophrenia, or significant suicidal risk, or had recent alcohol/substance abuse. There were no exclusionary criteria for children; the intent was to maximize generalizability of the sample to community populations of ADHD. Comorbidities in children were assessed with the Kiddie-SADS-Present and Lifetime Version.16 Thirty-five mother-child dyads were randomized in the current trial. See Table 1 for participant demographics. At baseline, all mothers met diagnostic criteria for ADHD, 23% had comorbid depressive disorder, and 14% had an anxiety disorder. For children, 85.71% met diagnostic criteria for ADHD at baseline, and 22.8% had a comorbid oppositional defiant disorder. The trial was registered at ClinicalTrials.gov (NCT01816074).
Table 1.
Descriptive Statistics of Study Sample and Study Variables, Stratified by Randomization Timea
| Variable | Overall (n = 35) | BPT-BPT (n = 8) | BPT-MED (n = 9) | MED-BPT (n = 9) | MED-MED (n = 9) |
|---|---|---|---|---|---|
| Baseline prerandomization 1 (BL) | |||||
| Child characteristics | |||||
| Age | 6.0 (1.4) | 5.8 (1.0) | 6.4 (1.3) | 6.0 (1.5) | 5.8 (1.9) |
| Gender male | 19 (54) | 6 (75) | 4 (44) | 5 (56) | 4 (44) |
| White (non-Hispanic) | 29 (83) | 8 (100) | 7 (78) | 6 (67) | 8 (89) |
| Hispanic/Latinx | 3 (9) | 0 | 1 (11) | 2 (22) | 0 |
| Conners-Early Childhood Global Index | 7.1 (1.2) | 6.3 (0.6) | 7.0 (1.4) | 7.5 (0.6) | 7.3 (2.1) |
| Conners 3 Global Index | 7.9 (0.9) | 7.8 (0.8) | 7.7 (1.1) | 8.0 (0.7) | 8.2 (1.2) |
| IRS | 4.1 (1.3) | 3.7 (1.6) | 4.4 (0.7) | 3.8 (1.9) | 4.4 (1.0) |
| CGI-S | 4.6 (1.2) | 4.5 (1.6) | 4.7 (1.4) | 4.4 (1.0) | 4.8 (1.0) |
| Oppositional defiant disorder | 8 (22.8) | 1 (12.5) | 2 (22.2) | 2 (22.2) | 3 (33.3) |
| Conduct disorder | 0 | 0 | 0 | 0 | 0 |
| Parent characteristics | |||||
| Age, y | 39.6 (6.4) | 39.5 (6.1) | 40.4 (6.9) | 36.6 (5.6) | 42.0 (6.4) |
| White (non-Hispanic) | 30 (86) | 8 (100) | 7 (78) | 7 (78) | 8 (89) |
| Hispanic/Latinx | 2 (6) | 0 | 1 (11) | 1 (11) | 0 |
| Married or living with partner | 28 (80) | 5 (63) | 8 (89) | 7 (78) | 8 (89) |
| Education | |||||
| Graduated high school or equivalent | 3 (9) | 1 (13) | 0 | 1 (11) | 1 (11) |
| Some college | 5 (14) | 0 | 1 (11) | 2 (22) | 2 (22) |
| Graduated 2-year college/technical school | 4 (11) | 1 (13) | 2 (22) | 1 (11) | 0 |
| Graduated 4-year college | 12 (34) | 4 (50) | 2 (22) | 3 (33) | 3 (33) |
| Some graduate/professional school | 5 (14) | 0 | 3 (33) | 1 (11) | 1 (11) |
| Completed graduate/professional school | 6 (17) | 2 (25) | 1 (11) | 1 (11) | 2 (11) |
| CAARS ADHD index | 20.6 (5.1) | 19.6 (4.2) | 20.7 (9.1) | 21.4 (5.1) | 20.7 (5.1) |
| Psychiatric comorbidities | |||||
| Depressive disorder | 19 (54) | 3 (28) | 6 (67) | 7 (78) | 3 (33) |
| Anxiety disorder | 3 (9) | 1 (13) | 0 | 1 (11) | 1 (11) |
| Substance use disorder | 4 (11) | 0 | 0 | 2 (22) | 2 (22) |
| History of previous stimulant treatment | 5 (14) | 3 (38) | 1 (11) | 0 | 1 (11) |
| Parenting | |||||
| APQ positive | 61.5 (6.6) | 55.7 (7.1) | 61.9 (5.7) | 65.4 (6.1) | 61.6 (4.0) |
| APQ negative | 36.8 (6.3) | 39.9 (4.3) | 33.9 (5.9) | 38.6 (7.5) | 34.9 (5.9) |
| DPICS positive | 26.4 (19.9) | 17.5 (11.2) | 21.8 (14.8) | 41.7 (27.6) | 23.6 (14.5) |
| DPICS negative | 9.6 (8.0) | 10.6 (6.7) | 8.0 (7.2) | 8.6 (5.5) | 11.3 (12.1) |
| Baseline prerandomization 2 (week 8) | |||||
| Child characteristics | |||||
| Conners-Early Childhood Global Index | 7.0 (1.2) | 6.5 (0.7) | 8.0b | 7.0 (2.0) | 7.0 (1.0) |
| Conners 3 Global Index | 7.2 (1.3) | 6.6 (1.1) | 6.7 (1.2) | 7.0 (1.4) | 8.3 (0.8) |
| IRS | 4.0 (1.4) | 3.9 (1.5) | 3.4 (1.2) | 4.1 (1.7) | 4.6 (1.3) |
| CGI-S | 3.9 (0.9) | 3.8 (1.2) | 3.8 (0.7) | 3.6 (1.1) | 4.3 (0.7) |
| Parent characteristics | |||||
| CAARS ADHD index | 16.9 (6.1) | 17.6 (5.2) | 22.0 (7.1) | 13.1 (4.1) | 15.3 (5.0) |
| Parenting | |||||
| APQ positive | 63.4 (6.3) | 64.3 (6.8) | 63.6 (5.3) | 62.6 (7.3) | 63.0 (7.0) |
| APQ negative | 30.8 (4.6) | 30.4 (3.8) | 28.0 (4.6) | 33.6 (4.7) | 31.2 (2.6) |
| DPICS positive | 35.1 (21.8) | 34.7 (15.8) | 46.3 (30.4) | 31.5 (21.2) | 27.2 (13.1) |
| DPICS negative | 11.3 (11.5) | 9.4 (4.8) | 5.0 (5.0) | 13.5 (15.6) | 17.0 (13.6) |
Values shown as mean (SD) or n (%).
No standard deviation is reported as only 1 participant completed the Conners-Early Childhood; the remaining participants in the BPT-MED sequence completed the Conners 3.
Abbreviations: ADHD = attention-deficit/hyperactivity disorder, APQ = Alabama Parenting Questionnaire, BPT = behavioral parenting training, CAARS = Conners Adult ADHD Rating Scale-Self Report, CGI-S = Clinical Global Impression-Severity, DPICS = Dyadic Parent-Child Interaction Coding System, IRS = Child Impairment Rating Scale, MED = maternal stimulant medication.
Study Design
The pilot SMART included 2 8-week phases: in the first phase, 18 families were randomized to stimulant medication (MED) treatment and 17 families were randomized to BPT treatment; in the second phase, half of the families were rerandomized to continue their initial treatment, and the other half were randomized to switch treatment. The design was an unrestricted SMART such that every family received a second randomization at week 8 regardless of their treatment response. The SMART design resulted in 4 treatment sequences: MED-BPT (n = 9), BPT-MED (n = 9), MED-MED (n = 9), and BPT-BPT (n = 8). See Figure 1 for CONSORT diagram. Families completed assessments at baseline, week 8, and week 16 by trained clinicians masked to treatment assignment. There was no statistically significant difference between the 4 treatment sequences in terms of child and maternal demographic and baseline primary child outcomes. All procedures were approved by the institutional review board at Seattle Children’s Hospital. All participants provided informed consent prior to participation.
Figure 1. CONSORT Diagram.

Treatments
Medication (MED).
Mothers assigned to medication received an initial dose of 20 mg/d of lisdexamfetamine, titrated weekly until optimal dose was reached (ie, Clinical Global Impression [CGI]-improvement score ≥2 with minimal side effects; maximum dose 70 mg/d). In cases of poor tolerability or poor response, mothers switched to an alternative stimulant at week 8 (second randomization). The average optimal dose was 41.76 mg/d. Medication adherence was monitored using medication dosing logs and pill counts collected at each medication visit with the research team psychiatrist.
Behavioral parent training (BPT).
Mothers assigned to BPT received 8 individual sessions of Barkley’s Your Defiant Child.17 BPT was delivered in person by clinical psychology doctoral students and psychologists, under the supervision of a licensed psychologist. BPT focused on teaching mothers behavioral skills to increase desirable behaviors (eg, labeled praise, positive attention) and decrease undesirable behaviors (eg, active ignoring, timeout) in their children. Fidelity to the BPT manual was monitored via weekly supervision with a licensed psychologist.
Measures
Child ADHD and impairment.
Primary outcomes were child ADHD symptoms and child impairment. Mothers completed the Conners 3rd edition18 for children ages 7–8 years or the Conners Early Childhood Scale19 for children ages 4–6 years to assess child ADHD symptoms. The Conners scales have demonstrated extensive reliability, validity, and clinical utility.18,19 The Global Index total raw score was used in analyses. Cronbach αs were 0.92 at baseline, 0.93 at week 8, and 0.93 at week 16 for the Conners 3. Cronbach αs were 0.89 at baseline, 0.88 at week 8, and 0.93 at week 16 for the Conners Early Childhood Scale. Mothers also completed the 7-item Child Impairment Rating Scale (IRS)20 to assess child impairment on a scale from 0 (no problem) to 6 (extreme problem). The IRS has demonstrated good stability and validity and can differentiate between children with and without ADHD.20 The average of nonmissing items was calculated for each participant who responded to at least 6 of 7 items. Cronbach αs were 0.80 at baseline, 0.91 at week 8, and 0.96 at week 16. Additionally, masked raters completed Clinical Global Impression-Severity (CGI-S) ratings for each child to assess severity of ADHD impairment (1 = normal/not ill; 7 = extremely ill).
Maternal ADHD.
Maternal ADHD symptoms were assessed with the 93-item Conners Adult ADHD Rating Scale-Self Report21 on a 4-point Likert scale (0 = not at all/never, 3 = very much/frequently). The scale has demonstrated good reliability and sensitivity to treatment in previous studies.22 The ADHD Index raw score was used. Cronbach αs were 0.92 at baseline, 0.96 at week 8, and 0.96 at week 16.
Parenting outcomes.
Parenting was assessed via self-report and observations of parent-child interactions. Mothers completed the 42-item Alabama Parenting Questionnaire.23 The measure has 5 subscales: involvement, positive parenting, poor monitoring/supervision, inconsistent discipline, and corporal punishment. The involvement and positive parenting subscales were aggregated into a positive parenting composite. The poor monitoring/supervision, inconsistent discipline, and corporal punishment subscales were aggregated into a negative parenting composite. Cronbach αs ranged from 0.74 to 0.87 across time points. Mother-child dyads were also observed in 3 structured laboratory tasks (eg, 5-minutes cleanup, 5-minutes free play, and 10-minutes homework) at each time point. The Dyadic Parent-Child Interaction Coding System, Fourth Edition,24 was applied to code the frequency of parenting behaviors across tasks. Two composites were generated: positive parenting (eg, praise, reflections, behavioral descriptions) and negative parenting (eg, negative talk). Intraclass correlations were 0.99 for positive parenting and 0.82 for negative parenting.
Analytic Plan
All 35 randomized participants were included in analyses. Missingness varied by variable and study time point, with most variables (21 out of 24) with missingness between 0% and 22%. The exception was the Conners measure, which had missingness in the 30%–40% range across time points. Importantly, CGI was structurally complete. Missing data were imputed using fully conditional specification methods. A total of 100 iterations were implemented. Each iteration included a single imputation of baseline measures followed by a single imputation of week 8 measures and a single imputation of week 16 measures. In addition to the identified outcome measures, additional measures (Beck Depression Inventory, Barkley Functional Impairment rating Scale (BFIS) number of domains impacted, Adult Clinician Diagnostic Scale (ACDS) hyperactive and inattentive symptoms) were included in the imputation models to improve estimation. Measures were selected based on preliminary correlations with the outcomes of interest at each timepoint. Distributions of imputed variables were compared with their associated unimputed distributions prior to analysis.
Treatment effects were estimated for each outcome, by imputation, using generalized estimating equations with sandwich estimator and exchangeable covariance. Meancentered covariates included baseline parent and child age, parent education, Beck Depression Inventory, and ACDS hyperactive symptoms. Model estimates were aggregated over imputations for each outcome. Least square mean estimates of outcomes were plotted for each treatment. We focus on interpreting treatment effect sizes (ESs), which were calculated as the mean difference between 2 conditions divided by the pooled standard deviation of the mean estimate. All analyses were implemented in SAS version 9.4.
RESULTS
Child ADHD Symptoms and Impairment
See Figure 2 for results on child ADHD symptoms and impairment. Children demonstrated the most reduction in Conners scores from baseline to week 16 when their mothers received BPT-MED (ES = −0.36). The ES for BPT-BPT was −0.28. Medication for the mother had minimal effect on child ADHD symptoms (ES = −0.07 for MED-BPT; ES = −0.18 for MED-MED). Similarly, children demonstrated the most reduction in IRS scores from baseline to week 16 when their mothers received BPT-MED (ES = −0.33). In comparison, the ESs for the other treatment sequences were −0.17 for BPT-BPT, −0.13 for MED-BPT, and 0.10 for MED-MED. Children also demonstrated the most reduction in CGI-S scores when their mothers received BPT-MED (ES = −0.51). In comparison, the ESs for the other treatment sequences were −0.36 for BPT-BPT, −0.37 for MED-BPT, and −0.12 for MED-MED. Overall, the BPT-MED demonstrated the most favorable outcomes for child ADHD symptoms and impairment, and all sequences involving BPT demonstrated larger impact on child outcomes than medication alone.
Figure 2. Trajectories of Child ADHD Symptoms and Impairment Across Treatment Sequences.
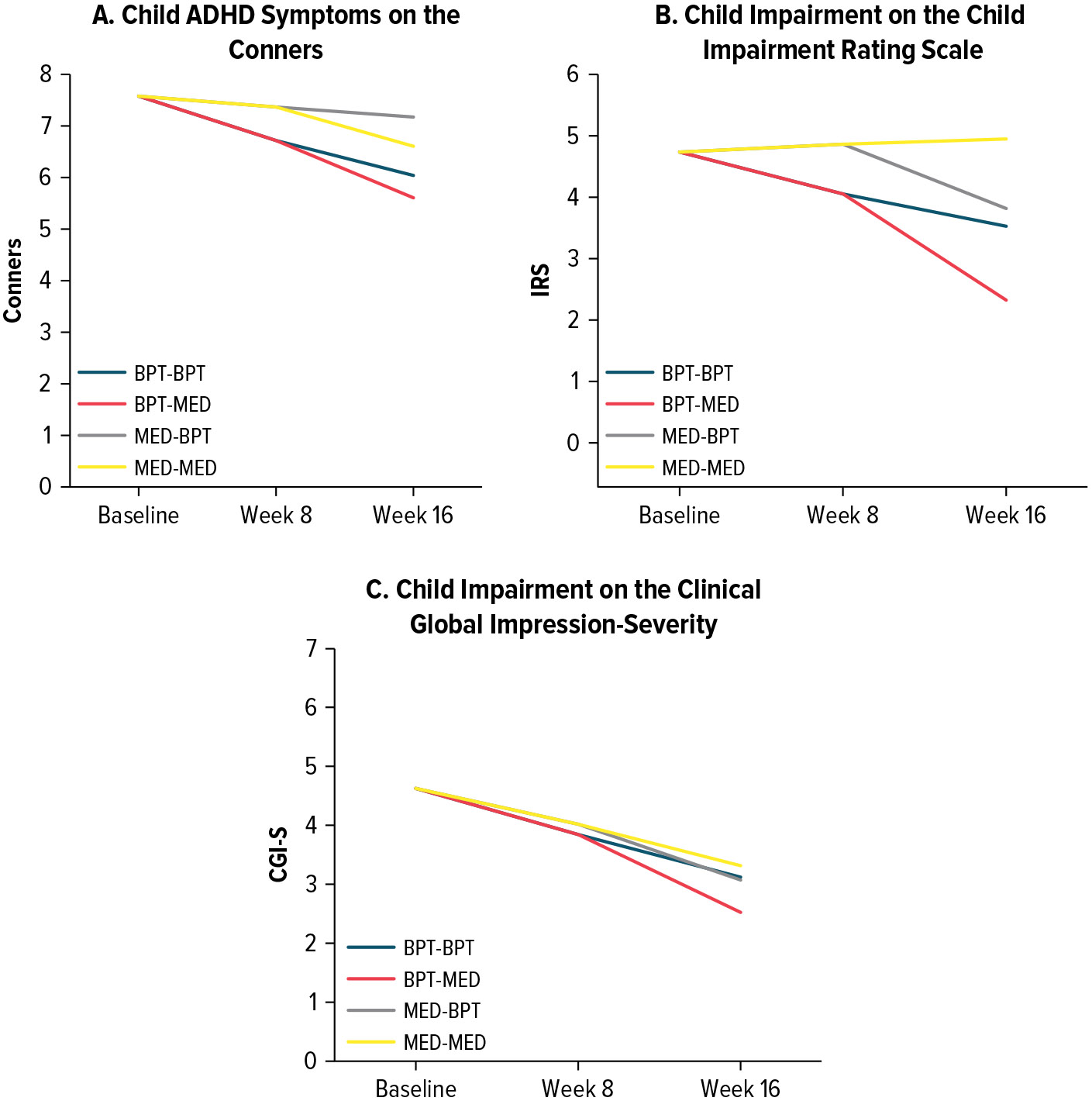
Abbreviations: ADHD = attention-deficit/hyperactivity disorder, BPT = behavioral parenting training, CGI-S = clinical global impressions scale-severity, Conners = Conners 3rd edition (ages 7–8 years) or Conners Early Childhood Scale (ages 4–6 years), MED = maternal stimulant medication, IRS = Child Impairment Rating Scale.
Maternal ADHD Symptoms
Mothers demonstrated the most reduction in their ADHD symptoms when they received medication (Figure 3). MED-MED (ES = −0.44) and MED-BPT (ES = −0.48) produced similar outcomes. For mothers in BPT-MED, ADHD symptoms only reduced from week 8 to 16 (ES = −0.32), during the active medication phase. BPT had minimal impact on maternal ADHD symptoms as indicated in the BPT-BPT sequence (ES = −0.08). Overall, the 3 sequences involving medication demonstrated similar impact on maternal ADHD symptoms compared to the BPT-BPT sequence.
Figure 3. Trajectories of Maternal ADHD Symptoms Across Treatment Sequences.
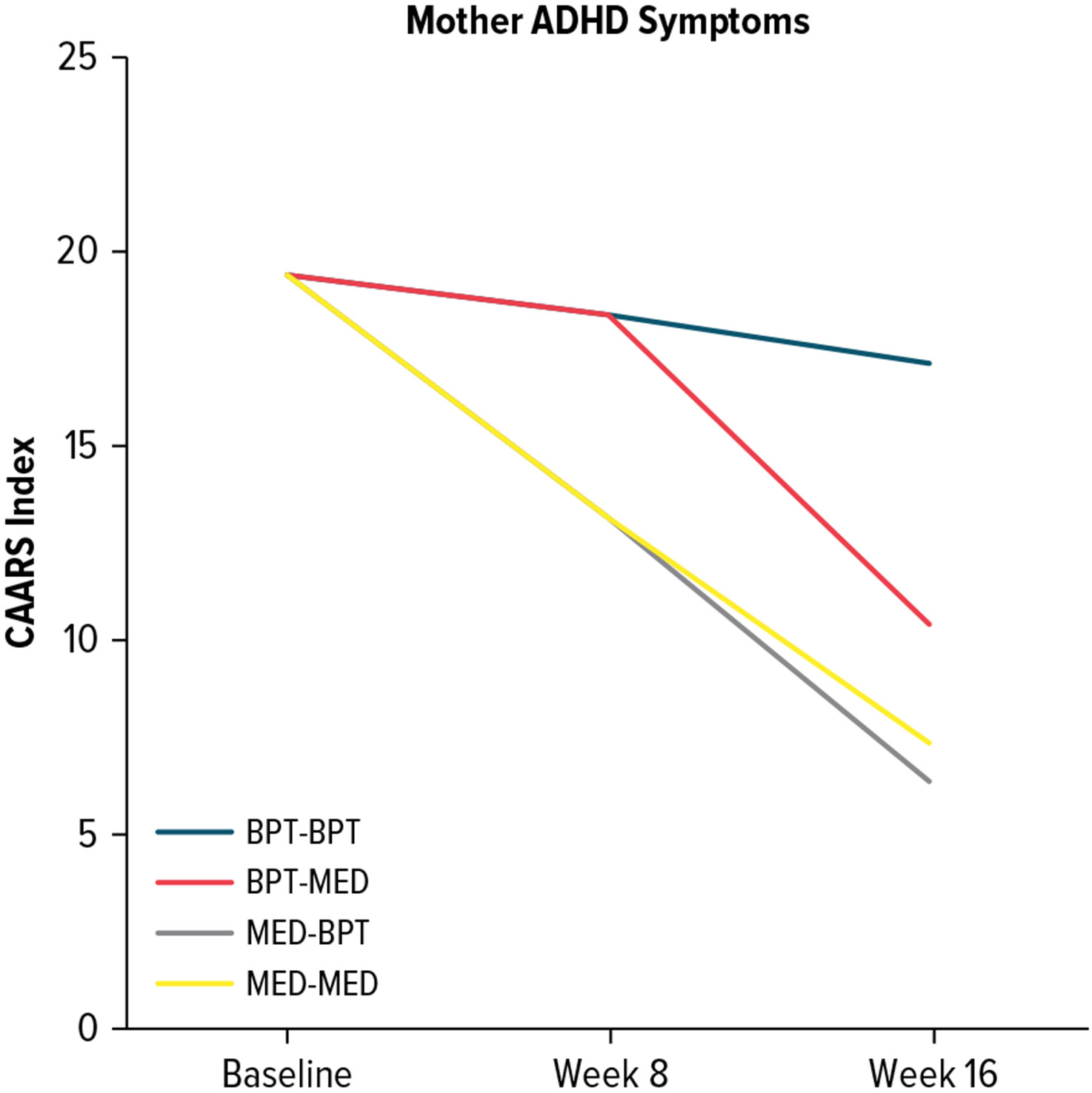
Abbreviations: ADHD = attention-deficit/hyperactivity disorder, BPT = behavioral parenting training, CAARS = Conners Adult ADHD Rating Scale, MED = maternal stimulant medication.
Parenting
See Figure 4 for results on parenting outcomes. For self-reported positive parenting, mothers in BPT-MED demonstrated the largest improvement (ES = 0.30). ESs for the other treatment sequences were 0.07 for BPT-BPT, −0.03 for MED-BPT, and 0.12 for MED-MED. For observed positive parenting, mothers in BPT-MED also demonstrated the largest improvement (ES = 0.35). ESs for the other treatment sequences were 0.32 for BPT-BPT, 0.16 for MED-BPT, and 0.01 for MED-MED. For self-reported negative parenting, BPT-MED (ES = −0.50) resulted in the largest improvement. ESs for the other treatment sequences were −0.30 for BPT-BPT, −0.33 for MED-BPT, and −0.31 for MED-MED. For observed negative parenting, BPT-BPT resulted in the largest improvement (ES = −0.08). ESs for the other treatment sequences were −0.01 for BPT-MED, 0.09 for MED-BPT, and 0.17 for MED-MED. Mothers who started medication first demonstrated increase in negative parenting behaviors based on observations, but the subsequent introduction of BPT reduced negative parenting behaviors. Overall, BPT-MED and BPT-BPT sequences had the most favorable effects on parenting outcomes.
Figure 4. Trajectories of Positive and Negative Parenting Across Treatment Sequences.

Abbreviations: ADHD = attention-deficit/hyperactivity disorder, APQ = Alabama Parenting Scale, BPT = behavioral parenting training, MED = maternal stimulant medication.
DISCUSSION
In this 16-week pilot SMART with multiplex ADHD families, 4 treatment sequences were compared: MED-MED, BPT-BPT, MED-BPT, and BPT-MED. Child ADHD symptoms, child impairment, maternal ADHD symptoms, and parenting were examined across 4 treatment sequences using a multimethod, multiinformant approach. Overall, BPT-MED favored child and parenting outcomes. For mothers who received 8 weeks of BPT then subsequently 8 weeks of medication for themselves, their children demonstrated the most improvements in ADHD symptoms and impairment, and they demonstrated the most improvements in positive and negative parenting, per self-report and observation. This finding is contrary to our hypothesis that MED-BPT would be associated with the most favorable outcomes. We reasoned that mothers treated with stimulants may be more attentive during BPT sessions, more able to consistently implement skills at home, and consistently inhibit reactive responses to child misbehaviors. Instead, our pilot findings suggest that starting BPT first before initiating medication for the parent may produce the strongest therapeutic effects, if the goal is to improve child and parenting outcomes. Although there were large observed effects for the BPT-MED sequence for child outcomes, it is important to note that many children remained impaired at the end of the 16-week treatment. Further fully powered SMARTs can investigate the appropriate dosage and intensity, in combination with the sequencing of treatment to optimize treatment effects for multiplex ADHD families.
In contrast, if the primary goal is to improve maternal ADHD symptoms, our findings suggest starting with parent medications may be appropriate. Both MED-MED and MED-BPT resulted in the large decreases in maternal ADHD symptoms, with minimal difference between the 2 sequences. Starting with BPT first then initiating medication also resulted in decreases in maternal ADHD symptoms, although the decrease was only evident during the active medication phase. Thus, medication had acute effects on improving maternal ADHD symptoms, but parent medication alone appears insufficient for improving child outcomes and parenting, consistent with previous literature.8,9
For parenting outcomes, mothers who received 8 weeks of BPT then subsequently 8 weeks of medication demonstrated the most improvements. Receiving BPT only for 16 weeks also resulted in improvements in parenting. Thus, aligned with extant literature, our findings suggest that BPT is critical to teach parents behavioral skills to support their young children with ADHD. Results are also consistent with a SMART in child ADHD treatment favoring BPT prior to child stimulant treatment.25 Although medicating parents alone did not have much impact on parenting behaviors, and in some cases, were associated with increased negative parenting, the addition of BPT as a second phase of treatment resulted in improvements in both positive and negative parenting. This finding is encouraging and suggests that a delayed initiation of BPT can still be beneficial for parents and their children.
It is somewhat surprising that MED-MED was associated with increased negative parenting, although this finding was only evident based on observations. Further studies are required to understand processes that may explain this provocative finding. It is possible that as parents’ own symptoms of attention and consistency improve, they are more aware of their children’s misbehaviors and/or are more consistently implementing discipline strategies, albeit negative parenting strategies in the absence of BPT treatment. Alternatively, irritability can be a side effect of stimulant and/or parents may experience “rebound irritability” when the effects of stimulants wear off,26 which likely coincide with increased time interacting with children after school and work. The experience of irritability may contribute to parents’ increased use of negative parenting strategies in response to child misbehaviors.
To implement a model for multiplex ADHD families that includes treatment for both parent and child, it becomes necessary to concurrently assess for parent and child ADHD. Research is needed to examine which setting may be most feasible and appropriate to implement this type of routine assessment. For example, pediatric primary care may be conducive to routinely screen for parent and child ADHD symptoms since parents often accompany their children during a pediatric visit and universal behavioral health screenings are increasingly becoming standard practice.27 Research is currently underway to examine screening for parent and child ADHD in pediatric primary care clinics to identify multiplex ADHD families for a randomized controlled trial examining the effects of 2 treatment strategies (NCT04240756). Family medicine may be another appropriate setting for screening and treating both parent and child ADHD where physicians routinely provide comprehensive care for the family unit.
LIMITATIONS
This was a pilot trial with a modest sample size and thus was not sufficiently powered to detect effects. An important next step is to conduct an adequately powered trial to fully investigate the appropriate sequence, dosage, and intensity of treatment for multiplex ADHD families. We also only included mothers in the sample. Future studies should explore whether fathers or other caregivers respond similarly to the treatment sequences. The trial did not include an option of combined BPT and child medication, which is often recommended. Children in the study only had to be stimulant naïve, and there was no exclusionary criteria regarding other treatment history. While it is possible that families may have had a previous course of behavioral intervention, children were nonetheless displaying sufficient impairment that prompted mothers to seek participation in a clinical trial and to meet entry into the trial. Indeed, baseline impairment is supported by a mean CGI-S score of 4.6 (at least moderately ill). Finally, this study examined immediate effects posttreatment. Future studies would benefit from examining the maintenance and generalization of therapeutic effects in the long term.
CONCLUSIONS
Unsurprisingly, treating mothers with medication reduced their ADHD symptoms but had little impact on children’s behavior, while BPT was associated with improvements in parenting and child behavior. Findings from the pilot SMART suggest that for multiplex ADHD families, sequencing treatments with BPT first followed by medication for the parent had the greatest overall impact. This represents a promising approach that awaits replication in a larger sample. The current pilot SMART represents an initial step in understanding how to best sequence treatments for families where both parent and children have ADHD.
Clinical Points.
Given high heritability, it is common for parent and children to both have ADHD. It remains unknown how to best sequence and personalize treatment for multiplex ADHD families.
We conducted a pilot Sequential Multiple Assignment Randomized Trial (SMART) to examine the effects of treatment sequence of parent stimulant treatment and behavioral parent training on child, maternal, and parenting outcomes among multiplex ADHD families.
The combination of maternal stimulant medication and behavioral parenting training is recommended for most multiplex ADHD families.
Sequencing treatments with behavioral parenting training first followed by stimulant medication for the mother may be the most promising approach to improve child ADHD symptoms, impairment, and parenting.
Funding/Support:
This study was supported by the National Institute of Mental Health (1R34MH099208; PIs: Drs Stein and Chronis-Tuscano), Seattle Children’s Research Institute (Dr Stein), and an investigator-initiated grant (Dr Stein) from Shire Development LLC, Lexington, Massachusetts, a member of the Takeda group of companies (Grant ID IIR-USA-001073).
Role of the Sponsor:
Study drug was provided by Shire Development LLC. The supporters had no role in the design, analysis, interpretation, or publication of this study.
Footnotes
Relevant Financial Relationships: Dr Chronis-Tuscano receives NIH funding and royalties from Oxford University Press. Dr Stein is an advisor/consultant to Maxis Health, Periapt Health, Medici, Supernus Pharmaceuticals, and Tris Pharma. Drs Stein, French, and Almirall receive research support from the National Institute of Mental Health. Dr Almirall additionally receives research support from the National Institute on Drug Abuse. Dr Lui and Ms Whitlock have no relevant financial relationships to disclose.
Previous Presentation: Presented at the 17th International Society for Child and Adolescent Psychopathology Scientific Meeting; July 2015; Portland, Oregon. The 63rd American Academy of Child and Adolescent Psychiatry Annual Meeting; October 2016; New York, New York. The Annual Association for Behavioral and Cognitive Therapies Convention; October 2016; New York, New York. The 4th International EUNYTHYDIS Conference on ADHD; October 2016; Berlin, Germany. The 18th International Society for Child and Adolescent Psychopathology Scientific Meeting; June 2017; Berlin, Germany. and The Annual Meeting of the American Professional Society on ADHD and Related Disorders; January 2021; held virtually.
Ethics Approval: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Seattle Children’s Hospital Institutional Review Board.
Participant Consent: Informed consent was obtained from all individual participants included in the study.
Contributor Information
Joyce H. L. Lui, Department of Psychology, Concordia University, Montreal, Quebec, Canada.
Andrea Chronis-Tuscano, Department of Psychology, University of Maryland, College Park, Maryland.
Daniel Almirall, Department of Statistics, Institute for Social Research, University of Michigan, Ann Arbor, Michigan.
Kathryn B. Whitlock, New Harmony Statistical Consulting LLC, Clinton, Washington.
William French, Department of Psychiatry and Behavioral Medicine, Seattle Children’s Hospital, Seattle, Washington.
Mark A. Stein, Department of Psychiatry and Behavioral Medicine, Seattle Children’s Hospital, Seattle, Washington.
References
- 1.Kleppesto TH, Eilertsen EM, van Bergen E, et al. Intergenerational transmission of ADHD behaviors: genetic and environmental pathways. Psychol Med. 2024;54(7):1309–1317. [DOI] [PubMed] [Google Scholar]
- 2.Faraone SV, Larsson H. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry. 2019;24(4):562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston C, Chronis-Tuscano A. Parental ADHD: relations to parenting, child behavior, and treatment outcomes. J Abnorm Child Psychol. 2017;45(3):411–413. [DOI] [PubMed] [Google Scholar]
- 4.Chronis-Tuscano A, Wang CH, Woods KE, et al. Parent ADHD and evidence-based treatment for their children: review and directions for future research. J Abnorm Child Psychol. 2017;45(3):501–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harold GT, Leve LD, Barrett D, et al. Biological and rearing mother influences on child ADHD symptoms: revisiting the developmental interface between nature and nurture. J Child Psychol Psychiatry. 2013;54(10):1038–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DuPaul GJ, Evans SW, Mautone JA, et al. Future directions for psychosocial interventions for children and adolescents with ADHD. J Clin Child Adolesc Psychol. 2020;49(1):134–145. [DOI] [PubMed] [Google Scholar]
- 7.Wolraich ML, Hagan JF, Allan C, et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2019;144(4):e20192528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chronis-Tuscano A, Seymour KE, Stein MA, et al. Efficacy of osmotic-release oral system (OROS) methylphenidate for mothers with attention-deficit/hyperactivity disorder (ADHD): preliminary report of effects on ADHD symptoms and parenting. J Clin Psychiatry. 2008;69(12):1938–1947. [DOI] [PubMed] [Google Scholar]
- 9.Chronis-Tuscano A, Rooney M, Seymour KE, et al. Effects of maternal stimulant medication on observed parenting in mother–child dyads with attention-deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. 2010;39(4):581–587. [DOI] [PubMed] [Google Scholar]
- 10.Waxmonsky JG, Waschbusch DA, Babinski DE, et al. Does pharmacological treatment of ADHD in adults enhance parenting performance? Results of a double-blind randomized trial. CNS Drugs. 2014;28(7):665–677. [DOI] [PubMed] [Google Scholar]
- 11.Jans T, Jacob C, Warnke A, et al. Does intensive multimodal treatment for maternal ADHD improve the efficacy of parent training for children with ADHD? A randomized controlled multicenter trial. J Child Psychol Psychiatry. 2015;56(12):1298–1313. [DOI] [PubMed] [Google Scholar]
- 12.Almirall D, Chronis-Tuscano A. Adaptive interventions in child and adolescent mental health. J Clin Child Adolesc Psychol. 2016;45(4):383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schoenfelder EN, Chronis-Tuscano A, Strickland J, et al. Piloting a sequential, Multiple assignment, randomized trial for mothers with attention-deficit/hyperactivity disorder and their at-risk young children. J Child Adolesc Psychopharmacol. 2019;29(4):256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chronis-Tuscano A, Wang CH, Strickland J, et al. Personalized treatment of mothers with ADHD and their young at-risk children: a SMART pilot. J Clin Child Adolesc Psychol. 2016;45(4):510–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. Biometrics Research, New York State: Psychiatric Institute; 2002. [Google Scholar]
- 16.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. [DOI] [PubMed] [Google Scholar]
- 17.Barkley R, Benton C. Your defiant child. In: Eight Steps to Better Behavior. 2nd ed. The Guilford Press; 2013. [Google Scholar]
- 18.Keith Conners C. Conners. 3rd ed. Multi-Health Systems; 2008. [Google Scholar]
- 19.Keith Conners C. Conners Early Childhood. Multi-Health Systems; 2009. [Google Scholar]
- 20.Fabiano GA, Pelham WE Jr, Waschbusch DA, et al. A practical measure of impairment: psychometric properties of the Impairment Rating Scale in samples of children with attention deficit hyperactivity disorder and two school-based samples. J Clin Child Adolesc Psychol. 2006;35(3):369–385. [DOI] [PubMed] [Google Scholar]
- 21.Conners CK, Erhardt D, Sparrow E. Conners’ Adult ADHD Rating Scale (CAARS). Multi-Health Systems; 1998. [Google Scholar]
- 22.Adler L, Cohen J. Diagnosis and evaluation of adults with attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2004;27(2):187–201. [DOI] [PubMed] [Google Scholar]
- 23.Frick PJ. Alabama Parenting Questionnaire. University of Alabama; 1991. [Google Scholar]
- 24.Eyberg S, Nelson M, Ginn N, et al. Dyadic Parent-Child Interaction Coding System: Comprehensive Manual for Research and Training. 4th ed. PCIT International; 2013. [Google Scholar]
- 25.Pelham WE, Fabiano GA, Waxmonsky JG, et al. Treatment sequencing for childhood ADHD: a multiple-randomization study of adaptive medication and behavioral interventions. J Clin Child Adolesc Psychol. 2016;45(4):396–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolar D, Keller A, Golfinopoulos M, et al. Treatment of adults with attention-deficit/hyperactivity disorder. Neuropsychiatr Dis Treat. 2008;4(2):389–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lui JHL, Danko CM, Triece T, et al. Screening for parent and child ADHD in urban pediatric primary care: pilot implementation and stakeholder perspectives. BMC Pediatr. 2023;23(1):354. [DOI] [PMC free article] [PubMed] [Google Scholar]


