Abstract
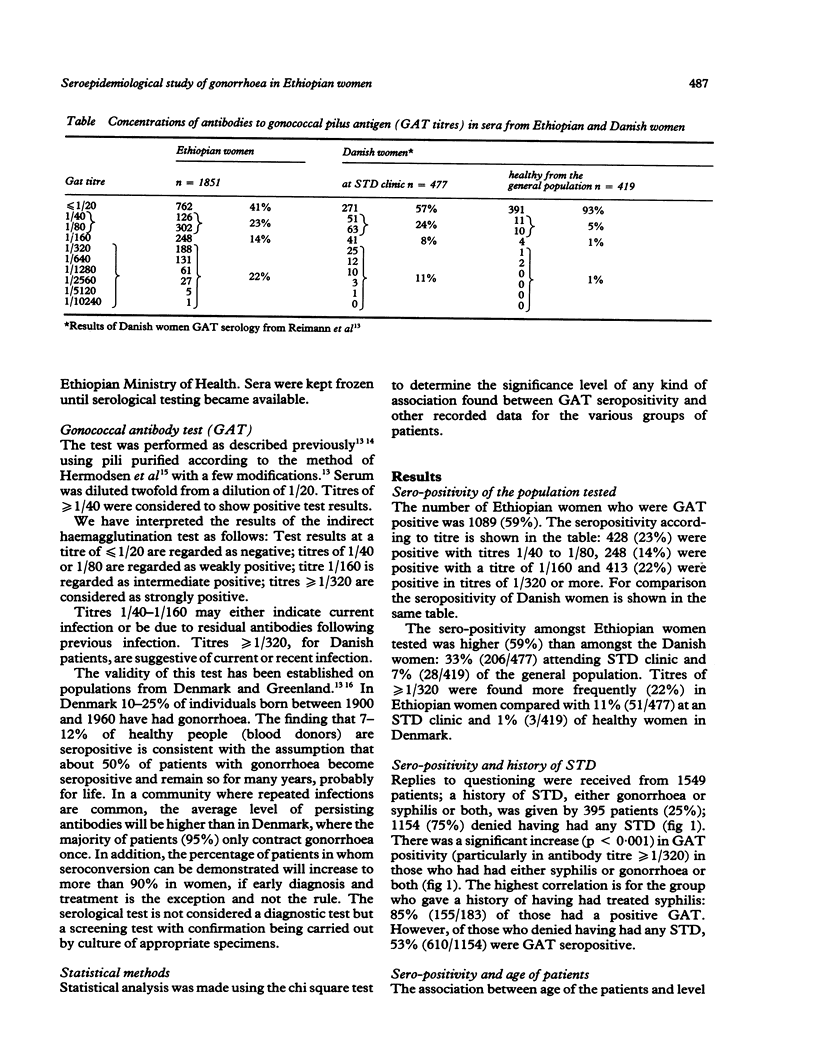
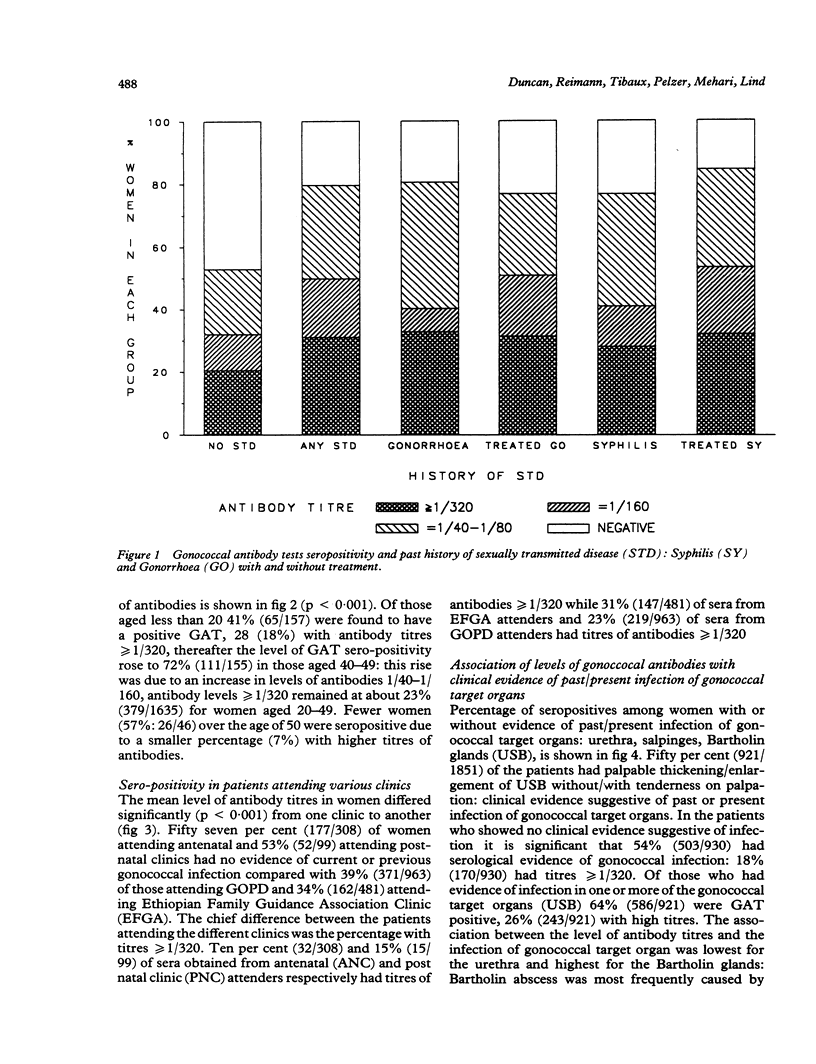
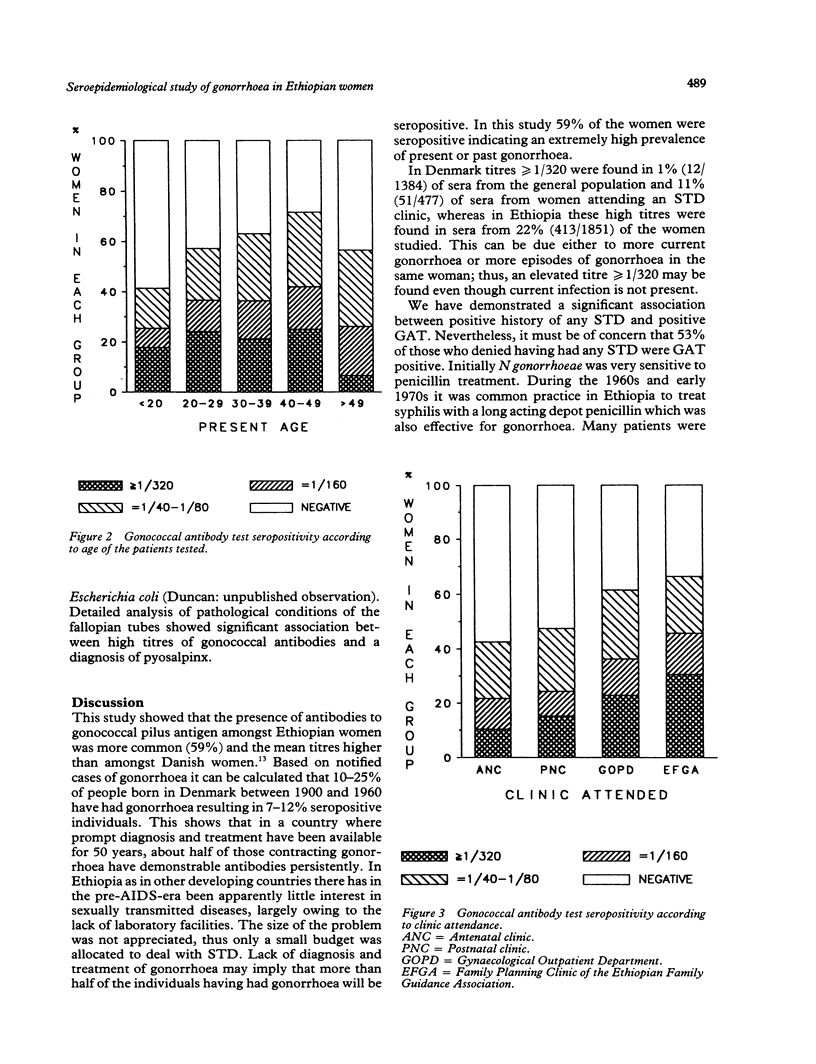
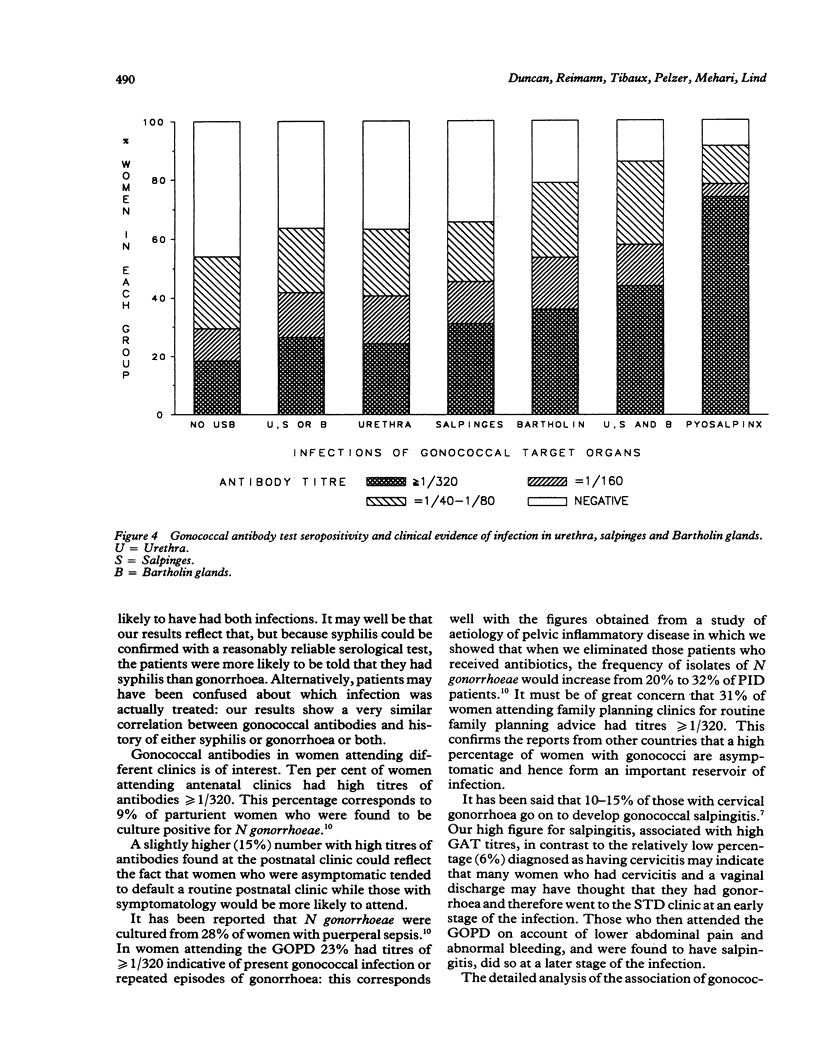
OBJECTIVE--To measure the prevalence of gonorrhoea in Ethiopian women attending gynaecologic, obstetric and family planning clinics: to determine the reliability of patient self history of sexually transmitted disease (STD); to correlate the serological diagnosis of gonorrhoea with clinical evidence of pelvic infection in order to define a reliable clinical diagnosis of gonorrhoea in a country where pelvic inflammatory disease is very common but where routine laboratory culture and serological tests for gonorrhoea are unavailable. SUBJECTS--1851 Ethiopian women: 50% symptomatic, 50% asymptomatic. SETTING--Gynaecological outpatient department, antenatal, postnatal and family planning clinics (Ethiopian Family Guidance Association (EFGA)), in two teaching hospitals and a mother and child health centre in Addis Ababa, Ethiopia. METHODS--The indirect haemagglutination test with gonococcal pilus antigen as an epidemiological tool was used in a cross-section study to screen 1851 sera for evidence of past or current gonococcal infection. The gonococcal antibody test (GAT) seropositivity was correlated with patient's history of STD, age, clinic attended and the clinical evidence of infection in "gonococcal target organs" urethra, salpinges or Bartholin glands. RESULTS--Fifty nine per cent of the study group were seropositive for the gonococcal antibody test, 22% with titres greater than or equal to 1/320, indicative of current, recent or recurrent infection. Seropositivity indicating past or present gonococcal infection was highest in those who gave a history of having had treated syphilis (85%), in women aged 40-49 (72%), and family planning attenders (EFGA) (66%) of whom 31% had titres greater than or equal to 1/320. Fifty per cent had clinical evidence of past or present infection in the urethra, salpinges or Bartholin glands. Gonococcal antibodies were present in 54% of women with no evidence of clinical infection, compared with 91% of those with pyosalpinx and 86% of those with triple infection of urethra, salpinges and Bartholin glands. CONCLUSION--The high prevalence of gonococcal antibodies in Ethiopian women, especially in asymptomatic clinic attenders must be of concern for all health workers especially those in gynaecology and obstetrics and the related disciplines of family planning and neonatal paediatrics. While seropositivity was highest in those giving a past history of syphilis, the patient's history of STD was unreliable, as of those who denied having any history of STD, fifty per cent were GAT seropositive. Despite a high correlation between GAT seropositivity with pyosalpinx and clinical evidence of infection in urethra, salpinges and bartholin glands, gonococcal antibodies were present in 54% of women with no clinical evidence of infection. Thus we were unable to define a diagnostic clinical picture of gonorrhoea in Ethiopian women.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler M. W. Trends for gonorrhea and pelvic inflammatory disease in England and Wales and for gonorrhea in a defined population. Am J Obstet Gynecol. 1980 Dec 1;138(7 Pt 2):901–904. doi: 10.1016/0002-9378(80)91079-0. [DOI] [PubMed] [Google Scholar]
- Clay J. C., Manuel A. R., Veeravahu M. Is gonorrhoea a good index of changed heterosexual behavior? Genitourin Med. 1988 Apr;64(2):135–135. doi: 10.1136/sti.64.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan M. E., Tibaux G., Pelzer A., Reimann K., Peutherer J. F., Simmonds P., Young H., Jamil Y., Daroughar S. First coitus before menarche and risk of sexually transmitted disease. Lancet. 1990 Feb 10;335(8685):338–340. doi: 10.1016/0140-6736(90)90617-e. [DOI] [PubMed] [Google Scholar]
- Hermodson M. A., Chen K. C., Buchanan T. M. Neisseria pili proteins: amino-terminal amino acid sequences and identification of an unusual amino acid. Biochemistry. 1978 Feb 7;17(3):442–445. doi: 10.1021/bi00596a010. [DOI] [PubMed] [Google Scholar]
- Mason P. R., Gwanzura L., Latif A. S., Marowa E. Genital infections in women attending a genito-urinary clinic in Harare, Zimbabwe. Genitourin Med. 1990 Jun;66(3):178–181. doi: 10.1136/sti.66.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårdh P. A., Lind I., From E., Andersen A. L. Prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae infections in Greenland. A seroepidemiological study. Br J Vener Dis. 1980 Oct;56(5):327–331. doi: 10.1136/sti.56.5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nsanze H. Problems and approaches in the surveillance and control of sexually transmitted agents associated with pelvic inflammatory disease in Africa. Am J Obstet Gynecol. 1980 Dec 1;138(7 Pt 2):1088–1090. doi: 10.1016/0002-9378(80)91113-8. [DOI] [PubMed] [Google Scholar]
- Osoba A. O. Epidemiology of urethritis in Ibadan. Br J Vener Dis. 1972 Apr;48(2):116–120. doi: 10.1136/sti.48.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osoba A. O. Microbiologic techniques for the diagnosis of pelvic inflammatory disease in developing countries. Am J Obstet Gynecol. 1980 Dec 1;138(7 Pt 2):1091–1095. doi: 10.1016/0002-9378(80)91114-x. [DOI] [PubMed] [Google Scholar]
- Pariser H. Asymptomatic gonorrhea. Med Clin North Am. 1972 Sep;56(5):1127–1132. doi: 10.1016/s0025-7125(16)32338-0. [DOI] [PubMed] [Google Scholar]
- Perine P. L., Duncan M. E., Krause D. W., Awoke S. Pelvic inflammatory disease and puerperal sepsis in Ethiopia. I. Etiology. Am J Obstet Gynecol. 1980 Dec 1;138(7 Pt 2):969–973. doi: 10.1016/0002-9378(80)91089-3. [DOI] [PubMed] [Google Scholar]
- Plorde J. J., Kidan T. G., Wright L. J. Penicillin sensitivity of gonococci in Ethiopia. Br J Vener Dis. 1973 Jun;49(3):260–262. doi: 10.1136/sti.49.3.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnam A. V., Din S. N., Chatterjee T. K. Gonococcal infection in women with pelvic inflammatory disease in Lusaka, Zambia. Am J Obstet Gynecol. 1980 Dec 1;138(7 Pt 2):965–968. doi: 10.1016/0002-9378(80)91088-1. [DOI] [PubMed] [Google Scholar]
- Reimann K., Odum L., Larsen S. O., Lind I. Indirect haemagglutination test using gonococcal pilus antigen: how useful to diagnose gonorrhoea? Genitourin Med. 1987 Aug;63(4):250–255. doi: 10.1136/sti.63.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz K. F., Cates W, O'mara P. R. A synopsis of the problems in Africa in syphilis and gonorrhoeae during pregnancy. Afr J Sex Transmi Dis. 1986 Oct;2(2):56-7, 60. [PubMed] [Google Scholar]
- Taha O. M., Ali M. H., Omer E. E., Ahmed M. A., Abbaro S. A. Study of STDs in patients attending venereal disease clinics in Khartoum, Sudan. Br J Vener Dis. 1979 Oct;55(5):313–315. doi: 10.1136/sti.55.5.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen A. R., Gemert W. Social and epidemiological determinants of gonorrhoea in an East African country. Br J Vener Dis. 1972 Aug;48(4):277–286. doi: 10.1136/sti.48.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]


