Abstract
OBJECTIVE--To measure the prevalence of chlamydial genital infection in Ethiopian women attending gynaecological, obstetric and family planning clinics; to identify the epidemiological, social and economic factors affecting the prevalence of infection in a country where routine laboratory culture and serological tests for chlamydial species are unavailable; to determine the risk factors for genital chlamydial infection in those with serological evidence of other sexually transmitted diseases. SUBJECTS--1846 Ethiopian women, outpatient attenders at two teaching hospitals and a mother and child health centre in Addis Ababa, Ethiopia. SETTING--Gynaecological outpatient department, antenatal, postnatal and family planning clinics. METHODS--Sera were tested for type-specific anti-chlamydial antibodies using purified chlamydial antigens (C. trachomatis A-C (CTA-C), C. trachomatis D-K (CTD-K), Lymphogranuloma venereum (LGV1-3), and C. pneumoniae (CPn)), in a micro-immunofluorescence test. The genital chlamydia seropositivity was analysed against patient's age, clinic attended, ethnic group, religion, origin of residence, age at first marriage and first coitus, income, number of sexual partners, duration of sexual activity, marital status/profession, obstetric and contraceptive history, and seropositivity for other sexually transmitted diseases. RESULTS--Overall exposure to chlamydia species was found in 84%, genital chlamydial infection in 62%, and titres suggestive of recent or present genital infection in 42% of those studied. Genital chlamydial infection was highest (64%) in family planning and lowest (54%) in antenatal clinic attenders. Exposure to genital chlamydia species was influenced by ethnic group and religion. Those married and sexually active under 13 years of age had greater exposure (69%) to genital chlamydial infection than those first sexually active aged over 18 (46%). Prevalence of infection was highest in those with more than five sexual partners (78%) and in bargirls (84%). The lowest income groups had a higher prevalence (65%) of genital chlamydial infection than the wealthiest (48%). Multivariate analysis showed the most important factors to be age at first coitus, religion, prostitution and present age of the woman in that order. Risk for genital chlamydial infection was increased in those with seropositivity for syphilis, gonorrhoea, HSV-2 but not HBV infection. CONCLUSION/APPLICATION--Chlamydial genital infections are highly prevalent in both symptomatic and asymptomatic Ethiopian women. The high prevalence of infection reported reflects a complexity of socioeconomic factors: very early age at first marriage and first coitus, instability of first marriage, subsequent divorce and remarriage or drift into prostitution, all of which are influenced by ethnic group, religion and poverty--together with transmission from an infected group of prostitutes by promiscuous males to their wives, lack of diagnostic facilities and inadequate treatment of both symptomatic and asymptomatic men and women. The problem of chlamydial disease in Ethiopia needs to be addressed urgently in the context of control of STD.
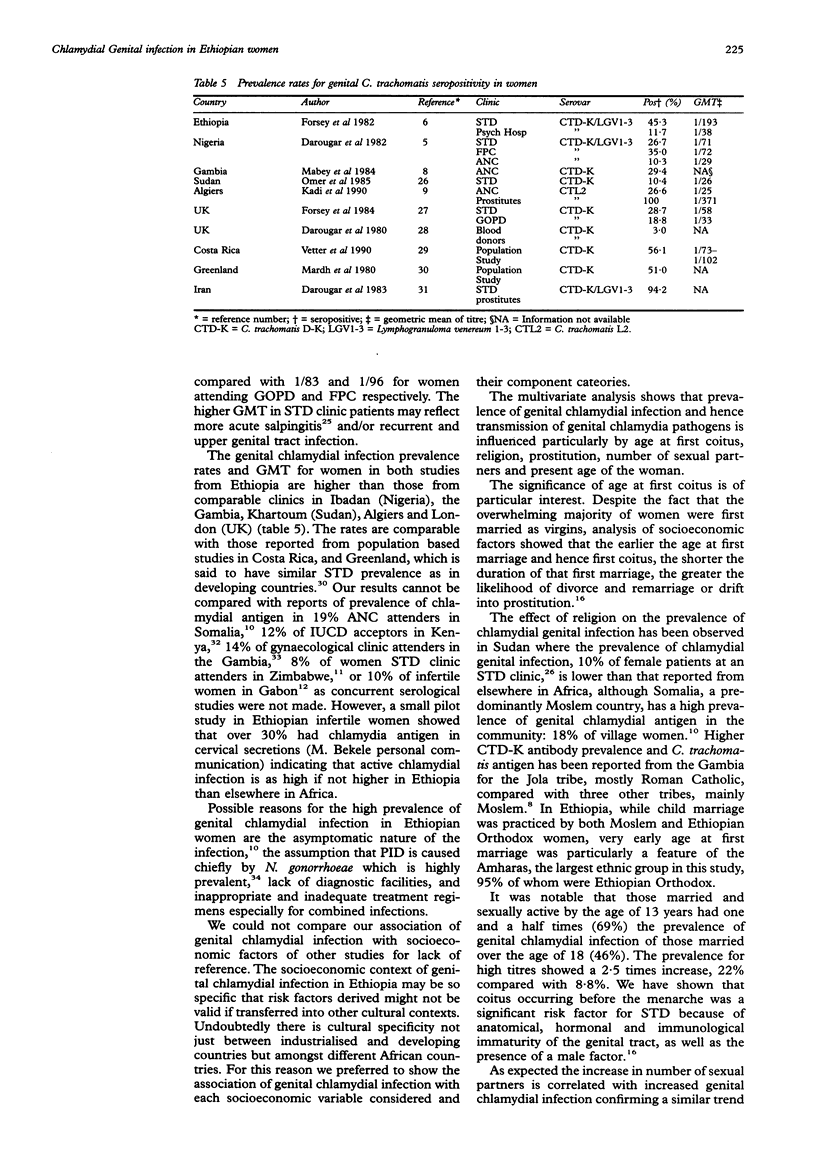
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Darougar S., Aramesh B., Gibson J. A., Treharne J. D., Jones B. R. Chlamydial genital infection in prostitutes in Iran. Br J Vener Dis. 1983 Feb;59(1):53–55. doi: 10.1136/sti.59.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darougar S., Forsey T., Brewerton D. A., Rogers K. L. Prevalence of antichlamydial antibody in London blood donors. Br J Vener Dis. 1980 Dec;56(6):404–407. doi: 10.1136/sti.56.6.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darougar S., Forsey T., Osoba A. O., Dines R. J., Adelusi B., Coker G. O. Chlamydial genital infection in Ibadan, Nigeria. A seroepidemiological survey. Br J Vener Dis. 1982 Dec;58(6):366–369. doi: 10.1136/sti.58.6.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan M. E., Perine P. L., Krause D. W., Awoke S., Zaidi A. A. Pelvic inflammatory disease and puerperal sepsis in Ethiopia. II. Treatment. Am J Obstet Gynecol. 1980 Dec 1;138(7 Pt 2):1059–1063. doi: 10.1016/0002-9378(80)91108-4. [DOI] [PubMed] [Google Scholar]
- Duncan M. E., Reimann K., Tibaux G., Pelzer A., Mehari L., Lind I. Seroepidemiological study of gonorrhoea in Ethiopian women. 1. Prevalence and clinical significance. Genitourin Med. 1991 Dec;67(6):485–492. doi: 10.1136/sti.67.6.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan M. E., Reimann K., Tibaux G., Pelzer A., Mehari L., Lind I. Seroepidemiological study of gonorrhoea in Ethiopian women. 2. Socioeconomic picture. Genitourin Med. 1991 Dec;67(6):493–497. doi: 10.1136/sti.67.6.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan M. E., Tibaux G., Pelzer A., Reimann K., Peutherer J. F., Simmonds P., Young H., Jamil Y., Daroughar S. First coitus before menarche and risk of sexually transmitted disease. Lancet. 1990 Feb 10;335(8685):338–340. doi: 10.1016/0140-6736(90)90617-e. [DOI] [PubMed] [Google Scholar]
- Forsey T., Darougar S., Dines R. J., Wright D. J., Friedmann P. S. Chlamydial genital infection in Addis Ababa, Ethiopia. A seroepidemiological survey. Br J Vener Dis. 1982 Dec;58(6):370–373. doi: 10.1136/sti.58.6.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsey T., Darougar S. Indirect micro-immunofluorescence test for detecting type-specific antibodies to herpes simplex virus. J Clin Pathol. 1980 Feb;33(2):171–176. doi: 10.1136/jcp.33.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R. S. Aspects of Bantu domestic life in relation to some gynaecological conditions. S Afr Med J. 1972 Sep 23;46(38):1383–1386. [PubMed] [Google Scholar]
- Hare M. J., Thin R. N. Chlamydial infection of the lower genital tract of women. Br Med Bull. 1983 Apr;39(2):138–144. doi: 10.1093/oxfordjournals.bmb.a071805. [DOI] [PubMed] [Google Scholar]
- Harnisch J. P., Berger R. E., Alexander E. R., Monda G., Holmes K. K. Aetiology of acute epididymitis. Lancet. 1977 Apr 16;1(8016):819–821. doi: 10.1016/s0140-6736(77)92773-8. [DOI] [PubMed] [Google Scholar]
- Ismail S. O., Ahmed H. J., Jama M. A., Omer K., Omer F. M., Brundin M., Olofsson M. B., Grillner L., Bygdeman S. Syphilis, gonorrhoea and genital chlamydial infection in a Somali village. Genitourin Med. 1990 Apr;66(2):70–75. doi: 10.1136/sti.66.2.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadi Z., Bouguermouh A., Djenaoui T., Allouache A., Dali S., Hadji N. Chlamydial genital infection in Algiers: a sero-epidemiological survey. Trans R Soc Trop Med Hyg. 1990 Nov-Dec;84(6):863–865. doi: 10.1016/0035-9203(90)90110-z. [DOI] [PubMed] [Google Scholar]
- Leclerc A., Frost E., Collet M., Goeman J., Bedjabaga L. Urogenital Chlamydia trachomatis in Gabon: an unrecognised epidemic. Genitourin Med. 1988 Oct;64(5):308–311. doi: 10.1136/sti.64.5.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabey D. C., Lloyd-Evans N. E., Conteh S., Forsey T. Sexually transmitted diseases among randomly selected attenders at an antenatal clinic in The Gambia. Br J Vener Dis. 1984 Oct;60(5):331–336. doi: 10.1136/sti.60.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabey D. C., Whittle H. C. Genital and neonatal chlamydial infection in a trachoma endemic area. Lancet. 1982 Aug 7;2(8293):300–301. doi: 10.1016/s0140-6736(82)90273-2. [DOI] [PubMed] [Google Scholar]
- Mason P. R., Gwanzura L., Latif A. S., Marowa E. Genital infections in women attending a genito-urinary clinic in Harare, Zimbabwe. Genitourin Med. 1990 Jun;66(3):178–181. doi: 10.1136/sti.66.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir D. G., Belsey M. A. Pelvic inflammatory disease and its consequences in the developing world. Am J Obstet Gynecol. 1980 Dec 1;138(7 Pt 2):913–928. doi: 10.1016/0002-9378(80)91082-0. [DOI] [PubMed] [Google Scholar]
- Mårdh P. A., Lind I., From E., Andersen A. L. Prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae infections in Greenland. A seroepidemiological study. Br J Vener Dis. 1980 Oct;56(5):327–331. doi: 10.1136/sti.56.5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårdh P. A., Ripa T., Svensson L., Weström L. Chilamydia trachomatis infection in patients with acute salpingitis. N Engl J Med. 1977 Jun 16;296(24):1377–1379. doi: 10.1056/NEJM197706162962403. [DOI] [PubMed] [Google Scholar]
- Omer E. E., Forsey T., Darougar S., Ali M. H., el-Naeem H. A. Seroepidemiological survey of chlamydial genital infections in Khartoum, Sudan. Genitourin Med. 1985 Aug;61(4):261–263. doi: 10.1136/sti.61.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peutherer J. F., MacKay P., Ross R., Stahl S., Murray K. Use of the hepatitis B core antigen produced in Escherichia coli in an assay for anti-HBc. Med Lab Sci. 1981 Oct;38(4):355–358. [PubMed] [Google Scholar]
- Plorde D. S. Sexually transmitted diseases in Ethiopia. Social factors contributing to their spread and implications for developing countries. Br J Vener Dis. 1981 Dec;57(6):357–362. doi: 10.1136/sti.57.6.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann K., Odum L., Larsen S. O., Lind I. Indirect haemagglutination test using gonococcal pilus antigen: how useful to diagnose gonorrhoea? Genitourin Med. 1987 Aug;63(4):250–255. doi: 10.1136/sti.63.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowen D., Carne C. A. Heterosexual transmission of human immunodeficiency virus. Int J STD AIDS. 1990 Jul;1(4):239–244. doi: 10.1177/095646249000100402. [DOI] [PubMed] [Google Scholar]
- Treharne J. D., Darougar S., Jones B. R. Modification of the microimmunofluorescence test to provide a routine serodiagnostic test for chlamydial infection. J Clin Pathol. 1977 Jun;30(6):510–517. doi: 10.1136/jcp.30.6.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treharne J. D., Ripa K. T., Mårdh P. A., Svensson L., Weström L., Darougar S. Antibodies to Chlamydia trachomatis in acute salpingitis. Br J Vener Dis. 1979 Feb;55(1):26–29. doi: 10.1136/sti.55.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treharne J. D., Ripa K. T., Mårdh P. A., Svensson L., Weström L., Darougar S. Antibodies to Chlamydia trachomatis in acute salpingitis. Br J Vener Dis. 1979 Feb;55(1):26–29. doi: 10.1136/sti.55.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen A. R., Gemert W. Social and epidemiological determinants of gonorrhoea in an East African country. Br J Vener Dis. 1972 Aug;48(4):277–286. doi: 10.1136/sti.48.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter K. M., Barnes R. C., Oberle M. W., Rosero-Bixby L., Schachter J. Seroepidemiology of chlamydia in Costa Rica. Genitourin Med. 1990 Jun;66(3):182–188. doi: 10.1136/sti.66.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H., Henrichsen C., Robertson D. H. Treponema pallidum haemagglutination test as a screening procedure for the diagnosis of syphilis. Br J Vener Dis. 1974 Oct;50(5):341–346. doi: 10.1136/sti.50.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]


