Abstract
Once one cognitive set dominates our behavior, it continues to influence subsequent behavior for a while even after a task to be performed is changed to another. Despite abundant knowledge of the inhibitory mechanisms that are recruited at the first trial after the change (the first inhibition trial), little is known about the inhibition of prolonged proactive interference from a previous set that lingers for several trials after the first inhibition trial. The present functional MRI study explored the neural mechanisms for inhibition of a previous set that were recruited after the first inhibition trial. A modified Wisconsin Card Sorting Test was used where “dual-match stimuli” were intermittently presented and allowed subjects to perform correctly based on previously appropriate, now inappropriate, responses. In response to the dual-match stimulus at “release” trials presented after the first inhibition trials, the subjects were transiently exempted from inhibiting the prolonged previous set. As expected from the exempted inhibitory demands, significant reaction time decrease was revealed in the release trials. Consistent with the behavioral results, transient signal decrease time-locked to the release trials was revealed in the left anterior part of the superior frontal sulcus. Moreover, the anterior prefrontal region was not sensitive to the task change, which exhibited a marked contrast to the left posterior inferior prefrontal region that showed significant signal changes in both events. These results revealed multiple inhibitory mechanisms in the lateral prefrontal cortex that are recruited in different temporal contexts of the interference from a previous cognitive set.
The prefrontal cortex supports flexible behavior by inhibiting proactive interference from a previously acquired cognitive set (1-3). It is well known that lesion to the lateral prefrontal cortex in both humans (4-6) and monkeys (7, 8) leads to perseverative errors in the Wisconsin Card Sorting Test (WCST) or its analogues modified for monkey studies, wherein the frontal patients characteristically adhere to previously appropriate responses based on a particular principle, or a “dimension.” Detailed behavioral analyses in normal human subjects have revealed that inhibition of proactive interference is one of the essential component processes recruited at the time of switching one task to another (9, 10). In support of the above findings, neuroimaging studies have demonstrated prominent activation in the lateral prefrontal cortex during set shifting/task switching (11-18).
Proactive interference from a previously acquired set lingers even after a task to be performed is changed to another. As has widely been held (4, 9, 10), the previously acquired set needs to be inhibited at the first trial after the change. However, previous behavioral studies have demonstrated that the interference continues even after the first inhibition trial over the time scale of a minute or so, and therefore the previous set needs to be inhibited even after the first trial to avoid perseverative errors (9). Previous investigations of inhibitory mechanisms have focused on the first inhibition trial, whether the first trial was given immediately after the change (11-18) or separated away from the change (19). Despite the abundant knowledge about the inhibitory mechanisms recruited at the first inhibition trial, little is known about the neural mechanisms responsible for the inhibition of the prolonged interference that persists long after the first inhibition trial. To explore the neural substrates for the inhibition of the prolonged interference, the present functional magnetic resonance imaging (fMRI) study used a “dual-match stimulus” in a modified version of the WCST (19) that exempted subjects from inhibiting a previously acquired set (Fig. 1). After a change from a previous dimension “color,” for example, subjects were exposed to proactive interference from the color dimension block during subsequent dimensions “form” and “number” (Fig. 1a). When the dual match stimuli were presented under the exposure of the previous set (a “release trial”), however, subjects were not required to inhibit the interference, because matching to the dual-match stimuli based on the previous dimension led to correct matching based on the current dimension (Fig. 1b). The release trials were compared with ordinary control trials in which subjects were required to inhibit proactive interference, and the control trials were matched with the release trials in terms of the temporal distance from the dimension change (DC) (19). Thus, the comparison of the control and release trials is expected to reveal cognitive components related to inhibition of proactive interference at the trials. The release trials were embedded in successive trials where the inhibition was required, and reaction time decrease at the release trials relative to control trials was predicted. The transient brain activity decrease at the release trials compared with the control trials was explored by using event-related fMRI [Experiment (Exp.) 1]. An additional confirmatory experiment (Exp. 2) also was conducted in a simpler design where proactive interference was derived from only one dimension block.
Fig. 1.
A modified WCST used in the present study. (a) The dual-match stimuli (D) were presented among successive single-match trials (S). In every trial, subjects were exposed to proactive interference (PI) (arrows) from a previous set acquired during the base dimension, but subjects were not required to inhibit the PI at D in red. (b) Examples of the dual-match stimuli (types A, B, and C presented in the first, second, and third blocks after the base dimension, respectively). In type A, the current dimension (form) and the last base dimension (color) in the case of a were matched in one reference card indicated by the black arrow. In type B, the current dimension (number) and the second-to-last base dimension (color) were matched. In type C, the dimensions (form and number) irrelevant to the current one (color) were matched.
Methods
Subjects and fMRI Procedures. Informed consent was obtained from 31 (15 males and 16 females; age 20-31 years, mean 22.6) and 20 (8 males and 12 females; age 20-25 years, mean 21.5) healthy right-handed subjects in Exp. 1 and 2, respectively. They were scanned by fMRI using experimental procedures approved by the institutional review board of the University of Tokyo School of Medicine. Scanning was conducted by using a 1.5-T fMRI system. Scout images were first collected to align the field of view centered on the subject's brain. Then, T2-weighted spin-echo images were obtained for anatomical reference [slice thickness = 2 mm; in-plane resolution = 2 × 2 mm; repetition time (TR) = 5.5 s; echo time (TE) = 30 ms]. For functional imaging, gradient echo echo-planar sequences were used (slice thickness = 4 mm; in-plane resolution = 4 × 4 ml; TE = 50 ms; flip angle = 90°; TR = 4 s and 3 s for Exp. 1 and 2, respectively). The first four functional images in each run were excluded from the analysis to take into account the equilibrium of longitudinal magnetization.
Behavioral Procedures. Visual stimuli were presented to subjects by projecting the stimuli onto a screen. Subjects viewed the screen through prism glasses. A magnet-compatible button press based on a fiber-optic switch was used to record the performance of the subjects. The tasks used in this study were derived from the WCST (20) modified in our previous studies (14, 19). In each WCST trial, a five-card display was presented until subjects responded to one of four reference card stimuli at the corner of the screen by matching the attribute of a central card on the basis of the dimension of color, form, or number. A four-channel button was pressed by using the right thumb for the choice of one of the four reference card stimuli. A feedback stimulus (right, O; wrong, X) was then presented. In Exp. 1, after 16 successive correct trials, the currently relevant dimension was changed to one of the others (Fig. 1a), and subjects were instructed of the subsequent dimension by visual presentation of the word “color,” “form,” or “number” (DC). After 8 successive correct trials, in the middle of the 16-trial block, the current dimension was presented to the subjects (“dimension repeat,” DR), and the subjects continued to perform based on the same dimension. The order of the dimensions was color-form-number-color-form-number... or color-number-form-color-number-form..., counterbalanced across runs. The task used a self-paced design, and the feedback and instruction stimuli were presented for 0.5 s, with each stimulus separated by a blank image for 0.25 s (therefore, the time between response and presentation of the next trial is 1.0 s).
There were two forms of card stimuli contained in the five-card display, “single match” and “dual match” (19). The single-match card stimulus was used in most of the card-sorting trials, and each one of its four reference card stimuli was matched to its central card stimulus on the basis of only one of the three dimensions. Conversely, the dual-match stimulus contained a reference card stimulus that was matched to the central card stimulus on the basis of two of the three dimensions (Fig. 1b). Moreover, there were three types of the dual-match stimuli that differed in the combinations of the two compatible dimensions. The combinations were as follows: the current and the last dimensions (type A), the current and the second-to-last dimensions (type B), and the two dimensions other than the current one (type C). Types A and B of the dual-match stimuli were used in release trials to examine the release from the inhibition of proactive interference from the last and the second-to-last dimension blocks, respectively. Type C of the dual-match stimuli was used in control trials as a control for the release trials: this type left the dual-match nature of the stimuli unchanged but required the inhibition because the combination of the compatible dimensions did not include the current dimension.
The release trials were presented at the third trial after the DC by using the dual-match stimuli (types A and B) (Fig. 1a). Importantly, the order of the dual-match type was varied systematically across the dimension blocks. Specifically, in the case of Fig. 1a, dual-match type A was presented in the form dimension, and type B was presented in the number dimension, such that the interference from the initial color dimension (a “base dimension”) could be examined in the subsequent form and number dimensions. In other words, the dual-match types A and B were presented in the first and second dimension blocks after the base dimension, respectively. Type C was presented at the third dimension block after the base dimension and served as a control for the release trials. In every fourth dimension block, only single-match stimuli were presented throughout the block, which counterbalanced the content of the base dimensions (color, form, or number) within fMRI runs (in the case of Fig. 1, the next base dimension was form).
Exp. 2 was similar to Exp. 1, but the task design was simplified to isolate the source of proactive interference. There were only three dimension blocks in one fMRI run, and the first and third dimensions were the same (for example, a dimension order was color-form-color). The second dimension block served as a base dimension block, and the release trials were presented in the third dimension block to examine proactive interference from the second dimension block. Thus, there was only one block (i.e., the third block) tested for the interference effect from the last base dimension. Inter-run interval was ≈5 min on average to allow proactive interference from the previous runs to decay considerably. These procedures limited the source of proactive interference examined in release trials to virtually only one dimension block (i.e., the second block). More specifically, the first and second dimension blocks consisted of eight single-match trials, and the third block consisted of 6 release trials (dual-match type A) that did not require the inhibition of proactive interference, 6 control trials (dual-match type C) that required the inhibition, and 12 single-match trials. The order of these trials in the third block was as follows: single-dual (type A)-single-dual (type C)-single... or single-dual (type C)-single-dual (type A)-single..., counterbalanced across runs.
Data Analysis. Data were analyzed by using spm99 (www.fil.ion.ucl.ac.uk/spm). Functional images were realigned to the first image in the first run after excluding the four initial images, and slice timing was corrected, normalized to the default template with interpolation to a 2 × 2 × 2-mm space, and spatially smoothed (full width at half-maximum = 8 mm). Then event timing was coded into a general linear model (21). The main regressors of interest in Exp. 1, the three kinds of dual-match trials, together with other events including the DC, DR, and error trials, were coded into a general linear model by using the canonical hemodynamic response function in spm99, time-locked to the onset of stimulus presentation. Main regressors of interest in Exp. 2 were also dual-match control and release trials, similar to Exp. 1. Images of parameter estimates for signal response magnitudes in these events then were analyzed for group analysis by using a random effect model. Peak coordinate locations in activation maps were generated by using a threshold of 19 or more contiguous significant voxels above P < 0.001 (z > 3.3) (each voxel: 2 × 2 × 2 mm3), calculated by using an empirical analysis of control data set (22, 23).
Results
Mean correct performance in Exp. 1 was 99.7 ± 0.2% (mean ± SD), and in the release trials only, mean correct performance was 99.8 ± 0.4%. Fig. 2a shows the time course of mean reaction time at each trial after the changes from the base dimensions. Mean reaction time decrease in correct release trials presented in the third trial in the first and second blocks, compared with correct control trials, was 53 ms [t(30) = 4.6; P < 0.001] in the first block and 29 ms [t(30) = 2.1; P < 0.05] in the second block (Fig. 2b).
Fig. 2.
Reaction time decrease at release trials. (a) Time course of mean reaction time at each of the trials after changes from the base dimensions. Mean reaction time in the release trials (dual-match type A or B) was smaller than that in the control trials (dual-match type C) (arrows). (b) The reaction time decrease at each of the release trials compared with the control trials. End stopped lines in the graph bars show standard error of the mean across subjects. *, P < 0.05; **, P < 0.001.
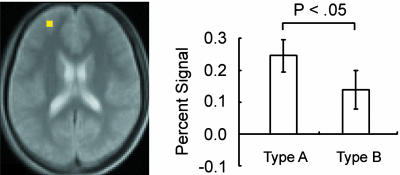
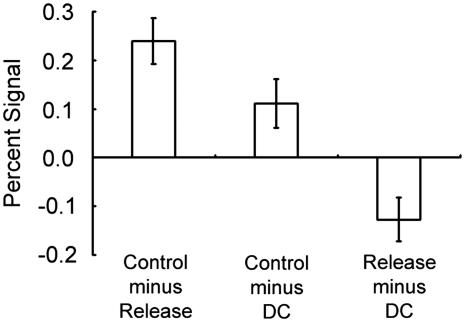
The image data set from a pool of the 31 subjects was analyzed by a general linear model implemented in spm99 and was applied to a random effect model. As shown in Fig. 3, the contrast “DC minus DR” elicited prominent activations in a set of mostly left-lateralized regions including the posterior inferior frontal sulcus regions, consistent with our previous study using a similar modification of the WCST (14, 19). More central to the activation of interest in the present study, the control dual-match trials (dual-match type C) in the third block was contrasted with the release dual-match trials (dual-match type A) in the first block after the base dimension. The contrast “control minus release trials” yielded prominent activation in the anterior part of the left superior frontal sulcus at (-30, 50, 18) [t(30) = 5.2] that corresponds approximately to Brodmann area 10 (24). The coordinates are in millimeters in Talairach's three-dimensional rectangular coordinate system. A list of other peak coordinates in the contrasts DC minus DR and control minus release are shown in Table 1. The contrast control minus release based on the release trials in the second block also yielded a significant activation peak in the adjacent anterior prefrontal region at (-30, 52, 18) [t(30) = 2.3]. The magnitude of the brain activity in the anterior prefrontal region was compared between the release trials at the first and second blocks (Fig. 4). For unbiased comparison, the region of interest was determined based on the average of the first and second blocks, and the resultant region was still at the coordinate (-30, 50, 18). The difference between type A and B release trials was significant [t(30) = 2.4; P < 0.05], suggesting that proactive interference from the base dimension was weakened after another DC. To describe the anterior prefrontal activity more thoroughly, the contrasts “control minus DC” and “release minus DC” were calculated (Fig. 5). This analysis indicated that the level of the anterior prefrontal activity during DC was near the middle of control and release trials.
Fig. 3.
Statistical activation maps for signal increase during DC minus DR and control minus release trials. The release trials in the first block after the base dimension (type A) were used for this analysis. The color scale in the maps reflects statistical significance, using the threshold of z > 3.3, P < 0.001 (uncorrected) for a display purpose. Activation maps are displayed as transverse sections and are overlaid on the anatomic image averaged across subjects. The transverse section level is indicated by the Z coordinates of Talairach space at the bottom.
Table 1. Activation peak coordinates in the contrasts DC minus DR and control minus release.
| Coordinates
|
t value
|
||||
|---|---|---|---|---|---|
| Contrast | X | Y | Z | BA/area | |
| DC minus DR | |||||
| Frontal cortex | 6 | 10 | 68 | 6.6 | 6 |
| −44 | 8 | 30 | 6.1 | 44 | |
| −20 | −2 | 64 | 6.0 | 6 | |
| Others | −12 | −80 | 44 | 4.8 | 7 |
| −54 | −10 | 54 | 4.7 | 3/4 | |
| −16 | −80 | −36 | 4.2 | Cerebellum | |
| 34 | −46 | −26 | 4.2 | Cerebellum | |
| 4 | −32 | −2 | 4.1 | Brain stem | |
| −42 | −30 | 66 | 4.0 | 2/5 | |
| −30 | −62 | 50 | 3.8 | 7 | |
| Control minus release | |||||
| Frontal cortex | −30 | 50 | 18 | 5.2 | 10 |
| Others | 56 | −66 | 18 | 5.4 | 39 |
| −18 | −46 | −22 | 4.4 | Cerebellum | |
BA, Brodmann area.
Fig. 4.
The signal decrease of the left anterior prefrontal activity at the release trials in the first and second blocks using dual-match types A and B, respectively. End stopped lines in the graph bars show standard error of the mean across subjects.
Fig. 5.
Signal magnitude in the anterior prefrontal activity (type A) in the contrasts control minus release, control minus DC, and release minus DC.
The left posterior part of the inferior frontal sulcus has been implicated in inhibitory functions (13, 14, 16-18). This inferior prefrontal region was tested for the contrast control minus release trials and also for the contrast DC minus DR (Fig. 6), which included the inhibition of a previous set in the first trial after the DC. The results also were compared with the left anterior prefrontal region reported in this work. For unbiased analyses, the coordinates for the inferior prefrontal region were represented based on our previous studies of inhibitory control at (-44, 16.5, 23) (22). For the anterior prefrontal region, the data set for the second block was used as region of interest delineation, and the signals in the first block were examined. In the anterior prefrontal region of interest determined at (-30, 52, 18), significant activation was similarly observed in the contrast control minus release trials [t(30) = 4.9; P < 0.001] but not in the contrast DC minus DR [t(30) = 0.6; P > 0.05], and the signal difference between these contrasts was significant [t(30) = 3.4; P < 0.05]. Conversely, in the inferior prefrontal region, significant activation was observed for both of the contrasts DC minus DR [t(30) = 2.7; P < 0.05] and control minus release trials [t(30) = 2.4; P < 0.05]. The two-way ANOVA (the inferior and anterior regions by the two contrasts) revealed significant interaction effects [F(1, 30) = 10.1; P < 0.005], indicating the functional dissociation of these two prefrontal regions in terms of the two contrasts.
Fig. 6.
Significant interaction of brain activity in two-way ANOVA with regions (inferior and anterior prefrontal) and contrasts (DC minus DR and control minus release trials) as main effects. The release trials in the first block after the base dimension (type A) were used for this analysis.
The anterior prefrontal activation was further investigated in a similar but simpler design in Exp. 2 where the source of proactive interference was restricted to only one dimension block. Mean reaction time decrease in correct release trials compared with correct control trials was 36 ms [t(19) = 7.6; P < 0.001]. As shown in Fig. 7, the left anterior prefrontal activation was observed at (-38, 52, 16), only ≈8 mm apart from that observed in Exp. 1. The two data sets from these experiments confirmed that the anterior prefrontal activation with a radius of 8 mm cleared the threshold level of P < 0.05 corrected for whole-brain multiple comparison; the activation based on Exp. 1 was significant in the data from Exp. 2 [t(19) = 3.1; P < 0.01], and the activation based on Exp. 2 was also significant in the data from Exp. 1 [t(30) = 2.2; P < 0.05].
Fig. 7.
Statistical activation maps for signal increase during control minus release trials in Exp. 1 and 2. The format is similar to Fig. 3.
Discussion
The present study used the modified WCST that contained release trials where subjects were not required to inhibit a previous set even after the first trial after the DC. Significant reaction time decrease was observed in the release trials relative to the control trials. In the neuroimaging data, the contrast control minus release trials revealed prominent activation in the left anterior part of the superior frontal sulcus. However, the brain activity in the anterior prefrontal region was not sensitive to the contrast DC minus DR that included the inhibition of a previous set in the first trial after the DC. Moreover, the results of the anterior prefrontal region were dissociable from those of the left posterior inferior frontal region that was sensitive to both the contrasts. These results suggest multiple inhibitory mechanisms within the lateral prefrontal cortex that are activated depending on the temporal contexts of the inhibition of proactive set interference.
The behavioral results of the present study fit well with previous behavioral studies using task switching paradigms that demonstrated prolonged interference effects that are attributed to residual switch cost (9, 10). It is thus suggested that the left anterior prefrontal region is involved in the inhibition of proactive interference that constitutes the residual switch constant. It is to be noted that the type of cognitive control presented here may be different from that revealed in the anterior prefrontal cortex in the right hemisphere (as opposed to the left hemisphere reported in the present work) that acts to reconfigure and maintain a set over the task periods (16, 25). It is also to be noted that the left anterior prefrontal region reported here is ≈2 cm lateral and superior to the one reported to be activated during DCs in visual singleton search tasks (11, 26) and during task switching under predictable task order and timing (27) that was attributed to memory retrieval (28-31). Although it is not yet clear whether these anterior prefrontal regions are distinct functional entities, further interrogation might reveal functional heterogeneity within the anterior prefrontal regions.
The dual-match stimuli have been used in our previous study and revealed left superior prefrontal activation during the first inhibition trial that took place several trials after the DC (19). Although the present study also examined the inhibitory mechanisms that were recruited several trials after the DC, the anterior prefrontal region reported in the present work was activated several trials after the first inhibition trial, not at the first inhibition trial. Moreover, these two inhibitory processes might be supported by distinct neural mechanisms in the anterior and superior prefrontal regions that are separated by ≈3 cm: the anterior prefrontal region was not significantly activated in the data set of ref. 19 (P > 0.05), and the superior prefrontal region was not significantly activated in the present data set either (P > 0.05). Thus, these two inhibitory processes appear orthogonal not only conceptually but also in neural correlates, but it is to be noted that all of the three inhibitory mechanisms in the lateral prefrontal cortex (inferior, superior, and anterior ones) are left-hemisphere dominant. One important caveat regarding the left prefrontal dominance in set shifting revealed by our previous studies is that our modified WCST allowed subjects to respond slowly (≈1,000 ms; see Fig. 2), unlike task switching paradigms in other studies where responses are made more rapidly, thus requiring inhibition of inappropriate responses implemented in the right prefrontal cortex (32-36). This task difference may account for the right frontal cortical involvement in task switching revealed by previous neuroimaging and neuropsychological studies (37, 38).
Previous studies have demonstrated that the posterior part of the inferior frontal sulcus is implicated in inhibitory control during set shifting/task switching (13, 14, 16-19, 39) and during other inhibitory situations such as the go/no-go (32-36), Stroop (40), and controlled retrieval tasks (41-44). The region of interest analysis in the present work also showed that, besides the left anterior prefrontal region, the release trials also involved the posterior inferior prefrontal region. However, the dissociable functional aspects of the left anterior and inferior prefrontal regions were revealed by the contrast DC minus DR (Fig. 6), a contrast typically investigated in previous fMRI studies of set shifting (14, 45). On one hand, the anterior prefrontal region did not show the sensitivity to this contrast, consistent with negative results in previous studies using set shifting/task switching paradigms (11-19, 32, 37, 46). The negative results suggest the functional specificity of the anterior prefrontal region: the region is not activated during the first trial that required the inhibition (DC minus DR) but is activated during the second or later inhibition trials (control minus release trials). On the other hand, the inferior prefrontal region showed significant sensitivity to both of the contrasts, demonstrating the functional dissociation between the two prefrontal regions (Fig. 6). One possible account for this anterior-inferior dual inhibitory mechanism depending on the temporal context might be related to the fact that the first inhibition trials are temporally close to DC. At the time of DC, subjects have to adapt to a new task by reconfiguring a new task set (47). Such executive processes are generally involved in various cognitive functions (48) and thus also might be involved in inhibition of proactive interference, which may result in a less significant role of the anterior inhibitory mechanism required for inhibition at the first trial. Although it remains to be seen how the two lateral prefrontal regions interact with each other, the present study revealed the functional dissociation between the anterior and inferior prefrontal regions that contribute to inhibitory processes in different temporal contexts of the interference from a previous cognitive set.
Acknowledgments
This work was supported by Ministry of Education, Culture, Sports, Science and Technology (Japan) Grant-in-Aid for Specially Promoted Research 14002005 (to Y.M.) and Grant 17500203 (to S.K.).
Author contributions: S.K. designed research; S.K., J.C., K.J., and T.A. performed research; S.K. analyzed data; and S.K. and Y.M. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: DC, dimension change; DR, dimension repeat; Exp., experiment; fMRI, functional MRI; WCST, Wisconsin Card Sorting Test.
References
- 1.Milner, B. (1964) in Frontal Granular Cortex and Behavior, eds. Warren J. M. & Akert, K. (McGraw-Hill, New York), pp. 313-334.
- 2.Mishkin, M. (1964) in Frontal Granular Cortex and Behavior, eds. Warren J. M. & Akert, K. (McGraw-Hill, New York), pp. 219-241.
- 3.Robbins, T. W. (1996) Philos. Trans. R. Soc. London B 351, 1463-1470. [DOI] [PubMed] [Google Scholar]
- 4.Milner, B. (1963) Arch. Neurol. 9, 90-100. [Google Scholar]
- 5.Janowski, J. S., Shimamura, A. P., Kritchevski, M. & Squire, L. R. (1989) Behav. Neurosci. 103, 548-560. [DOI] [PubMed] [Google Scholar]
- 6.Owen, A. M., Roberts, A. C., Hodges, J. R., Summers, B. A., Polkey, C. E. & Robbins, T. W. (1993) Brain 116, 1159-1175. [DOI] [PubMed] [Google Scholar]
- 7.Passingham, R. E. (1972) Neuropsychologia 10, 41-46. [DOI] [PubMed] [Google Scholar]
- 8.Dias, R., Robbins, T. W. & Roberts, A. C. (1996) Nature 380, 69-72. [DOI] [PubMed] [Google Scholar]
- 9.Allport, A., Styles, E. A. & Hsieh, S. L. (1994) in Attention and Performance, eds. Umilta, C. & Moscovitch, M. (MIT Press, Cambridge, MA), Vol. XV, pp. 421-452. [Google Scholar]
- 10.Meiran, N., Chorev, Z. & Sapir, A. (2000) Cognit. Psychol. 41, 211-253. [DOI] [PubMed] [Google Scholar]
- 11.Pollmann, S., Weidner, R., Muller, H. J. & von Cramon, D. Y. (2000) J. Cognit. Neurosci. 12, 480-494. [DOI] [PubMed] [Google Scholar]
- 12.Sohn, M.-H., Ursu, S., Anderson, J. R., Stenger, V. A. & Carter, C. S. (2000) Proc. Natl. Acad. Sci. USA 97, 13448-13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monchi, O., Petrides, M., Petre, V., Worsley, K. & Dagher, A. (2001) J. Neurosci. 21, 7733-7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konishi, S., Hayashi, T., Uchida, I., Kikyo, H., Takahashi, E. & Miyashita, Y. (2002) Proc. Natl. Acad. Sci. USA 99, 7803-7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rushworth, M. F. S., Passingham, R. E. & Nobre, A. C. (2002) J. Cognit. Neurosci. 14, 1139-1150. [DOI] [PubMed] [Google Scholar]
- 16.Braver, T. S., Reynolds, J. R. & Donaldson, D. I. (2003) Neuron 39, 713-726. [DOI] [PubMed] [Google Scholar]
- 17.Brass, M. & von Cramon, Y. (2004) J. Neurosci. 24, 8847-8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cools, R., Clark, L. & Robbins, T. W. (2004) J. Neurosci. 24, 1129-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konishi, S., Jimura, K., Asari, T. & Miyashita, Y. (2003) J. Neurosci. 23, 7776-7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grant, D. A. & Berg, E. A. (1948) J. Exp. Psychol. 38, 404-411. [DOI] [PubMed] [Google Scholar]
- 21.Worsley, K. J. & Friston, K. J. (1995) NeuroImage 2, 173-181. [DOI] [PubMed] [Google Scholar]
- 22.Konishi, S., Donaldson, D. I. & Buckner, R. L. (2001) NeuroImage 13, 364-374. [DOI] [PubMed] [Google Scholar]
- 23.Lustig, C. & Buckner, R. L. (2004) Neuron 42, 865-875. [DOI] [PubMed] [Google Scholar]
- 24.Talairach, J. & Tournoux, P. (1988) Co-planar Stereotaxic Atlas of the Human Brain (Thieme Medical, New York).
- 25.Sakai, K. & Passingham, R. E. (2003) Nature Neurosci. 6, 75-81. [DOI] [PubMed] [Google Scholar]
- 26.Weidner, R., Pollmann, S., Muller, H. J. & von Cramon, D.Y. (2002) Cereb. Cortex 12, 318-328. [DOI] [PubMed] [Google Scholar]
- 27.Dreher, J. C., Koechlin, E, Ali, S. O. & Grafman, G. (2002) NeuroImage 17, 95-109. [DOI] [PubMed] [Google Scholar]
- 28.Buckner, R. L., Koutstaal, W., Schacter, D. L., Wagner, A. D. & Rosen, B. R. (1998) NeuroImage 7, 151-162. [DOI] [PubMed] [Google Scholar]
- 29.Duzel, E., Cabeza, R., Picton, T. W., Yonelinas, A. P., Scheich, H., Heinze, H. J. & Tulving, E. (1999) Proc. Natl. Acad. Sci. USA 96, 1794-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henson, R. N., Rugg, M. D., Shallice, T. & Dolan, R. J. (2000) J. Cognit. Neurosci. 12, 913-923. [DOI] [PubMed] [Google Scholar]
- 31.Konishi, S., Wheeler, M. E., Donaldson, D. I. & Buckner, R. L. (2000) NeuroImage 12, 276-286. [DOI] [PubMed] [Google Scholar]
- 32.Konishi, S., Nakajima, K., Uchida, I., Kikyo, H., Kameyama, S. & Miyashita, Y. (1999) Brain 122, 981-991. [DOI] [PubMed] [Google Scholar]
- 33.Bunge, S. A., Dudukovic, N. M., Thomason, M. E., Vaidya, C. J. & Gabrieli, J. D. E. (2002) Neuron 33, 301-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durston, S., Thomas, K. M., Worden, M. S., Yang, Y. & Casey, B. J. (2002) NeuroImage 16, 449-453. [DOI] [PubMed] [Google Scholar]
- 35.Aron, A. R., Fletcher, P. C., Bullmore, E. T., Sahakian, B. J. & Robbins, T. W. (2003) Nature Neurosci. 6, 115-116. [DOI] [PubMed] [Google Scholar]
- 36.Hester, R. L., Murphy, K., Foxe, J. J., Foxe, D. M., Javitt, D. C. & Garavan, H. (2004) J. Cognit. Neurosci. 16, 776-785. [DOI] [PubMed] [Google Scholar]
- 37.Dove, A., Pollmann, S., Schubert, T., Wiggins, C. J. & von Cramon, D. Y. (2000) Cognit. Brain Res. 9, 103-109. [DOI] [PubMed] [Google Scholar]
- 38.Aron, A. R., Monsell, S., Sahakian, B. J. & Robbins, T. W. (2004) Brain 127, 1561-1573. [DOI] [PubMed] [Google Scholar]
- 39.Nakahara, K., Hayashi, T., Konishi, S. & Miyashita, Y. (2002) Science 295, 1532-1536. [DOI] [PubMed] [Google Scholar]
- 40.Taylor, S. F., Kornblum, S., Lauber, E. J., Minoshima, S. & Koeppe, R. A. (1997) NeuroImage 6, 81-92. [DOI] [PubMed] [Google Scholar]
- 41.Thompson-Schill, S. L., D'Esposito, M., Aguirre, G. K. & Farah, M. J. (1997) Proc. Natl. Acad. Sci. USA 94, 14792-14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sohn, M.-H., Goode, A., Stenger, V. A., Carter, C. S. & Anderson, J. R. (2003) Proc. Natl. Acad. Sci. USA 100, 7412-7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Konishi, S., Uchida, I., Okuaki, T., Machida, T., Shirouzu, I. & Miyashita, Y. (2002) J. Neurosci. 22, 9549-9555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wheeler, M. E. & Buckner, R. L. (2003) J. Neurosci. 23, 3869-3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asari, T., Konishi, S., Jimura, K. & Miyashita, Y. (2005) NeuroImage 26, 694-702. [DOI] [PubMed] [Google Scholar]
- 46.Barcelo, F., Sanz, M., Molina, V. & Rubia, F. J. (1997) Neuropsychologia 35, 399-408. [DOI] [PubMed] [Google Scholar]
- 47.Rogers, R. D. & Monsell, S. (1995) J. Exp. Psychol. Gen. 124, 207-231. [Google Scholar]
- 48.Duncan, J. & Owen, A. M. (2000) Trends Neurosci. 23, 475-483. [DOI] [PubMed] [Google Scholar]