Abstract
Background
Waist circumference (WC) and body mass index (BMI) are considered useful for the diagnosis of metabolic dysfunction-associated steatotic liver disease (MASLD). This study aimed to investigate the association between the body roundness index (BRI) and a body shape index (ABSI) with incident MASLD, in comparison to WC and BMI.
Methods
Retrospective cohort data from a Japanese health check-up program were analyzed. Logistic regression model was used to evaluate the associations between anthropometric measure quartiles and MASLD, and receiver operating characteristic (ROC) analysis was performed to evaluate the associations between anthropometric measures and incident MASLD, stratified by sex.
Results
A total of 10,561 males and 7,187 females were included, of whom 3,182 males and 914 females developed MASLD. Multivariate analysis revealed that higher WC, BMI, and BRI were associated with incident MASLD in both sexes, whereas higher ABSI was significantly associated with incident MASLD only in females. Among males, the area under the ROC curve (AUC) of BMI was higher than that of WC, BRI, and ABSI. Conversely, among females, the AUC of BMI was higher than that of ABSI, whereas it was comparable to that of WC or BRI. The AUC and the optimal cut-off values of BMI for predicting incident MASLD were 0.77 and 23.9 kg/m2 in males, and 0.86 and 22.2 kg/m2 in females. The optimal cut-off values of WC were 82.0 cm for males and 76.3 cm for females, respectively.
Conclusions
We demonstrated the strongest association between BMI and incident MASLD compared to other measures, particularly in males, while also showing a strong association in females. Additionally, specific WC criteria for Asians to improve MASLD diagnosis are needed.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-025-03780-8.
Keywords: Anthropometric measure, MASLD, Japan, Cohort
Introduction
In recent years, concerns about the stigma associated with the terms “non-alcoholic fatty liver disease” (NAFLD) and “nonalcoholic steatohepatitis” (NASH) have led to the introduction of the term “metabolic dysfunction-associated steatotic liver disease” (MASLD) by the Delphi consensus statement [1]. The clinical profile of MASLD has been reported to closely resemble that of NAFLD [2, 3]. The diagnosis of MASLD requires evidence of hepatic steatosis accompanied by at least one cardiometabolic factor, such as overweight, impaired glucose metabolism, hypertension, hypertriglyceridemia, or reduced high-density lipoprotein (HDL) cholesterol levels, in the absence of significant alcohol intake [1]. The prevalence of MASLD has risen from 17.6 to 23.4% over the past two decades [4], and previous studies have revealed that MASLD is linked not only to cirrhosis and hepatocellular carcinoma, but also to an increased incidence of cardiovascular disease and extrahepatic malignancies [4, 5]. Therefore, early diagnosis and intervention to prevent the onset of MASLD-related complications are critically important.
Ultrasonography, computed tomography, and magnetic resonance imaging are essential modalities for screening hepatic lipidosis [6], one of the criteria for MASLD; however, it is difficult to perform in all individuals. Therefore, it is desirable to identify individuals at high risk of incident MASLD. Previous studies have highlighted the utility of body indices in screening for NAFLD [7], demonstrating their potential value in the diagnosis of MASLD as well.
Currently, waist circumference (WC) and body mass index (BMI) are recommended as the anthropometric measures for the diagnosis of MASLD [1]. However, recent studies have highlighted the utility of new anthropometric measures such as body roundness index (BRI) and a body shape index (ABSI) for clinical practice [8, 9]. These indicators have been reported to be potentially more useful than other anthropometric measures with respect to cardiovascular events and mortality [10, 11, 12, 13]. Additionally, higher BRI has been linked to an increased risk of NAFLD [14], and BRI demonstrates a higher predictive ability for NAFLD than BMI [15]. Kuang et al. [16] also reported that the combination of BMI and ABSI was associated with NAFLD prevalence. Thus, there is a possibility that BRI or ABSI is also useful as well as WC or BMI in predicting incident MASLD.
In light of these findings, our study investigated the association between WC, BMI, BRI, and ABSI with incident MASLD.
Methods
Study population and design
We have been conducting the NAGALA study (NAfld in Gifu Area, Longitudinal Analysis), a longitudinal cohort with recruitment spanning from 1994 to 2023. The study design employed an opt-out recruitment design. The ethics committee at Asahi university hospital approved the study protocol (ID:2018-09-01). The research samples were the medical examiners who underwent health check-ups at Asahi university hospital (Gifu, Japan). For this study, we included medical examiners from 2004 to 2018 who had no missing data in the sections listed below and who were still undergoing examinations five years later.
Data collection and measurements
The methods for data collection and measurements were described in detail previously [17]. Briefly, we used a standardized self-administered questionnaire to collect information on medical history and lifestyle factors, such as alcohol intake, smoking habits, and physical activity [17, 18]. We estimated the mean ethanol intake per week based on the amounts and types of alcoholic beverages consumed and categorized participants into three groups: none-to-light drinkers (< 210 g/week for men and < 140 g/week for women), moderate drinkers (210–420 g/week for men and 140–280 g/week for women), and heavy drinkers (≥ 420 g/week for men and ≥ 280 g/week for women) [17]. Smoking status was classified as never-smokers, past smokers, or current smokers. Regular exercisers were defined as participants who exercised regularly for ≥ 1 day/week [19].
Waist circumference was measured at the midpoint between the inferior margin of the last rib and the iliac crest in the horizontal plane while the participant was standing. Body mass index was calculated as weight (kg) divided by height squared. The ABSI and BRI were calculated using the following equation [9, 20]:
 |
 |
Definition of metabolic dysfunction-associated steatotic liver disease incidence
Hepatic steatosis was evaluated using abdominal ultrasound, which was performed by a trained technician and independently reviewed by two hepatologists, as detailed previously [21].
Metabolic dysfunction-associated steatotic liver disease was defined according to the multi-society Delphi consensus statement from the American Association for the Study of Liver Diseases (AASLD) [1]. According to this definition, MASLD requires hepatic steatosis along with at least one of five cardiometabolic risk factors: (1) BMI ≥ 23 kg/m² (specific to the Asian population) or WC > 94 cm for men and > 80 cm for women; (2) fasting serum glucose ≥ 100 mg/dL, 2-hour post-load glucose ≥ 140 mg/dL, HbA1c ≥ 5.7%, or treatment for type 2 diabetes; (3) blood pressure ≥ 130/85 mmHg or antihypertensive treatments; (4) plasma triglycerides ≥ 150 mg/dL or lipid-lowering treatments; and (5) plasma HDL cholesterol ≤ 40 mg/dL for men and ≤ 50 mg/dL for women, or lipid-lowering treatments. Individuals consuming < 210 g of alcohol per week for male and < 140 g per week for female were classified as having true MASLD within this classification.
Statistical analysis
Baseline differences in continuous variables among the participants were assessed using the t-test if normally distributed and the Wilcoxon rank-sum test if not. Differences in categorical variables were evaluated using the chi-square test. Continuous variables were summarized as mean ± standard deviation (SD) or median (25th, 75th quartile), while categorical variables were presented as a number (percentage).
Logistic regression was used to assess the longitudinal relationship between baseline quartile values of four anthropometric measures—WC, BMI, BRI, and ABSI—and incident MASLD, stratified by sex. To examine the effects of the quartiles of the four anthropometric measures on MASLD, we included the following factors as independent variables in multivariate logistic regression analyses. In Model 1, we adjusted for age. In Model 2, we additionally adjusted for confounding variables, including systolic and diastolic blood pressure, aspartate aminotransferase, alanine aminotransferase, gamma-glutamyltransferase, triglycerides, HDL-cholesterol, low-density lipoprotein (LDL) cholesterol, fasting plasma glucose, drinking habits, smoking status, and physical exercise. These were included along with the variables in Model 1. The associations were presented as odds ratios (ORs) with 95% confidence intervals (CIs).
The area under the curve (AUC) of BMI, WC, BRI and ABSI for predicting the incidence of MASLD was calculated using the receiver operating characteristic curve (ROC) analysis and optimal cut-off values for the incidence of MASLD were determined with separate sex-specific analyses conducted for male and female participants. Additionally, we compared the AUC of BMI, WC, BRI, and ABSI using the DeLong method [22] with the Bonferroni correction, separately for male and female participants. A value of p < 0.05 was considered statistically significant. However, after applying the Bonferroni correction, a value of p < 0.008 was considered statistically significant. Calculations were performed with JMP Pro, version 17.2.0 (SAS Institute, Cary, NC, USA) and R version 4.3.2 (R Foundation for Statistical Computing, Vienna, Austria); analyses were performed in RStudio.
Results
In total, 10,561 male participants and 7,187 female participants were included in the study. The baseline characteristics of the participants are shown in Table 1.
Table 1.
Participant characteristics
| Male | ALL | Incident MASLD (+) | Incident MASLD (-) | p value |
|---|---|---|---|---|
| n | 10,561 | 3,182 | 7,379 | |
| Age (years) | 44.0 (37.0–51.0) | 44.0 (38.0–51.0) | 44.0 (37.0–52.0) | 0.335 |
| Waist circumference (cm) | 81.0 (76.0-86.8) | 86.0 (81.0–91.0) | 79.0 (74.0–84.0) | < 0.001 |
| Body weight (kg) | 67.3 (61.3–74.5) | 73.5 (66.9–80.9) | 65.0 (59.6–71.3) | < 0.001 |
| Body mass index (kg/m2) | 23.1 (21.3–25.2) | 25.2 (23.3–27.3) | 22.3 (20.7–24.1) | < 0.001 |
| Body roundness index | 2.9 (2.4–3.5) | 3.4 (2.9-4.0) | 2.7 (2.2–3.2) | < 0.001 |
| A body shape index | 0.076 (0.074–0.078) | 0.076 (0.075–0.079) | 0.076 (0.074–0.078) | < 0.001 |
| Systolic blood pressure (mmHg) | 120.0 (111.0-130.0) | 124.5 (115.5-133.5) | 118.5 (109.5-128.5) | < 0.001 |
| Diastolic blood pressure (mmHg) | 75.5 (69.0–83.0) | 78.5 (72.0-85.5) | 74.5 (68.0–82.0) | < 0.001 |
| AST (IU/L) | 19.0 (15.0–23.0) | 21.0 (16.0–27.0) | 18.0 (15.0–22.0) | < 0.001 |
| ALT (IU/L) | 21.0 (16.0–30.0) | 28.0 (20.0–41.0) | 19.0 (15.0–25.0) | < 0.001 |
| GGT (IU/L) | 21.0 (15.0–33.0) | 24.5 (18.0–36.0) | 20.0 (14.0–30.0) | < 0.001 |
| Triglyceride (mg/dL) | 84.0 (57.0-127.0) | 109.0 (76.0-157.0) | 75.0 (52.0-112.0) | < 0.001 |
| HDL-cholesterol (mg/dL) | 49.3 (41.5–59.0) | 44.2 (38.6–52.0) | 52.0 (43.5–62.0) | < 0.001 |
| LDL-cholesterol (mg/dL) | 127.0 (107.2–148.0) | 134.3 (114.4-155.5) | 124.0 (104.2-144.7) | < 0.001 |
| Fasting plasma glucose (mg/dL) | 97.0 (92.0-104.0) | 100.0 (95.0-107.0) | 96.0 (91.0-102.0) | < 0.001 |
|
Drinking habits (none to light/moderate/heavy) (%) |
8,880/1,248/433 (84.1/11.8/4.1) |
3,012/136/34 (94.7/4.3/1.1) |
5,868/1,112/399 (79.5/15.1/5.4) |
< 0.001 |
|
Smoking status (never/past/current) (%) |
3,516/3,435/3,610 (33.3/32.5/34.2) |
1,149/1,027/1,006 (36.1/32.3/31.6) |
2,367/2,408/2,604 (32.1/32.6/35.3) |
< 0.001 |
| Physical exercise (-/+) (%) |
8,607/1,954 (81.5/18.5) |
2,700/482 (84.9/15.1) |
5,907/1,472 (80.1/20.0) |
< 0.001 |
| Female | ALL | Incident MASLD (+) | Incident MASLD (-) | p value |
| n | 7,187 | 914 | 6,273 | |
| Age (years) | 43.0 (38.0–50.0) | 48.0 (41.0–53.0) | 42.0 (37.0–49.0) | < 0.001 |
| Waist circumference (cm) | 71.5 (66.5–77.5) | 82.0 (77.0–88.0) | 70.1 (66.0-75.5) | < 0.001 |
| Body weight (kg) | 52.1 (47.5–57.5) | 61.4 (55.4–68.8) | 51.2 (47.0-55.9) | < 0.001 |
| Body mass index (kg/m2) | 20.7 (19.1–22.8) | 24.8 (22.6–27.5) | 20.3 (18.9–22.0) | < 0.001 |
| Body roundness index | 2.5 (2.0-3.2) | 3.8 (3.1–4.5) | 2.4 (1.9–2.9) | < 0.001 |
| A body shape index | 0.075 (0.072–0.078) | 0.077 (0.074–0.080) | 0.075 (0.072–0.078) | < 0.001 |
| Systolic blood pressure (mmHg) | 109.0 (100.5–120.0) | 119.5 (110.0-130.0) | 107.5 (99.5–118.0) | < 0.001 |
| Diastolic blood pressure (mmHg) | 67.5 (61.0-74.5) | 74.5 (67.0–82.0) | 66.5 (60.5–73.5) | < 0.001 |
| AST (IU/L) | 16.0 (13.0–19.0) | 17.0 (14.0–22.0) | 16.0 (13.0–19.0) | < 0.001 |
| ALT (IU/L) | 13.0 (11.0–17.0) | 18.0 (13.0–24.0) | 13.0 (10.0–16.0) | < 0.001 |
| GGT (IU/L) | 12.0 (10.0–15.0) | 15.0 (12.0–21.0) | 12.0 (10.0–15.0) | < 0.001 |
| Triglyceride (mg/dL) | 51.0 (36.0–73.0) | 81.0 (59.0-114.0) | 48.0 (35.0–67.0) | < 0.001 |
| HDL-cholesterol (mg/dL) | 64.0 (54.2–75.0) | 54.1 (46.3–65.0) | 65.0 (56.0–76.0) | < 0.001 |
| LDL-cholesterol (mg/dL) | 117.5 (98.4-139.3) | 132.3 (112.8-155.8) | 115.4 (96.8-136.8) | < 0.001 |
| Fasting plasma glucose (mg/dL) | 90.0 (86.0–96.0) | 96.0 (91.0-103.0) | 90.0 (85.0–94.0) | < 0.001 |
|
Drinking habits (none to light/moderate/heavy) (%) |
6,901/196/90 (96.0/2.7/1.3) |
899/12/3 (98.4/1.3/0.3) |
6,002/184/87 (95.7/2.9/1.4) |
< 0.001 |
|
Smoking status (never/past/current) (%) |
6,268/503/416 (87.2/7.0/5.8) |
796/60/58 (87.1/6.6/6.3) |
5,472/443/358 (87.2/7.1/5.7) |
0.655 |
| Physical exercise (-/+) (%) |
6104/1083 (84.9/15.1) |
782/132 (85.6/14.4) |
5,322/951 (84.8/15.2) |
0.571 |
Data were presented as n (%), median (25th, 75th)
Abbreviations: MASLD, Metabolic dysfunction-associated steatotic liver disease; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyltransferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein
WC, BMI, BRI and ABSI were higher at baseline in the individuals with incident MASLD than in those without MASLD. The ORs of univariate and multivariate logistic regression models for incident MASLD for male and female participants are presented in Tables 2 and 3, respectively.
Table 2.
Odds ratios for associations between anthropometric measures quartiles and the risk of MASLD incidence in male participants (95% CI)
| Q1 | Q2 | Q3 | Q4 | P-values for trend | |
|---|---|---|---|---|---|
| Waist circumference (cm) | -76.0 | 76.0–81.0 | 81.0-86.8 | 86.8- | |
| Crude | Ref. | 3.07 (2.62–3.60)** | 5.46 (4.70–6.38)** | 13.14 (11.32–15.29)** | < 0.001 |
| Model 1 | Ref | 3.21 (2.74–3.77)** | 5.93 (5.09–6.94)** | 14.35 (12.34–16.75)** | < 0.001 |
| Model 2 | Ref. | 2.35 (1.99–2.78)** | 3.25 (2.75–3.86)** | 6.28 (5.28–7.48)** | < 0.001 |
| Body mass index (kg/m2) | -21.3 | 21.3–23.1 | 23.1–25.2 | 25.2- | |
| Crude | Ref. | 2.82 (2.39–3.35)** | 5.74 (4.89–6.76)** | 16.73 (14.28–19.69)** | < 0.001 |
| Model 1 | Ref. | 2.87 (2.42–3.40)** | 5.90 (5.02–6.96)** | 17.09 (14.58–20.12)** | < 0.001 |
| Model 2 | Ref. | 2.16 (1.81–2.58)** | 3.56 (2.99–4.24)** | 7.54 (6.29 − 0.07)** | < 0.001 |
| Body Roundness Index | -2.37 | 2.37–2.91 | 2.91–3.49 | 3.49- | |
| Crude | Ref. | 3.42 (2.89–4.06)** | 6.96 (5.92–8.22)** | 14.54 (12.39–17.16)** | < 0.001 |
| Model 1 | Ref. | 3.74 (3.15–4.45)** | 8.23 (6.97–9.75)** | 18.05 (15.28–21.41)** | < 0.001 |
| Model 2 | Ref. | 2.63 (2.20–3.15)** | 4.39 (3.66–5.27)** | 7.36 (6.09–8.94)** | < 0.001 |
| A Body Shape Index | -0.074 | 0.074–0.076 | 0.076–0.078 | 0.078- | |
| Crude | Ref. | 1.44 (1.28–1.62)** | 1.45 (1.29–1.64)** | 1.41 (1.25–1.60)** | < 0.001 |
| Model 1 | Ref. | 1.46 (1.30–1.65)** | 1.51 (1.33–1.70)** | 1.51 (1.33–1.71)** | < 0.001 |
| Model 2 | Ref. | 1.24 (1.08–1.43)* | 1.15 (1.00-1.33)* | 1.10 (0.95–1.27) | 0.45 |
Model 1 was adjusted for age
Model 2 was adjusted for age, systolic blood pressure, diastolic blood pressure, aspartate aminotransferase, alanine aminotransferase, gamma-glutamyltransferase, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, fasting plasma glucose, drinking habits, smoking status, and the presence of physical exercise
**p < 0.001. *p < 0.05
Abbreviations: MASLD, Metabolic dysfunction-associated steatotic liver disease
Table 3.
Odds ratios for associations between anthropometric measures quartiles and the risk of MASLD incidence in female participants (95% CI)
| Q1 | Q2 | Q3 | Q4 | P-values for trend | |
|---|---|---|---|---|---|
| Waist circumference (cm) | -66.5 | 66.5–71.5 | 71.5–77.5 | 77.5- | |
| Crude | Ref. | 4.37 (2.59–7.84)** | 12.52 (7.72–21.82)** | 66.27 (41.57–114.10)** | < 0.001 |
| Model 1 | Ref | 4.28 (2.54–7.68)** | 11.51 (7.09–20.06)** | 58.68 (36.76-101.13)** | < 0.001 |
| Model 2 | Ref. | 3.62 (2.13–6.51)** | 7.49 (4.58–13.13)** | 25.38 (15.70-44.15)** | < 0.001 |
| Body mass index (kg/m2) | -19.1 | 19.1–20.7 | 20.7–22.8 | 22.8- | |
| Crude | Ref. | 3.01 (1.82–5.22)** | 9.85 (6.27–16.41)** | 55.37 (35.93–90.99)** | < 0.001 |
| Model 1 | Ref. | 2.84 (1.71–4.93)** | 8.77 (5.57–14.63)** | 48.79 (31.62–80.25)** | < 0.001 |
| Model 2 | Ref. | 2.35 (1.41–4.09)* | 5.89 (3.72–9.88)** | 20.53 (13.10-34.17)** | < 0.001 |
| Body Roundness Index | -1.97 | 1.97–2.49 | 2.49–3.15 | 3.15- | |
| Crude | Ref. | 3.76 (2.10–7.26)** | 15.38 (9.09–28.50)** | 82.05 (49.24-150.43)** | < 0.001 |
| Model 1 | Ref. | 3.68 (2.05–7.10)** | 14.44 (8.52–26.78)** | 73.76 (44.15-135.49)** | < 0.001 |
| Model 2 | Ref. | 3.19 (1.77–6.18)** | 9.58 (5.62–17.86)** | 32.38 (19.15-60.00)** | < 0.001 |
| A Body Shape Index | -0.072 | 0.072–0.075 | 0.075–0.078 | 0.078- | |
| Crude | Ref. | 1.31 (1.04–1.64)* | 1.89 (1.53–2.35)** | 2.54 (2.07–3.14)** | < 0.001 |
| Model 1 | Ref. | 1.31 (1.04–1.65)* | 1.81 (1.46–2.26)** | 2.10 (1.70–2.60)** | < 0.001 |
| Model 2 | Ref. | 1.29 (1.00-1.66) | 1.80 (1.41–2.30)** | 1.90 (1.49–2.42)** | < 0.001 |
Model 1 was adjusted for age
Model 2 was adjusted for age, systolic blood pressure, diastolic blood pressure, aspartate aminotransferase, alanine aminotransferase, gamma-glutamyltransferase, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, fasting plasma glucose, drinking habits, smoking status, and the presence of physical exercise
**p < 0.001. *p < 0.05
Abbreviations: MASLD, Metabolic dysfunction-associated steatotic liver disease
For male participants, Model 2 of multivariate logistic regression analysis showed that higher WC, BMI, and BRI were significantly associated with the risk of incident MASLD, (p for trend < 0.001). Similarly, for female participants, Model 2 of multivariate logistic regression analysis showed that higher WC, BMI, BRI, and ABSI were significantly associated with the risk of incident MASLD, (p for trend < 0.001).
We present the AUC and optimal cut-off values using ROC curve analysis, with separate analyses conducted for male and female participants, which are shown in Table 4.
Table 4.
Area under the curve and optimal cut-off values for incidence MASLD in male and female participants
| Male | AUC (95% CI) | Sensitivity | Specificity | Cut-off value |
|---|---|---|---|---|
| Waist circumference | 0.75 (0.74–0.76) | 72.0% | 64.4% | 82.0 |
| Body mass index | 0.77 (0.76–0.78) | 67.1% | 72.7% | 23.9 |
| Body roundness index | 0.75 (0.74–0.76) | 71.8% | 64.9% | 2.98 |
| A body shape index | 0.53 (0.52–0.55) | 77.5% | 29.5% | 0.074 |
| Female | AUC (95% CI) | Sensitivity | Specificity | Cut-off value |
| Waist circumference | 0.85 (0.84–0.86) | 76.4% | 78.4% | 76.3 |
| Body mass index | 0.86 (0.85–0.87) | 80.6% | 76.4% | 22.2 |
| Body roundness index | 0.86 (0.85–0.87) | 80.2% | 78.1% | 3.02 |
| A body shape index | 0.60 (0.59–0.62) | 63.3% | 53.2% | 0.075 |
Abbreviations: AUC, area under the curve, CI, confidence intervals
In male participants, the AUC and optimal cut-off values for predicting incident MASLD were 0.75 and 82.0 cm for WC, 0.77 and 23.9 kg/m2 for BMI, 0.75 and 2.98 for BRI and 0.53 and 0.074 for ABSI, respectively. In female participants, the corresponding values were 0.85 and 76.3 cm for WC, 0.86 and 22.2 kg/m2 for BMI, and 0.86 and 3.02 for BRI, and 0.60 and 0.075 for ABSI.
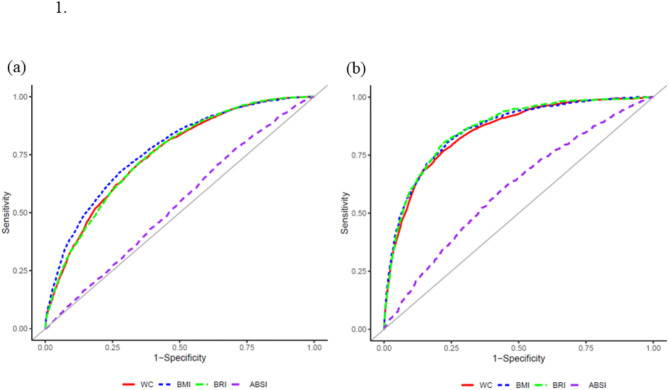
Figure 1 shows the ROC curve indicating the ability of WC, BMI, BRI, and ABSI to predict the development of MASLD for male and female participants. Table 5 shows the results of the comparison of the AUC for WC, BMI, BRI, and ABSI among male participants, with BMI showing the highest AUC, followed by WC, BRI, and ABSI. (Table 5. Comparison of area under the curve for body indices with incidence MASLD in male and female participants)
Fig. 1.
Receiver operating characteristic curve showing the ability of WC, BMI, BRI, ABSI to determine the incidence of MASLD with (a) male participants and (b) female participants
WC, Waist circumference; BMI, Body mass index; BRI, Body round index; ABSI, A body shape index
Table 5.
Comparison of area under the curve for body indices with incidence MASLD in male and female participants
| Male | vs. WC | vs. BMI | vs. BRI | |||
|---|---|---|---|---|---|---|
| Difference value (95% CI) | p value | Difference value (95% CI) | p value | Difference value (95% CI) | p value | |
| WC | reference | — | — | |||
| BMI | 0.020 (0.014 to 0.025) | < 0.001 | reference | — | ||
| BRI | 0.000 (-0.004 to 0.004) | 0.913 | -0.020 (-0.025 to -0.014) | < 0.001 | reference | |
| ABSI | -0.215 (-0.226 to -0.203) | < 0.001 | -0.235 (-0.250 to -0.219) | < 0.001 | -0.215 (-0.227 to -0.203) | < 0.001 |
| Female | vs. WC | vs. BMI | vs. BRI | |||
| Difference value (95% CI) | p value | Difference value (95% CI) | p value | Difference value (95% CI) | p value | |
| WC | reference | — | — | |||
| BMI | 0.009 (-0.000 to 0.018) | 0.054 | reference | — | ||
| BRI | 0.012 (0.007 to 0.017) | < 0.001 | 0.003 (-0.005 to 0.012) | 0.455 | reference | |
| ABSI | -0.246 (-0.264 to -0.228) | < 0.001 | -0.255 (-0.279 to -0.231) | < 0.001 | -0.258 (-0.276 to -0.240) | < 0.001 |
Abbreviations: WC, Waist circumference; BMI, Body mass index; BRI, Body roundness index; ABSI, A body shape index
In contrast, the AUC of BMI among female participants was higher than that of ABSI, whereas it was comparable to that of WC or BRI. The AUC of ABSI was lower than that of WC, BMI, and BRI among both male and female participants.
Discussion
Our major findings were as follows: (1) BMI, WC, and BRI were associated with the incidence of MASLD in male participants, whereas, all anthropometric indices were significantly associated with MASLD incidence in female participants; (2) BMI showed a stronger association with incident MASLD compared to WC, BRI, and ABSI in males and was also strongly associated in females; (3) The optimal cut-off values of BMI were 23.9 kg/m2 for males and 22.2 kg/m2 for females; (4) The optimal cut-off values of WC were 82.0 cm for males and 76.3 cm for females, both lower than conventional criteria.
Previous studies suggested that MASLD was associated with a higher risk of various complications, including metabolic and cardiovascular outcomes [4]. Moreover, fibrosis progression has been observed in 39% of MASLD cases over a 14-year period [23], and the presence of significant liver fibrosis in MASLD is associated with an elevated mortality risk [24]. These findings highlighted the critical importance of effective management and early diagnosis for MASLD.
Obesity is a significant global health concern [25], with its prevalence having tripled worldwide since 1980 [26]. Obesity, defined by the excessive accumulation of body fat, has been linked to metabolic dysfunction. Obesity-induced inflammation has been reported to lead to insulin resistance [27], and this condition was linked with cardiovascular disease [28]. Similarly, insulin resistance has been found to play a pivotal role in the development of MASLD [29]. Hepatic insulin resistance enhances anabolic reactions, leading to elevated levels of circulating free fatty acids, causing insulin resistance in adipose tissue, and increasing de novo lipogenesis. Notably, this accumulation of fatty acids in the liver constitutes a central pathological mechanism. Additionally, β-oxidation is primarily carried out in hepatocyte mitochondria, where fatty acids are converted to triglycerides and released into systemic circulation as components of very-low-density lipoproteins (VLDL). When the balance between these mechanisms is disturbed, triglycerides accumulate in hepatocytes as lipid droplets, ultimately leading to hepatic steatosis. Clinically, Jiang et al. [30] reported that anthropometric indices such as BMI and WC have a positive association with insulin resistance. Regarding MASLD, patients with severe obesity, as defined by BMI, are known to face a higher mortality risk than lean patients [31]. Therefore, the assessment of BMI is crucial in the management of MASLD. In fact, this study revealed that BMI demonstrated a stronger association with the incident MASLD compared to WC, BRI, and ABSI in male participants and there was also a strong association between BMI and incident MASLD in female participants.
The diagnosis of obesity is typically based on BMI, with a threshold of BMI ≥ 30 kg/m2 generally indicating obesity [32]. However, in East Asia, including Japan, a BMI threshold of ≥ 25 kg/m2 was proposed for diagnosing obesity [33], as obesity-related health complications were observed at lower BMI thresholds than in Western populations [34, 35].
With respect to the diagnosis of MASLD, BMI threshold of ≥ 23 kg/m2 was proposed for the Asian populations [1]. In our study, the optimal BMI cut-off for incident MASLD was identified as 23.9 kg/m2 in male participants and 22.2 kg/m2 in female participants. This was closely aligned with the BMI threshold proposed in previous consensus statements for the diagnosis of MASLD [1]. In the Asian population, individuals with MASLD at lower BMI could present with more severe manifestations than other ethnic groups [36]. Therefore, careful monitoring is warranted for those with MASLD and BMI ≥ 23 kg/m2 in the Asian population.
Recently, the clinical utilities of anthropometric measures such as BRI and ABSI have been highlighted.8920 BRI has shown a particularly strong association with the risk of NAFLD [14]. Conversely, ABSI has been reported to have a weaker correlation with NAFLD compared to BRI [37]. In the present study, BRI demonstrated a stronger association with incident MASLD compared to ABSI. These findings are consistent with the previous study by Motamed et al. [37].
The previous study indicated that increased WC was associated with the onset of NAFLD in the Asian population [38]. Although the diagnostic criteria for MASLD suggest a WC threshold of > 94 cm for male participants and > 80 cm for female participants [1], the present study identified lower cut-off values of 82.0 cm in male participants and 76.3 cm in female participants for predicting incident MASLD, compared to the conventional criteria. Previously, Suzuki et al. [39] reported that the diagnostic criteria based on thresholds for metabolic syndrome in Japanese populations (specifically, WC ≥ 85 cm for males and ≥ 90 cm for females) might be useful for diagnosing MASLD, in contrast to conventional diagnostic standards. In this study, the cut-off values were derived from a longitudinal study, which complicates direct comparison with the diagnostic criteria for MASLD. However, the cut-off values of BMI in our study were close to the proposed diagnostic criteria for MASLD in the Asian populations. Thus, for WC as well, it may be necessary to consider lower cut-off values compared to the diagnostic criteria specific to the Asian populations.
The limitations of our study are as follows. Initially, this study was conducted at a single center with participants solely from the Japanese population. Consequently, the potential for selection bias cannot be excluded, limiting the generalizability of the findings to other ethnic groups. Second, hepatic steatosis was not assessed using scoring methods. However, since we evaluated fatty liver based on standardized diagnostic criteria, we were able to identify a certain degree of hepatic steatosis. Thirdly, smoking status and alcohol consumption were self-reported, which might not be entirely accurate. Nevertheless, previous studies have shown that self-reported data can be reliable for assessing smoking status and alcohol consumption behaviors [40, 41], and the lifestyle data collected in the present study is considered valid. Despite these limitations, to the best of our knowledge, our study was the first to compare WC, BMI, BRI and BMI in relation to incident MASLD. This represented a significant strength of our study.
In conclusion, BMI, WC, and BRI were associated with the incidence of MASLD in male participants, with BMI demonstrating a stronger association compared with WC, BRI, and ABSI. In females, all anthropometric indices were significantly associated with MASLD incidence, with BMI also showing a strong association with incident MASLD. Additionally, the lower cut-off values for WC compared to the conventional criteria for incident MASLD in this study suggest that there may be a need to develop specific criteria for WC, in addition to BMI, which already has established criteria for diagnosing MASLD in the Asian populations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Abbreviations
- MASLD
Metabolic dysfunction-associated steatotic liver disease
- WC
Waist circumference
- BMI
Body mass index
- BRI
Body roundness index
- ABSI
A body shape index
- HDL
High-density lipoprotein
- LDL
Low-density lipoprotein
- SD
Standard deviation
- ORs
Odds ratios
- CIs
Confidence intervals
- AUC
Area under the curve
- ROC
Receiver operating characteristic curve
Author contributions
T.I. conceptualized the present study and conducted the data analysis and wrote the original manuscript. Y.H. conceptualized the present study and contributed the discussion. H.O. and T.O. contributed the discussion. A.O. and T.K. contributed to originate the study. M.H. and M.F. collected the data, contributed the discussion and supervised this study. All authors reviewed and approved the revised manuscript.
Funding
This work was supported by MHLW Comprehensive Research Project for Measures against Cardiovascular Diseases, Diabetes and Other Lifestyle Related Diseases Program Grant Number JPMH 24FA1008.
Data availability
The datasets utilized in this study are accessible from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The protocol for the present research project was approved by Asahi University Hospital Ethics Committee, (approval number:2018-09-01) in accordance with the provisions of the Declaration of Helsinki. Informed consent was obtained from all participants and/or their guardians.
Consent for publication
Not applicable.
Competing interests
Ichikawa T grants from Abbott Japan Co. Ltd. Hashimoto Y received personal fees from Novo Nordisk Pharma Ltd., Sanofi K.K., Sumitomo Dainippon Pharma Co., Ltd., Nippon Boehringer Ingelheim Co., Mitsubishi Tanabe Pharma Corp., Kowa Company, Ltd., Taisho Pharma Co., Eli Lilly Japan K.K. and Daiichi Sankyo Co. Hamaguchi M received grants from AstraZeneca K.K., Ono Pharma Co. Ltd., Kowa Pharma Co. Ltd. Fukui M received grants from Ono Pharma Co. Ltd., Oishi Kenko inc., Yamada Bee Farm, Nippon Boehringer Ingelheim Co. Ltd., Kissei Pharma Co. Ltd., Mitsubishi Tanabe Pharma Corp., Daiichi Sankyo Co. Ltd., Sanofi K.K., Takeda Pharma Co. Ltd., Astellas Pharma Inc., MSD K.K., Kyowa Kirin Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Kowa Pharma Co. Ltd., Novo Nordisk Pharma Ltd., Sanwa Kagagu Kenkyusho CO., Ltd., Eli Lilly, Japan, K.K., Taisho Pharma Co., Ltd., Terumo Corp., Tejin Pharma Ltd., Nippon Chemiphar Co., Ltd., Abbott Japan Co. Ltd., and Johnson & Johnson K.K. Medical Co., TERUMO CORPORATION. All authors’ grants were outside the submitted work.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rinella ME, Lazarus JV, Ratziu V, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79(6):1542–56. 10.1016/j.jhep.2023.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Paik JM, Stepanova M, Ong J, Alqahtani S, Henry L. Clinical profiles and mortality rates are similar for metabolic dysfunction-associated steatotic liver disease and non-alcoholic fatty liver disease. J Hepatol. 2024;80(5):694–701. 10.1016/j.jhep.2024.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Hagström H, Vessby J, Ekstedt M, Shang Y. 99% of patients with NAFLD Meet MASLD criteria and natural history is therefore identical. J Hepatol. 2024;80(2):e76–7. 10.1016/j.jhep.2023.08.026. [DOI] [PubMed] [Google Scholar]
- 4.Miao L, Targher G, Byrne CD, Cao YY, Zheng MH. Current status and future trends of the global burden of MASLD. Trends Endocrinol Metabolism. 2024;35(8):697–707. 10.1016/j.tem.2024.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Zhou BG, Jiang X, She Q, Ding YB. Association of MASLD with the risk of extrahepatic cancers: A systematic review and meta-analysis of 18 cohort studies. Eur J Clin Invest Published Online. 2024. 10.1111/eci.14276. [DOI] [PubMed] [Google Scholar]
- 6.Sidhu PS, Fang C. Us-based hepatic fat quantification: an emerging technique and game changer? Radiology. 2023;307(1). 10.1148/radiol.223002. [DOI] [PubMed]
- 7.Singh A, Parida S, Narayan J, et al. Simple anthropometric indices are useful for predicting Non-alcoholic fatty liver disease [NAFLD] in Asian Indians. J Clin Exp Hepatol. 2017;7(4):310–5. 10.1016/j.jceh.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Ma N, Lin Q, et al. Body roundness index and All-Cause mortality among US adults. JAMA Netw Open. 2024;7(6):E2415051. 10.1001/jamanetworkopen.2024.15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS ONE. 2012;7(7). 10.1371/journal.pone.0039504. [DOI] [PMC free article] [PubMed]
- 10.Kajikawa M, Maruhashi T, Kishimoto S, et al. A body shape index as a simple anthropometric marker of abdominal obesity and risk of cardiovascular events. J Clin Endocrinol Metab. 2024;109(12):3272–81. 10.1210/clinem/dgae282. [DOI] [PubMed] [Google Scholar]
- 11.Yang N, Zhuo J, Xie S, et al. A body shape index and its changes in relation to All-Cause mortality among the Chinese elderly: A retrospective cohort study. Nutrients. 2023;15(13):2943. 10.3390/nu15132943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji M, Zhang S, An R. Effectiveness of A body shape index (ABSI) in predicting chronic diseases and mortality: a systematic review and meta-analysis. Obes Rev. 2018;19(5):737–59. 10.1111/obr.12666. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, He Y, Yang L, et al. Body roundness index and Waist–Hip ratio result in better cardiovascular disease risk stratification: results from a large Chinese Cross-Sectional study. Front Nutr. 2022;9. 10.3389/fnut.2022.801582. [DOI] [PMC free article] [PubMed]
- 14.Zhao E, Wen X, Qiu W, Zhang C. Association between body roundness index and risk of ultrasound-defined non-alcoholic fatty liver disease. Heliyon. 2024;10(1):e23429. 10.1016/j.heliyon.2023.e23429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian X, Ding N, Su Y, Qin J. Comparison of Obesity-Related indicators for nonalcoholic fatty liver disease diagnosed by transient elastography. Turkish J Gastroenterol. 2023;34(10):1078–87. 10.5152/tjg.2023.23101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuang M, Sheng G, Hu C, Lu S, Peng N, Zou Y. The value of combining the simple anthropometric obesity parameters, body mass index (BMI) and a body shape index (ABSI), to assess the risk of non-alcoholic fatty liver disease. Lipids Health Dis. 2022;21(1):104. 10.1186/s12944-022-01717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto Y, Hamaguchi M, Kojima T, et al. The modest alcohol consumption reduces the incidence of fatty liver in men: A population-based large-scale cohort study. J Gastroenterol Hepatol (Australia). 2015;30(3):546–52. 10.1111/jgh.12786. [DOI] [PubMed] [Google Scholar]
- 18.Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143(10):722. 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 19.Ryu S, Chang Y, Kim DI, Kim WS, Suh BS. γ-Glutamyltransferase as a predictor of chronic kidney disease in nonhypertensive and nondiabetic Korean men. Clin Chem. 2007;53(1):71–7. 10.1373/clinchem.2006.078980. [DOI] [PubMed] [Google Scholar]
- 20.Thomas DM, Bredlau C, Bosy-Westphal A, et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity. 2013;21(11):2264–71. 10.1002/oby.20408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamaguchi M, Kojima T, Itoh Y, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. 2007;102(12):2708–15. 10.1111/j.1572-0241.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 22.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 1988;44(3):837. 10.2307/2531595. [PubMed] [Google Scholar]
- 23.Lekakis V, Papatheodoridis GV. Natural history of metabolic dysfunction-associated steatotic liver disease. Eur J Intern Med. 2024;122:3–10. 10.1016/j.ejim.2023.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Han E, Lee BW, Kang ES, et al. Mortality in metabolic dysfunction-associated steatotic liver disease: A nationwide population-based cohort study. Metabolism. 2024;152:155789. 10.1016/j.metabol.2024.155789. [DOI] [PubMed] [Google Scholar]
- 25.Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378(9793):804–14. 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 26.Boutari C, Mantzoros CS. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to Rage on. Metabolism. 2022;133:155217. 10.1016/j.metabol.2022.155217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127(1):1–4. 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–80. [DOI] [PubMed] [Google Scholar]
- 29.Maldonado-Rojas A, del Zuarth-Vázquez C, Uribe JM, Barbero-Becerra M. Insulin resistance and metabolic dysfunction-associated steatotic liver disease (MASLD): pathways of action of hypoglycemic agents. Ann Hepatol. 2024;29(2). 10.1016/j.aohep.2023.101182. [DOI] [PubMed]
- 30.Jiang J, Cai X, Pan Y, et al. Relationship of obesity to adipose tissue insulin resistance. BMJ Open Diabetes Res Care. 2020;8(1):e000741. 10.1136/bmjdrc-2019-000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Behari J, Wang R, Luu HN, McKenzie D, Molinari M, Yuan JM. Severe obesity is associated with worse outcomes than lean metabolic dysfunction–associated steatotic liver disease. Hepatol Commun. 2024;8(7). 10.1097/HC9.0000000000000471. [DOI] [PMC free article] [PubMed]
- 32.Obesity and overweight. Accessed August 24. 2024. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 33.Ogawa W, Hirota Y, Miyazaki S, et al. Definition, criteria, and core concepts of guidelines for the management of obesity disease in Japan. Endocr J. 2024;71(3):EJ23–0593. 10.1507/endocrj.EJ23-0593. [DOI] [PubMed] [Google Scholar]
- 34.Shai I, Jiang R, Manson JE, et al. Ethnicity, obesity, and risk of type 2 diabetes in women. Diabetes Care. 2006;29(7):1585–90. 10.2337/dc06-0057. [DOI] [PubMed] [Google Scholar]
- 35.Huxley R, James WPT, Barzi F, et al. Ethnic comparisons of the cross-sectional relationships between measures of body size with diabetes and hypertension. Obes Rev. 2008;9(s1):53–61. 10.1111/j.1467-789X.2007.00439.x. [DOI] [PubMed] [Google Scholar]
- 36.Truong E, Yeo YH, Cook-Wiens G, et al. Nonalcoholic fatty liver disease prevalence and severity in Asian Americans from the National health and nutrition examination surveys 2017–2018. Hepatol Commun. 2022;6(9):2253–61. 10.1002/hep4.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Motamed N, Rabiee B, Hemasi GR, et al. Body roundness index and Waist-to-Height ratio are strongly associated with Non-Alcoholic fatty liver disease: A Population-Based study. Hepat Mon. 2016;16(9). 10.5812/hepatmon.39575. [DOI] [PMC free article] [PubMed]
- 38.Lee JH, Jeon S, Lee HS, Kwon YJ. Association between waist circumference trajectories and incident non-alcoholic fatty liver disease. Obes Res Clin Pract. 2023;17(5):398–404. 10.1016/j.orcp.2023.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki K, Tamaki N, Kurosaki M, et al. Concordance between metabolic dysfunction-associated steatotic liver disease and nonalcoholic fatty liver disease. Hepatol Res. 2024;54(6):600–5. 10.1111/hepr.14011. [DOI] [PubMed] [Google Scholar]
- 40.Wong SL, Shields M, Leatherdale S, Malaison E, Hammond D. Assessment of validity of self-reported smoking status. Health Rep. 2012;23(1):47–53. [PubMed] [Google Scholar]
- 41.Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: state of the science and challenges for research. Addiction. 2003;98(s2):1–12. 10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets utilized in this study are accessible from the corresponding author upon reasonable request.