Abstract
In this study, we used functional MRI (fMRI) at high field (3T) to track the time course of activation in the entire basal ganglia circuitry, as well as other motor-related structures, during the explicit learning of a sequence of finger movements over a month of training. Fourteen right-handed healthy volunteers had to practice 15 min daily a sequence of eight moves using the left hand. MRI sessions were performed on days 1, 14 and 28. In both putamen, activation decreased with practice in rostrodorsal (associative) regions. In contrast, there was a significant signal increase in more caudoventral (sensorimotor) regions of the putamen. Subsequent correlation analyses between signal variations and behavioral variables showed that the error rate (movement accuracy) was positively correlated with signal changes in areas activated during early learning, whereas reaction time (movement speed) was negatively correlated with signal changes in areas activated during advanced learning stages, including the sensorimotor putamen and globus pallidus. These results suggest the possibility that motor representations shift from the associative to the sensorimotor territories of the striato-pallidal complex during the explicit learning of motor sequences, suggesting that motor skills are stored in the sensorimotor territory of the basal ganglia that supports a speedy performance.
Keywords: functional MRI, human, subthalamic nucleus
There is now ample evidence from a number of sources that indicates that the basal ganglia are implicated in the formation of motor skills (1). In human studies using brain-imaging techniques, for example, changes of activity have been observed in the basal ganglia at different stages of the acquisition of motor abilities. Decreases of activity in the basal ganglia were reported during the early phase of trial-and-error learning of sequential movements (2). Using a similar task, Toni et al. (3) also reported a decrease of activity in the caudate nucleus, pre-supplementary motor area (SMA), and prefrontal cortex during the first hour of the acquisition process (3). By contrast, studies of implicit motor learning showed that the striatum was more active when subjects reached asymptotic performance, thus suggesting that this structure may be critical for the long-term storage of motor sequences (4-7). However, other investigations (8, 9) failed to document the presence of a greater increase in activity during the execution of well-learned sequential movements compared with the early learning phase.
Although still conjectural, differences in the patterns of activation described above may reflect the involvement of different cortico-basal ganglia circuits during the acquisition process, because it is now known that cortical areas project to the striatum in separate associative, premotor, and sensorimotor circuits (10, 11). Indeed, previous imaging works have shown that rostral striatal areas are activated during learning of new motor sequences (2, 3) whereas the execution of well learned sequential movements involved only the sensorimotor loop, including the SMA and putamen (4-7). Thus, despite some conflicting observations (8, 12), these results suggest that anterior (associative) striatal regions are implicated during the acquisition of new motor skills, whereas posterior (sensorimotor) regions may be critical for the long-term storage of those skilled behaviors (2). Further support to this hypothesis comes from animal studies in monkeys, where reversible pharmacological blockade of the anterior striatum has been shown to lead to deficits in learning of new sequences, whereas blockade in the posterior striatum impaired the execution of well learned sequence of movements (13). Based on such findings, Hikosaka et al. (14) have proposed that early and advanced learning of motor skills depends, respectively, upon two independent circuits within the basal ganglia, one involving the anterior associative/premotor loop, and the other implicating the posterior sensorimotor-basal ganglia loop (14, 15). If this hypothesis is true, a shift in motor representations from the associative to the sensorimotor territories of the striatum during the course of motor sequence learning should be expected. However, the time course of such a functional shift has yet to be demonstrated. Furthermore, although animal studies suggest that other critical basal ganglia nuclei like the substantia nigra (SN) (16, 17), and probably the subthalamic nucleus (STN), are also engaged in motor learning, little is known with respect to the functional contribution of these structures in humans because they have been beyond reach of imaging approaches like functional MRI (fMRI) up to now.
In the present study, we thus used fMRI at high field (3T) to track the time course of cerebral plasticity during extended practice of an explicitly known sequence of finger movements. Therefore, improvements in performance in our paradigm reflect sequential learning that is implicit in nature. Most previous learning studies have focused on the fast early learning phase (2, 3, 18-20) or have monitored activity changes in the primary motor cortex only (21). To reveal the possible shift of motor representations from the associative to the sensorimotor territories of the basal ganglia during the course of learning, subjects were followed over a period of training of 4 weeks, and scanning parameters were set to include all of the basal ganglia structures, as well as the entire motor-related circuitry. In addition, at 4 weeks of training, automaticity was evaluated by using a dual-task to determine whether subjects could perform the tasks with minimal interference.
Methods
Subjects. Fourteen right-handed healthy volunteers participated in the present study (6 men; mean age, 23.7 ± 4.2 yr; age range, 19-34 yr). None of the subjects was a musician or a professional typist. One subject had previously played the piano, but stopped practicing >7 yr ago. The Local Ethics Committee from the University of Minnesota approved the study, and the subjects gave their informed consent. All subjects were right handed as confirmed by the Edinburgh Handedness Inventory.
Imaging Parameters. The MR protocol was carried out with a 3-T whole-body system (Siemens, Erlangen, Germany) by using blood oxygen level-dependent (BOLD) fMRI. The head of the subject was immobilized by using foam cushions and tape, with the ears plugged. The protocol lasted 90 min and included (i) one sagittal T1-weighted image to localize functional and anatomical axial slices; (ii) 43 oblique axial gradient echo echo-planar imaging (EPI) images [repetition time (TR) 4.5 s/echo time (TE) 40 ms/α 90°; bandwidth, 1,562 Hz per pixel; field of view (FOV), 192 × 192 mm2; voxel size, 1.5 × 1.5 × 2.5 mm3; partial Fourier imaging 6/8]. For each series, 123 EPI volumes were acquired over 9 min and 13 s. The first three volumes of each run were discarded to reach signal equilibrium; (iii) 144 sagittal 3D magnetization prepared rapid gradient echo (MP-RAGE) images (1 mm thick; FOV, 256 × 256 mm2; matrix size, 256 × 256) for anatomical localization.
Tasks. Subjects were asked to practice a trained sequence (T-sequence) of eight moves by using fingers 2 to 5 of the left hand over a period of 4 weeks. Subjects were asked to practice this sequence during 10-20 min daily, during which they were instructed to repeatedly tap a sequence in a rapid self-paced and accurate manner. Seven different sequences were generated by using matlab (Mathworks, Natick, MA). Within each sequence, the order of finger movements was pseudorandomly generated such that each finger was used twice in each sequence. For each subject, one of these sequences was randomly chosen to be the T-sequence, whereas the others served as untrained control sequences (U-sequences).
The subjects' performance was assessed inside and outside the scanner by using a four-key keyboard (Electrical Geodesics, Eugene, OR) that was MR compatible. The keyboard allowed recording of the subjects' response accuracy and timing. Subjects were required to keep their fingers on the keys to minimize amplitude variation. The amount of force required to press the keys was minimal. Outside the scanner, subjects' speed and accuracy were assessed by using 30-s duration tests. During these speed tests, subjects were instructed to tap the sequence as rapidly as possible while making as few errors as possible. Five speed tests were performed weekly, as well as before and after each scanning session. Subjects underwent three scanning sessions on day 1, day 14, and day 28, respectively. On day 1, they were asked to practice two sequences (i.e., the T-sequence and a control U-sequence) until they could perform them from memory. They were then required to produce each sequence during five speed tests (i.e., baseline performance). After the scan session, they performed five other speed tests with the T-sequence (i.e., re-test). On day 14 and day 28, the procedure was repeated again, except that subjects were tested with a new U-sequence on each occasion.
To assess how performance became automatic after 4 weeks of training, subjects were then asked to perform a dual-task paradigm before the MR session. It was expected that the trained sequence would be performed with minimal interference while subjects were doing a secondary task (8). On day 28, subjects were thus asked to perform the speed test with the T-sequence and a new U-sequence while reading aloud a simple text. The number of words that the subject could read during the 30-s period was recorded and used as an index of their level of automatization.
To control for potential differences in amplitude of the subjects' finger movements before and after training, the amplitude of their movements was also measured outside the MR unit by using a single-axis goniometer (F35) and angle display unit (ADU301) as described in Supporting Text, which is published as supporting information on the PNAS web site.
MR Protocol. On each scanning session, subjects performed two different runs by using the T- and the U-sequences separately. The order of the runs was counterbalanced across subjects. Movements were audio-paced with computer generated sounds at a fixed frequency of 2 Hz and transmitted to the subjects by using headphones. For each run, subjects alternated 10 epochs of 27 s (6 volumes) of rest and 27 s of the motor conditions. During the rest condition, subjects were told to remain in a resting awake state while listening to the beat of the metronome. On day 1, subjects were given two more runs of the T-sequence: one before (i.e., 10 min) and one immediately after 30 min of additional practice on the T-sequence (i.e., 50 min) while they were still lying on the scanner's bed, but without scanning. Thus, there were a total of five runs of the T-sequence (three in session 1 on day 1, one in session 2 on day 14, and one in session 3 on day 28).
Behavioral Data Analysis. Behavioral variables (key pressed, movement frequency, and reaction times) were automatically recorded based on the subjects' responses by using matlab-written software. For each subject, this software compared the sequence of key presses produced by the subject to the sequence template to be performed, and thus detected any incorrect tap (discordance between the real and expected taps within a given sequence). These behavioral variables were analyzed by using repeated-measure ANOVA (RM-ANOVA).
fMRI Data Analysis. Functional data analyses were first performed with SPM99 (Wellcome Department of Cognitive Neuroscience, London). Anatomical images were normalized to Montreal Neurological Institute coordinates (MCs), with a final voxel size of 1.5 × 1.5 × 1.5 mm3. The functional scans, corrected for the subjects' motion, were then normalized by using the same transformation and smoothed with an isotropic Gaussian spatial filter [full-width half-maximum (FWHM) = 8 mm]. Data were analyzed across subjects (group analysis with random effects). A 240-s temporal cut-off was applied to filter out subject-specific low-frequency drifts of the signal. Blood oxygen level-dependent (BOLD) signals from each voxel were modeled by using the general linear model with separate hemodynamic response functions and one-time derivative for the action and rest periods of the tasks. Overall signal differences between runs were also modeled. To identify the location of brain areas involved in each task, one-sample t tests were used to compare the motor conditions with the rest condition (condition - rest, which will be referred to as T1 to T5 for the T-sequences and U1 to U3 for the U-sequences), as well as the motor conditions against each other [e.g., (condition 1 - rest 1) versus (condition 2 - rest 2) = T1 - T2].
To identify voxels that were activated within runs when comparing each condition with the rest period, we used a stringent height threshold (P < 0.0001). Comparisons of the functional data between runs and between sessions were assessed statistically at lower thresholds (P < 0.001 and P < 0.01). In these maps, activated clusters were considered significant at P < 0.05, corrected for multiple comparisons inside the volume of the whole brain (for cortical activation) or inside the volume of the basal ganglia or dentate nucleus (DN) (small volume correction). Regression analyses between signal variations in the areas activated in these maps and behavioral variables were assessed statistically at lower thresholds (P < 0.001 and P < 0.01, uncorrected for multiple comparisons).
Results
Behavioral Data. Behavioral data are shown in Table 1 and Fig. 3, which are published as supporting information on the PNAS web site.
Speed Tests. During the pre-MRI training session, subjects' performances improved slightly over the first five speed tests, with a mean 16% increase in tapping frequency (RM-ANOVA, F = 4.1, P < 0.05), with no significant difference between the T-sequences and the U-sequences. Over the 4 weeks of practice with the T-sequence, subjects made 72% fewer errors, tapped 2.2 times faster and more regularly, with a decrease in SD of the inter-tap intervals of 55%. All parameters significantly improved between the first and last MR Sessions (RM-ANOVA, all P values <0.05). As expected, speed parameters reached a plateau after 4 weeks of training. Subjects' performances also improved with the U-sequences, but the differences did not reach significance (RM-ANOVA, all P values >0.05). They made 14% more errors and tapped only 1.26 times faster, and the SD of the inter-tap interval decreased by merely 21% after 4 weeks of training.
Dual Task Performance. With the U-sequence, subjects made 9.38 ± 6.66% errors and were 45% slower during the dual than during the single task (all P values <0.05). With the T-sequence, subjects made only 1.22 ± 1.21% errors (i.e., not significant compared with the single task) and were only 19% slower during the dual than during the single task (P = 0.02) (see Table 3).
Goniometer Data. Angle measurements (mean ± SD after training = 3.16 ± 0.50; before training = 3.13 ± 0.52) were entered in a general linear model with subjects as random effect, sequence (before vs. after training) as fixed effect, and time as a covariate (to remove possible linear trends over time). This model yielded a nonsignificant effect (F = 0.973, P = 0.344), suggesting that there was no difference in movement amplitude before and after training.
MR Sessions. There was no difference in the tapping frequencies observed between the T- and U-sequences before training. For the T-sequence, reaction times were significantly longer during the first run of day 1 than during all subsequent runs, but there was no difference between these subsequent runs (RM-ANOVA, P = 0.045, see Fig. 3). This rapid, within run, decrease in reaction times was also observed during the U-sequences across the three MR sessions, but there were no between-run differences. On day 28, reaction times for the T-sequence were shorter than for the U-sequence (P = 0.0003). For the T-sequence, the number of errors also decreased significantly with training from 2.29 ± 1.05% on day 1 to 0.85 ± 0.85% on day 28 (RM-ANOVA, P = 0.009), whereas there was no change for the U-sequences.
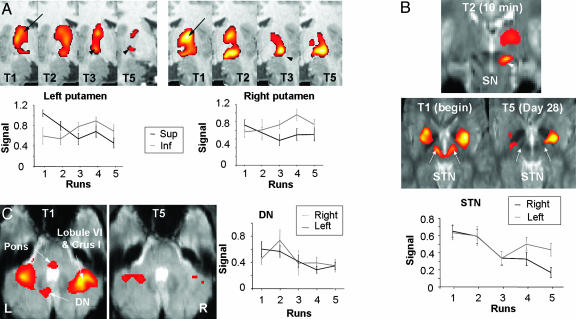
fMRI Data. As expected, there were dynamic changes in activation within the striatum during the course of learning. When the T-sequences were compared with rest, there were two main foci of activation in the rostrodorsal (MC, +27, 0, +12 in T1) and more ventral posterior parts (MC, +29, -6, -5 in T5) of the putamen (Fig. 1A). There was an inverse relationship between the levels of activation and training in these two areas. A regression analysis on the percentage of signal increase across the three MR sessions showed that the level of activity decreased with practice in the rostrodorsal part of both putamen (F = 8.99, P = 0.030 and F = 7.90, P = 0.048 for the right and left putamen, respectively), whereas the level of activation increased bilaterally in the ventral posterior compartments (F = 8.13, P = 0.046 and F = 8.57, P = 0.043 for the right and left putamen, respectively). This shift of activation was more pronounced on the right side, was already completed after 50 min of training (run 3 on day 1), and persisted after 4 weeks of practice. Comparisons between sessions also revealed that the extent of area activated in the rostrodorsal putamen declined significantly from run 1 to run 3 (T1 - T3, P < 0.001), run 2 to run 3 (T2 - T3, P < 0.01), and run 4 to run 5 (T4 - T5, P < 0.01). By contrast, activation in ventral posterior parts of the putamen increased with practice, became significant as soon as 10 min after the beginning of training in the contralateral hemisphere (T2 - T1, P < 0.01), and persisted after 2 weeks (T4 - T1 and T4 - T3, P < 0.05) and 4 weeks (T5 - T1, P < 0.01) of training (Fig. 1).
Fig. 1.
Activation patterns in the basal ganglia and cerebellum. (A Upper) Activation maps obtained in the putamen superimposed on a coronal T1-weighted image. There was a progressive activation decrease in the dorsal part of the putamen (arrows) and an increase in a more ventrolateral area (arrowheads) bilaterally, which persisted after 4 weeks of training. (Lower) Percentage signal increase ± SEM averaged across all subjects for each run of the trained sequence confirmed the activation decrease in the dorsal putamen and increase in the ventral putamen (RM-ANOVA). (B Top) Activation maps obtained in the SN (arrowhead, coronal level y = -20 on T2 day 1) and STN (arrows, axial level z = -3 on T1 day 1 and T5 day 28) superimposed on EPI images. During session 1, STN activation was observed during the first run of T-sequence (T1). After 4 weeks of training, these areas were no more activated during the T-sequence. There was no significant signal change in the SN across runs. (Bottom) Signal-to-time curves ± SEM in the STN averaged across all subjects and epochs confirm the activation decrease (RM-ANOVA). (C Left) Activation maps obtained in the cerebellum during the T-sequence (T1 on day 1 and T5 on day 28). Activation in the lateral cerebellar hemispheres, the left DN, and the pons decreased with training. (Right) Percentage signal increase ± SEM averaged across all subjects for each run of the trained sequence in the left and right DN. In the right DN, activation increased transiently during T2 (10 min of practice) and returned to pretraining values. All activation maps are corrected for cluster extent at P < 0.05 (height threshold P < 0.0001).
To test further whether this differential pattern of activations within the two regions of the putamen was significant, the mean percentage of signal increase in these two regions for both hemispheres, and for all runs of the T sequence, was analyzed by using a RM-ANOVA over the three scanning sessions. The result of this analysis showed a significant effect of regions (signal in the ventral posterior > rostrodorsal compartment, F = 12.9, P = 0.011) and interaction runs × regions (F = 21.4, P = 0.045).
In addition to the signal changes in the dorsal putamen, there was a progressive decrease of activation in the associative/premotor cortico-subcortical circuits. In subcortical areas, the direct comparison between sessions showed that activation decreased with learning bilaterally in the ventral anterior (VA)/ventral lateral (VL) and lateropolar (LP) nuclei of the thalamus, the left STN (T2 - T3, T4 - T5, P < 0.01), and the right red nucleus (T2 - T3, P < 0.01). Similarly, regression analysis on percentage signal changes showed a linear decrease of activation across all runs of the three MR sessions in the VA/VL and LP thalamus (MC during T1, -18, -12, +15; +20, -9, +12; F = 12.4, P = 0.024), the left STN (MC, -10, -15, -2; F = 16.6, P = 0.015), and the right red nucleus (MC, +7, -23, -11; F = 14.1, P = 0.020) (Fig. 1B). The SN was activated during all tasks compared with rest, but not in the direct comparison between runs. Activation changes in the cortex are presented in Supporting Text and Fig. 4, which is published as supporting information on the PNAS web site).
A dissociation of signal changes was also found in the cerebellum (see ref. 22 for nomenclature of the cerebellum). Comparisons between sessions showed that activity in lobules V and VI and Crus I in the right hemisphere (T1 - T2, T1 - T3, and T4 - T5, P < 0.01), lobules V and VI in the left hemisphere (MC, -21, -55, -23; T1 - T3, T3 - T4, and T4 - T5, P < 0.01), the pons (T1 - T3 and T4 - T5, P < 0.01), and the left DN (T3 - T4, P < 0.01) also decreased as learning progressed. As expected, regression analyses on percentage signal increase versus the number of runs showed that the activation level decreased with practice over the three MR sessions in the right lateral cerebellar hemisphere (MC during T1, +31, -57, -29, F = 8.72, P = 0.040), the pons (MC, -5, -29, -44, F = 16.7, P = 0.015), and the left inferior DN (MC, -12, -64, -42, F = 16.3, P = 0.016, Fig. 1C). By contrast, activity within the anterodorsal paravermian cortex (MC, -8, -50, -18) of the ipsilateral hemisphere (lobules III and IV) did not vary significantly during learning. Activation in the right DN followed a different pattern, because greater activity was observed on run 2 of the trained sequence on day 1 (MC, +11, -54, -32, T2 - T1), but then decreased to pretraining values in the subsequent runs (Fig. 1C).
Importantly, the same comparisons as described above using the successive U-sequences did not reveal significant activation foci in the above-mentioned regions (except in the left amygdala and anterior cingulate cortex, right hippocampus and bilateral insula during the U1 - U2 comparison, and the right SMA and hippocampus during the U2 - U3 comparison, P < 0.01), showing that the pattern of activations described above was not due to confounding habituation effects.
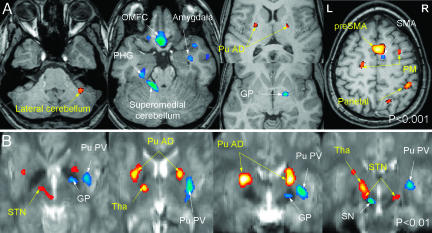
Finally, multiple regression analyses between signal variation and behavioral variables (errors and reaction times recorded during the fMRI sessions) yielded the following. Errors were positively correlated with signal changes in areas activated during early learning (i.e., bilateral pre-SMA, lateral premotor, dorsal putamen, and the right intraparietal sulcus and lobule VI of the cerebellum at P < 0.001; bilateral STN and red nuclei, as well as the lobule V and VI of the left cerebellum, at P < 0.01, Fig. 2 A). By contrast, reaction times were negatively correlated with signal changes in areas activated during late learning stages (i.e., contralateral SMA and posteroventral pallidum, ipsilateral lobules III and IV of the cerebellum, red nucleus, and hippocampus, as well as bilateral amygdala and orbitomedial frontal cortex at P < 0.001, contralateral inferior putamen and red nucleus, and ipsilateral substantia nigra at P < 0.01, Fig. 2B).
Fig. 2.
Multiple regression analysis between signal variation across all runs of all MR sessions and behavioral variables (errors and reaction times). Activation maps are superimposed to a T1-weighted (Upper) or an EPI (Lower) template. Shown are whole brain (A) (P < 0.001) and basal ganglia (B)[P < 0.01; two axial views (Left) and two coronal views (Right)] analysis showing areas in which signal changes positively correlated with the number of errors (orange scale) and negatively correlated with reaction times (blue scale). Errors were positively correlated with signal changes in areas activated during early learning. Reaction times were negatively correlated with signal changes in areas activated during late learning.
Discussion
This study was designed to identify the dynamic activation changes within the basal ganglia and related motor regions during the extended practice of a sequential known sequence of finger movements. After one month of training, subjects' speed, accuracy, and regularity greatly improved. They were capable of performing the task with a good level of automaticity (little interference when performing a secondary task) and without any changes in basic movement parameters (frequency, amplitude, and the amount of force required to press the keys was minimal).
The main findings of this study were that two distinct sets of regions were activated in the basal ganglia and thalamus. The associative/premotor territories of the basal ganglia, including the dorsal parts of the putamen and more rostral striatal areas, the anterodorsal globus pallidus, the corresponding output nuclei of the thalamus, as well as the STN, were active only during the early learning stage. In contrast, activations in the sensorimotor territory of the putamen and globus pallidus (i.e., posteroventral regions) increased with practice. This increase was observed early during training (after the first 10 min) and reached a plateau after 2 weeks. The results suggest that there was a dynamic shift of activation from the associative/premotor to the sensorimotor territories of the striato-pallidal complex during the course of sequence learning. These two striato-pallidal territories closely corresponded to the anterior premotor and sensorimotor territories described in humans by using diffusion tensor imaging (23). Most of the activation changes were observed during the fast, within-session learning stage (14, 24), which corresponded to the first hour of training, whereas fewer activation changes were observed during the following weeks of training. Some degree of learning also occurred during the five speed tests (2.5 min) pre-MRI training session as shown by the increase in tapping frequency between the first and fifth tests.
Such a functional dissociation between the two territories is consistent with previous work in animals and humans. In monkeys, the learning of new sequences was impaired after injections of muscimol (a GABA agonist) in the associative but not the sensorimotor striatum, whereas the execution of well learned sequences was much more impaired after injections into the sensorimotor than the associative striatum (13). Similarly, recordings studies in the monkey's striatum showed that the associative and sensorimotor regions of the striatum contribute preferentially to the early and late stages of procedural learning, respectively (25). In rats, widely distributed changes in neuronal activity patterns have also been observed in the sensorimotor striatum during the course of learning (26, 27), suggesting that the long-term representation of actions is built up through dynamic reorganization of neuronal activity in this territory. Finally, previous studies in humans have also reported activations within the associative striatum during the early stage of motor sequence learning (2, 3, 19). This activity was probably related to the use of cognitive strategies and working memory, as suggested by other studies in which such activation was also observed (2, 28). By contrast, an increase in striatal activation has been reported in other studies when subjects reach asymptotic performance of sequential movements (4, 6, 7) or during a visually tracking task (29), thus suggesting that the striatum is involved in the long-term storage of motor actions.
The STN was recruited during the early learning stage, but then activation decreased with practice. The STN plays an important integrative role in information processing in the indirect basal ganglia circuit (30-32). A leading hypothesis of the function of the indirect pathway is that it is involved in the inhibition of competing motor programs (32-34). According to this hypothesis, the STN could act by inhibiting competing finger movements, thereby contributing to building a precise sequence of temporally ordered inhibition and activation of motor programs.
Activation was observed in the SN during the entire course of motor learning. There were also dynamic signal changes in the amygdala and the orbitomedial frontal cortex (OMFC), which are known to be involved in reward-based learning (35, 36). Our results are thus consistent with models of basal ganglia function that propose that basal ganglia neurons integrate cortical signals with reward error signals carried by dopamine neurons during learning to build learned behaviors (26, 27, 37).
During the fast learning stage (within the first MRI session), there was additional activation in the rostral premotor and prefrontal areas as well as the left anterior cingulate cortex (ACC), which decreased with practice. Numerous studies have shown the contribution of rostral premotor and prefrontal areas during the early stage of explicit learning of motor procedures, including serial reaction time tasks (4, 7), learning with trial and errors (2, 3, 18, 19), sequential finger movement (8), and visuomotor association tasks (12, 38). Anatomically, these regions are connected to the associative territory of the striatum, which projects back to the frontal cortex through the ventral anterior/ventral lateral nuclei of the thalamus (11, 39). This finding indicates that, in agreement with previous models of motor skill learning (14, 15), the entire associative/anterior premotor cortico-basal ganglia circuit is activated during the early stages of explicit learning. This model suggests that this circuit contains a spatial representation of the sequence, which requires spatial attention and working memory (14, 15).
Correlation analyses showed dissociation between regions in which signal changes correlated with the number of errors during sequence execution (reflecting movement accuracy) and the reaction times (reflecting movement speed). These results are consistent with previous studies, which demonstrated that the accuracy and speed of movement may be acquired and retained in different brain regions (15, 40). Areas contributing to new learning, such as the pre-SMA (18, 38, 41, 42) and the associative striatum (2, 13), would be related to the accuracy of motor memory. By contrast, areas contributing to the execution of well learned sequences, such as the SMA (4, 6, 42) and the posterior striatum (13) (4), would mediate speed in motor memory. According to this model, motor skills would be stored in the sensorimotor cortico-basal ganglia circuit, which supports a speedy performance (14, 15). The present findings are consistent with such a view and thus suggest that multiple neural systems are necessary for motor skill learning, each contributing to a different aspect of learning (18, 43-45).
Bilateral activations in lobules V and VI of the cerebellum as well as in the pons and the left DN were observed during the first session, but then decreased over a month of practice, as previously reported (4, 46, 47). Furthermore, activation in lobule VI was proportional to performance accuracy, thus suggesting that the lateral cerebellum, in particular, is critical for building up an accurate motor sequential routine. An additional increase in signal was observed in the right DN, but, as reported previously using other sequence (4) and motor adaptation paradigms (29, 48), this increase was transient (between 10 and 20 min of practice) and returned to pretraining levels, suggesting that a time-dependent transfer of activation occurred during the course of learning between the cortex and the deep nuclei.
Contrary to the modulation described above, activation in the left superomedial and anterior cerebellum (lobules III and IV) remained stable across the entire month of training when the T-sequence and rest conditions were compared. Although, the activity within these regions was proportional to the subjects' speed of performance on the task, it did not increase with learning. Consequently, we propose instead that these regions are part of the neuronal system engaged in the motoric execution of the sequence of finger movements, and probably not in its learning per se. This hypothesis is in contrast to previous studies that used a motor adaptation task and reported increased activation in the cerebellum (4, 46, 47). Overall, such findings are in agreement with the model of motor skill learning proposed by Doyon et al. (4), who have suggested that the long-lasting retention of the skill is believed to involve representational changes in the striatum and associated motor cortical regions, whereas the cerebellum would be more engaged in motor adaptation skills (43).
Conclusion
This study reveals the dynamic changes in activation pattern from the associative/anterior premotor to the sensorimotor territories of the basal ganglia during the acquisition of the long-term representation of a sequence of finger movements. The associative cortico-basal ganglia circuit is thus believed to be engaged at the beginning of learning and to contribute to the acquisition of an accurate representation of the sequence, whereas the sensorimotor circuit is thought to maintain a speedy representation of that skill when it is well learned and has become automatic. These results, together with the modulation of activity with the cerebellum and motor-related structures, extend the model of skill learning (43) of Doyon et al. (4), as they demonstrate that the plasticity associated with the long-term representation of a motor sequence takes place within the sensorimotor territory of the basal ganglia.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health Grants BTRR P41-RR08079 and RO1 EB000331, the Institut Fédératif de Recherche 49, and by grants from the Keck Foundation and the Mental Illness and Neuroscience Discovery Institute.
Author contributions: S.L., H.B., K.U., and J.D. designed research; S.L. performed research; S.L., H.B., P.-F.V.d.M., M.P.-I., and T.W. analyzed data; and S.L., H.B., and J.D. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: DN, dentate nucleus; MC, Montreal Neurological Institute coordinate; SMA, supplementary motor area; SN, substantia nigra; STN, subthalamic nucleus; fMRI, functional MRI; EPI, echo-planar imaging; RM-ANOVA, repeated-measure ANOVA; U-sequence, untrained sequence; T-sequence, trained sequence.
Data deposition: The neuroimaging data have been deposited with the fMRI Data Center, www.fmridc.org (accession no. 2-2005-119 KH).
References
- 1.Graybiel, A. M. (1995) Curr. Opin. Neurobiol. 5, 733-741. [DOI] [PubMed] [Google Scholar]
- 2.Jueptner, M., Frith, C. D., Brooks, D. J., Frackowiak, R. S. & Passingham, R. E. (1997) J. Neurophysiol. 77, 1325-1337. [DOI] [PubMed] [Google Scholar]
- 3.Toni, I., Krams, M., Turner, R. & Passingham, R. E. (1998) NeuroImage 8, 50-61. [DOI] [PubMed] [Google Scholar]
- 4.Doyon, J., Song, A. W., Karni, A., Lalonde, F., Adams, M. M. & Ungerleider, L. G. (2002) Proc. Natl. Acad. Sci. USA 99, 1017-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deiber, M. P., Wise, S. P., Honda, M., Catalan, M. J., Grafman, J. & Hallett, M. (1997) J. Neurophysiol. 78, 977-991. [DOI] [PubMed] [Google Scholar]
- 6.Grafton, S. T., Mazziotta, J. C., Presty, S., Friston, K. J., Frackowiak, R. S. & Phelps, M. E. (1992) J. Neurosci. 12, 2542-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hazeltine, E., Grafton, S. T. & Ivry, R. (1997) Brain 120, 123-140. [DOI] [PubMed] [Google Scholar]
- 8.Wu, T., Kansaku, K. & Hallett, M. (2004) J. Neurophysiol. 91, 1690-1698. [DOI] [PubMed] [Google Scholar]
- 9.Jansma, J. M., Ramsey, N. F., Slagter, H. A. & Kahn, R. S. (2001) J. Cognit. Neurosci. 13, 730-743. [DOI] [PubMed] [Google Scholar]
- 10.Alexander, G. E., DeLong, M. R. & Strick, P. L. (1986) Annu. Rev. Neurosci. 9, 357-381. [DOI] [PubMed] [Google Scholar]
- 11.Inase, M., Tokuno, H., Nambu, A., Akazawa, T. & Takada, M. (1999) Brain Res. 833, 191-201. [DOI] [PubMed] [Google Scholar]
- 12.Toni, I., Ramnani, N., Josephs, O., Ashburner, J. & Passingham, R. E. (2001) NeuroImage 14, 1048-1057. [DOI] [PubMed] [Google Scholar]
- 13.Miyachi, S., Hikosaka, O., Miyashita, K., Karadi, Z. & Rand, M. K. (1997) Exp. Brain Res. 115, 1-5. [DOI] [PubMed] [Google Scholar]
- 14.Hikosaka, O., Nakamura, K., Sakai, K. & Nakahara, H. (2002) Curr. Opin. Neurobiol. 12, 217-222. [DOI] [PubMed] [Google Scholar]
- 15.Hikosaka, O., Rand, M. K., Nakamura, K., Miyachi, S., Kitaguchi, K., Sakai, K., Lu, X. & Shimo, Y. (2002) Exp. Brain Res. 147, 494-504. [DOI] [PubMed] [Google Scholar]
- 16.Schultz, W. (2000) Nat. Rev. Neurosci. 1, 199-207. [DOI] [PubMed] [Google Scholar]
- 17.Hollerman, J. R. & Schultz, W. (1998) Nat. Neurosci. 1, 304-309. [DOI] [PubMed] [Google Scholar]
- 18.Jueptner, M., Stephan, K. M., Frith, C. D., Brooks, D. J., Frackowiak, R. S. & Passingham, R. E. (1997) J. Neurophysiol. 77, 1313-1324. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins, I. H., Brooks, D. J., Nixon, P. D., Frackowiak, R. S. & Passingham, R. E. (1994) J. Neurosci. 14, 3775-3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grafton, S. T., Woods, R. P. & Mike, T. (1994) Hum. Brain Mapp. 1, 221-234. [DOI] [PubMed] [Google Scholar]
- 21.Karni, A., Meyer, G., Jezzard, P., Adams, M. M., Turner, R. & Ungerleider, L. G. (1995) Nature 377, 155-158. [DOI] [PubMed] [Google Scholar]
- 22.Schmahmann, J. D., Doyon, J., McDonald, D., Holmes, C., Lavoie, K., Hurwitz, A. S., Kabani, N., Toga, A., Evans, A. & Petrides, M. (1999) NeuroImage 10, 233-260. [DOI] [PubMed] [Google Scholar]
- 23.Lehéricy, S., Ducros, M., Krainik, A., Francois, C., Van de Moortele, P. F., Ugurbil, K. & Kim, D. S. (2004) Cereb. Cortex 14, 1302-1309. [DOI] [PubMed] [Google Scholar]
- 24.Karni, A., Meyer, G., Rey-Hipolito, C., Jezzard, P., Adams, M. M., Turner, R. & Ungerleider, L. G. (1998) Proc. Natl. Acad. Sci. USA 95, 861-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyachi, S., Hikosaka, O. & Lu, X. (2002) Exp. Brain Res. 146, 122-126. [DOI] [PubMed] [Google Scholar]
- 26.Jog, M. S., Kubota, Y., Connolly, C. I., Hillegaart, V. & Graybiel, A. M. (1999) Science 286, 1745-1749. [DOI] [PubMed] [Google Scholar]
- 27.Aosaki, T., Graybiel, A. M. & Kimura, M. (1994) Science 265, 412-415. [DOI] [PubMed] [Google Scholar]
- 28.Dagher, A., Owen, A. M., Boecker, H. & Brooks, D. J. (1999) Brain 122, 1973-1987. [DOI] [PubMed] [Google Scholar]
- 29.Floyer-Lea, A. & Matthews, P. M. (2004) J. Neurophysiol. 92, 2405-2412. [DOI] [PubMed] [Google Scholar]
- 30.Nambu, A., Tokuno, H., Hamada, I., Kita, H., Imanishi, M., Akazawa, T., Ikeuchi, Y. & Hasegawa, N. (2000) J. Neurophysiol. 84, 289-300. [DOI] [PubMed] [Google Scholar]
- 31.DeLong, M. R., Crutcher, M. D. & Georgopoulos, A. P. (1985) J. Neurophysiol. 53, 530-543. [DOI] [PubMed] [Google Scholar]
- 32.Mink, J. W. (1996) Progr. Neurobiol. 50, 381-425. [DOI] [PubMed] [Google Scholar]
- 33.Penney, J. B., Jr., & Young, A. B. (1983) Annu. Rev. Neurosci. 6, 73-94. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell, I. J., Jackson, A., Sambrook, M. A. & Crossman, A. R. (1989) Brain 112, 1533-1548. [DOI] [PubMed] [Google Scholar]
- 35.Kelley, A. E., Andrzejewski, M. E., Baldwin, A. E., Hernandez, P. J. & Pratt, W. E. (2003) Ann. N.Y. Acad. Sci. 1003, 159-168. [DOI] [PubMed] [Google Scholar]
- 36.Tremblay, L. & Schultz, W. (1999) Nature 398, 704-708. [DOI] [PubMed] [Google Scholar]
- 37.Doya, K. (2000) Curr. Opin. Neurobiol. 10, 732-739. [DOI] [PubMed] [Google Scholar]
- 38.Sakai, K., Hikosaka, O., Miyauchi, S., Sasaki, Y., Fujimaki, N. & Putz, B. (1999) J. Neurosci. 19, RC1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Middleton, F. A. & Strick, P. L. (2000) Brain Res. Brain Res. Rev. 31, 236-250. [DOI] [PubMed] [Google Scholar]
- 40.Rand, M. K., Hikosaka, O., Miyachi, S., Lu, X. & Miyashita, K. (1998) Exp. Brain Res. 118, 293-297. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura, K., Sakai, K. & Hikosaka, O. (1999) J. Neurophysiol. 82, 1063-1068. [DOI] [PubMed] [Google Scholar]
- 42.Tanji, J. (2001) Annu. Rev. Neurosci. 24, 631-651. [DOI] [PubMed] [Google Scholar]
- 43.Doyon, J., Penhune, V. & Ungerleider, L. G. (2003) Neuropsychologia 41, 252-262. [DOI] [PubMed] [Google Scholar]
- 44.Shadmehr, R. & Holcomb, H. H. (1997) Science 277, 821-825. [DOI] [PubMed] [Google Scholar]
- 45.Krakauer, J. W., Ghilardi, M. F. & Ghez, C. (1999) Nat. Neurosci. 2, 1026-1031. [DOI] [PubMed] [Google Scholar]
- 46.Imamizu, H., Miyauchi, S., Tamada, T., Sasaki, Y., Takino, R., Putz, B., Yoshioka, T. & Kawato, M. (2000) Nature 403, 192-195. [DOI] [PubMed] [Google Scholar]
- 47.Flament, D., Ellerman, J. M., Kim, S. G., Ugurbil, K. & Ebner, T. J. (1996) Hum. Brain Mapp. 4, 210-226. [DOI] [PubMed] [Google Scholar]
- 48.Nezafat, R., Shadmehr, R. & Holcomb, H. H. (2001) Exp. Brain Res. 140, 66-76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.