Abstract
PURPOSE
Datopotamab deruxtecan (Dato-DXd) is a trophoblast cell-surface antigen-2–directed antibody-drug conjugate with a highly potent topoisomerase I inhibitor payload. The TROPION-Lung05 phase II trial (ClinicalTrials.gov identifier: NCT04484142) evaluated the safety and clinical activity of Dato-DXd in patients with advanced/metastatic non–small cell lung cancer (NSCLC) with actionable genomic alterations progressing on or after targeted therapy and platinum-based chemotherapy.
PATIENTS AND METHODS
Patients received Dato-DXd 6 mg/kg once every 3 weeks. The primary end point was objective response rate (ORR) by blinded independent central review. Secondary end points included duration of response (DOR), safety, tolerability, and survival.
RESULTS
Among 137 patients who received at least 1 dose of Dato-DXd, 71.5% received at least three lines of prior therapies for advanced/metastatic disease. Overall, 56.9% had EGFR mutations and 24.8% had ALK rearrangements. Median treatment duration was 4.4 months (range, 0.7-20.6). The confirmed ORR was 35.8% (95% CI, 27.8 to 44.4) overall, and 43.6% (95% CI, 32.4 to 55.3) and 23.5% (95% CI, 10.7 to 41.2) in those with EGFR mutations and ALK rearrangements, respectively. The median DOR was 7.0 months (95% CI, 4.2 to 9.8), and the overall disease control rate was 78.8% (95% CI, 71.0 to 85.3). Grade ≥3 treatment-related adverse events (TRAEs) occurred in 28.5% of patients. The most common TRAE was stomatitis (preferred term; any grade: 56.2%; grade ≥3: 9.5%). Five (3.6%) patients experienced adjudicated treatment-related interstitial lung disease/pneumonitis, with 1 (0.7%) grade 5 event.
CONCLUSION
Encouraging and durable antitumor activity was observed with Dato-DXd in this heavily pretreated advanced/metastatic NSCLC population with actionable genomic alterations. The rate of treatment-related grade ≥3 toxicities was comparable with previous observations, and no new safety signals were observed.
INTRODUCTION
Lung cancer is the leading cause of cancer mortality worldwide.1,2 Non–small cell lung cancer (NSCLC) accounts for approximately 85% of all patient cases, many of which bear genomic alterations for which standard of care involves targeted therapies.2,3 For patients with advanced/metastatic NSCLC, recommendations for biomarker testing exist for EGFR mutations, ALK translocations, and other actionable genomic alterations.4 Approximately one third of patients with NSCLC harbor EGFR mutations, whereas 5% harbor ALK rearrangements.5,6
CONTEXT
Key Objective
A considerable unmet need remains for patients with non–small cell lung cancer (NSCLC) and actionable genomic alterations who progress on front-line targeted therapies. This phase II study evaluated datopotamab deruxtecan (Dato-DXd) monotherapy in patients with pretreated advanced/metastatic NSCLC and actionable genomic alterations.
Knowledge Generated
Dato-DXd is clinically active in heavily pretreated patients with NSCLC and actionable genomic alterations, with objective response rates of 36% observed for the overall population, 44% in patients with EGFR mutations (including four complete responders), and 24% in patients with ALK rearrangements. The safety profile was manageable, with grade ≥3 treatment-related adverse events seen in 29% of patients.
Relevance (T.E. Stinchcombe)
Based upon these results, a phase III trial of Dato-DXd compared to platinum-based chemotherapy in patients with actionable genomic alterations with disease progression on targeted therapies is warranted. Studies investigating combination therapy are ongoing.*
*Relevance section written by JCO Associate Editor Thomas E. Stinchcombe, MD.
First-line and second-line treatment approaches for NSCLC with actionable genomic alterations consist mostly of targeted treatment with tyrosine kinase inhibitors (TKIs).2,4 Targeted therapies have significantly improved outcomes compared with conventional chemotherapy; however, resistance eventually develops, and subsequent treatment options remain limited.2 There remains an unmet need for novel therapies to treat patients with NSCLC with actionable genomic alterations in later-line settings.
Trophoblast cell-surface antigen-2 (TROP2) is broadly expressed in various tumor types, including NSCLC.7-9 Datopotamab deruxtecan (Dato-DXd) is an antibody-drug conjugate (ADC) consisting of a humanized anti-TROP2 monoclonal antibody covalently linked to a highly potent topoisomerase I inhibitor payload using a plasma-stable, tumor-selective, tetrapeptide-based cleavable linker.10 In the first-in-human TROPION-PanTumor01 study, Dato-DXd showed promising efficacy and manageable safety in patients with NSCLC, including those with actionable genomic alterations.11 In this study, we describe the efficacy and safety of Dato-DXd in patients with pretreated advanced/metastatic NSCLC with actionable genomic alterations enrolled in the phase II TROPION-Lung05 study.
PATIENTS AND METHODS
Study Design
TROPION-Lung05 (ClinicalTrials.gov identifier: NCT04484142) is a global, phase II, single-arm, open-label study of Dato-DXd in patients with advanced or metastatic NSCLC with actionable genomic alterations who progressed on or after targeted therapy and platinum-based chemotherapy. The study design is shown in Data Supplement (Fig S1, online only).
Patients
Adults were eligible if they had pathologically documented advanced or metastatic NSCLC and any of the following actionable genomic alterations: EGFR, ALK, ROS1, NTRK, BRAF, MET exon 14 skipping, or RET. Patients were required to have received prior treatment with 1-2 cytotoxic therapies (including 1 platinum-containing regimen) in the metastatic setting and 1-2 targeted therapies specific for the actionable genomic alteration harbored. Documentation of radiographic disease progression while on or after receiving the most recent treatment of advanced/metastatic NSCLC, measurable disease based on local imaging assessment using RECIST v1.1, and Eastern Cooperative Oncology Group performance status of 0 or 1 were required.
Exclusion criteria included prior treatment with topoisomerase I-targeted chemotherapeutic agent or TROP2-directed therapy, clinically active CNS metastases (untreated and symptomatic, or requiring therapy with corticosteroids or anticonvulsants to control symptoms), history of interstitial lung disease (ILD)/pneumonitis which required steroids, current ILD/pneumonitis, or suspected ILD/pneumonitis. Patients with KRAS mutations were excluded as no targeted therapy was approved for this group at trial initiation. A full list of inclusion and exclusion criteria is provided in the Data Supplement.
Study Treatment
Dato-DXd 6.0 mg/kg was administered intravenously once every 3 weeks on day 1 of each 21-day cycle. The initial dose was infused over 90 minutes and subsequent doses infused over 30 minutes if no infusion-related reactions (IRRs) occurred. Patients who experienced clinical benefit continued to receive Dato-DXd until the first of investigator-assessed radiographic or clinical disease progression, death, pregnancy, withdrawal of consent, loss to follow-up, unacceptable toxicity, or study termination. Study duration was defined as the interval between the first dose and data cutoff, and treatment duration was defined as the interval between the first and last dose.
End Points and Assessments
The primary efficacy end point was objective response rate (ORR) assessed by blinded independent central review, according to RECIST v1.1.12 Secondary end points included evaluation of duration of response (DOR), disease control rate (DCR), clinical benefit rate, progression-free survival (PFS), time to response, overall survival (OS), and safety. Definitions of tumor response end points are included in the Data Supplement.
Adverse events (AEs) were coded using the Medical Dictionary for Regulatory Activities, graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v5.0, and monitored continuously from the time the patient signs the informed consent form until 35 days after the last dose.
Adverse events of special interest (AESIs) for Dato-DXd were oral mucositis/stomatitis, ocular surface events, treatment-related ILD/pneumonitis, mucosal inflammation other than oral mucositis/stomatitis, and IRRs—each a grouped term consisting of any treatment-emergent event coded from a list of Medical Dictionary for Regulatory Activities preferred terms. An independent adjudication committee reviewed all investigator-reported patient cases of potential ILD/pneumonitis; further details are given in the Data Supplement. On the basis of previous clinical evaluations of Dato-DXd, specific guidelines for prophylaxis and management of AESIs were implemented and revised during this study.13
Statistical Analysis
The data cutoff date for the primary analysis (ie, when all patients had ≥9 months of follow-up after treatment initiation or had discontinued from the study, whichever occurred first) was December 14, 2022. The primary end point of ORR was summarized with exact two-sided CIs estimated using the Clopper-Pearson method. DOR, PFS, and OS were summarized and presented graphically using the Kaplan-Meier method; median event time with two-sided 95% CI, estimated using the Brookmeyer and Crowley methods, is presented. Descriptive statistics were used to summarize trial results. Statistical analyses were performed using SAS version 9.4. Sample size determination is described in the Data Supplement.
Biomarker Analysis
Tumor biopsies collected within 3 months of screening were evaluated centrally for tumor cell membrane TROP2 expression by immunohistochemistry using the monoclonal antibody EPR20043. Descriptive statistics of immunohistochemistry scoring (H-score) were summarized by best overall response categories and stratified according to exploratory descriptive categories (H-score: low, 0-100; moderate, 101-200; high, 201-300). Genomic alterations were locally confirmed. Further details are provided in the Data Supplement.
Trial Oversight
The study was approved by the institutional review board at each participating site and conducted in compliance with the ethical principles of the Declaration of Helsinki; the International Council for Harmonisation Consolidated Guideline E6 for Good Clinical Practice; and applicable US, European, and Japanese regulatory requirements.
RESULTS
Patients
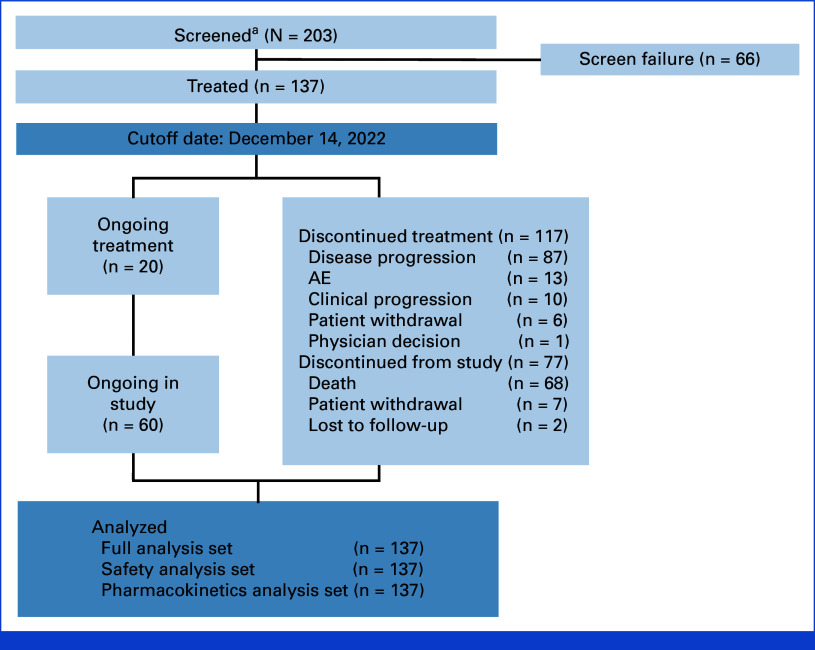
Between March 29, 2021, and December 14, 2022, 137 patients were treated with Dato-DXd (Fig 1). The median study duration was 15.2 months (range, 9.1-20.5), and the median treatment duration was 4.4 months (range, 0.7-20.6). At the data cutoff, 20 patients remained on treatment and 60 were ongoing in the study. The most common reason for treatment discontinuation was disease progression (70.8%; including progressive disease and clinical progression).
FIG 1.
Patient disposition. aPatients who signed the informed consent form and who were screened for eligibility. AE, adverse event.
Baseline demographics and disease characteristics are summarized in Table 1. Median age was 61 years (range, 29-79). More than half of patients (56.9%) had EGFR mutations; 49.6% of whom had exon 19 deletions, L858R mutations, and/or T790M mutations. ALK rearrangements occurred in 24.8% of patients. Patients were heavily pretreated, with 71.5% having received three or more lines of prior therapies for advanced/metastatic disease. In line with inclusion criteria, all patients received the required previous treatment with platinum-based chemotherapy and actionable genomic alteration–specific targeted therapy. In addition, 99.3% of patients had received other types of chemotherapy agents and 35.8% of patients had received prior immunotherapy. Overall, 51.1% had a history of brain metastases. A higher proportion of patients with ALK rearrangements had stage IVB disease at study entry compared with patients with EGFR mutations (76.5% v 62.8%) and a history of liver metastases (35.3% v 20.5%); patients with ALK rearrangements had also received more prior therapies in the advanced/metastatic setting (median [range], 4 [2-9] v 3 [1-5]).
TABLE 1.
Baseline Demographics and Disease Characteristics
| Patient Characteristic | N = 137 |
|---|---|
| Age, years, median (range) | 61 (29-79) |
| Female, No. (%) | 83 (60.6) |
| Race,a No. (%) | |
| White | 43 (31.4) |
| Asian | 78 (56.9) |
| Black or African American | 0 |
| Otherb | 15 (10.9) |
| ECOG PS, No. (%) | |
| 0 | 45 (32.8) |
| 1 | 92 (67.2) |
| Smoking history, No. (%) | |
| Never | 76 (55.5) |
| Former | 61 (44.5) |
| Current | 0 |
| Histology, No. (%) | |
| Adenocarcinoma | 130 (94.9) |
| Squamous | 3 (2.2) |
| Stage at study entry, No. (%) | |
| IIICc | 1 (0.7) |
| IVd | 20 (14.6) |
| IVA | 25 (18.2) |
| IVB | 91 (66.4) |
| History of metastases, No. (%) | |
| Bone | 78 (56.9) |
| Brain | 70 (51.1) |
| Liver | 34 (24.8) |
| Summary of mutation types,e No. (%) | |
| EGFR | 78 (56.9) |
| Exon 19 deletion | 41 (29.9) |
| Exon 20 T790M | 26 (19.0) |
| Exon 21 L858R | 25 (18.2) |
| Exon 18 G719 | 5 (3.6) |
| Exon 21 L861Q | 3 (2.2) |
| Exon 20 insertion | 2 (1.5) |
| ALK rearrangement | 34 (24.8) |
| ROS1 rearrangement | 10 (7.3) |
| RET rearrangement | 8 (5.8) |
| MET exon 14 skipping | 5 (3.6) |
| BRAF mutation | 4 (2.9) |
| MET amplificationf | 3 (2.2) |
| Prior cytotoxic systemic therapy,g No. (%) | |
| Platinum-based chemotherapy | 137 (100) |
| Other chemotherapy | 136 (99.3) |
| Anti–PD-1/anti–PD-L1 immunotherapy | 49 (35.8) |
| Other | 47 (34.8) |
| Prior systemic therapies for advanced/metastatic disease | |
| Median (range) | 3 (1-9) |
| 1-2, No. (%) | 39 (28.5) |
| ≥3, No. (%) | 98 (71.5) |
| Prior targeted therapy for advanced/metastatic disease | |
| Specific for the actionable genomic alteration harbored,h No. (%) |
137 (100) |
| Median (range) | 2 (1-6) |
| EGFR TKI, No. (%) | 89 (65.0) |
| Osimertinib | 61 (44.5) |
| Gefitinib | 19 (13.9) |
| Afatinib | 17 (12.4) |
| ALK inhibitor, No. (%) | |
| Alectinib | 31 (22.6) |
| Lorlatinib | 28 (20.4) |
| Crizotinib | 22 (16.1) |
| Prior radiation therapy, No. (%) | 80 (58.4) |
| Post-treatment systemic therapies for advanced/metastatic disease, No. (%) |
|
| Platinum-based chemotherapy | 14 (10.2) |
| Other chemotherapy | 41 (29.9) |
| Anti–PD-1/anti–PD-L1 immunotherapy | 12 (8.8) |
| Targeted therapy | 56 (40.9) |
Abbreviations: ALK, anaplastic lymphoma kinase; ECOG PS, Eastern Cooperative Oncology Group Performance Status; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
One patient had missing race data.
“Other” race as specified in the electronic data capture.
One patient had bilateral lung lesions at baseline that were incorrectly reported as stage IIIC by the investigator and should have been changed to IVA disease to align with the protocol.
Patients had nonspecified/not further classified A or B disease.
Samples were analyzed for mutations at diagnosis, study entry, or during the course of disease; the most recent mutations were recorded for patients with samples at multiple timepoints.
Patients with MET amplification also had RET rearrangement or EGFR exon 19 deletion.
Patients may have received more than 1 type of therapy.
Permitted targeted therapies for patients with alterations in ROS1, NTRK, BRAF, MET exon 14 skipping, and RET are listed in the Data Supplement (Table S1).
Efficacy
The efficacy analyses included all 137 patients. The confirmed ORR was 35.8% (95% CI, 27.8 to 44.4), including four complete responses (CR; 2.9%) and 45 partial responses (PR; 32.8%), observed across a range of mutation types (Table 2; Fig 2). The DCR was 78.8% (95% CI, 71.0 to 85.3). Best change from baseline in target lesion size for all patients is shown in Figure 2A. Changes in tumor burden over time for responders and those with stable disease as best response are shown in Figure 2B. Responses were durable, with a median DOR of 7.0 months (95% CI, 4.2 to 9.8). At data cutoff, the median PFS was 5.4 months (95% CI, 4.7 to 7.0; number of patients with events, 99 [72%]; Fig 3A) and median OS was 13.6 months (95% CI, 9.9 to not evaluable [NE]; number of censored patients, 69 [50%]; Fig 3B). Baseline TROP2 tumor membrane expression by immunohistochemistry was moderate to high in most patients, regardless of genomic alteration status, and did not show a significant association with either response or PFS (Data Supplement, Figs S2A and S2B). Similarly, no differences were observed between the subsets of patients with and without EGFR-mutated disease (Data Supplement, Fig S2C).
TABLE 2.
Antitumor Activity Assessed by Blinded Independent Central Review
| Variable | Overall (N = 137) | EGFR Mutations (n = 78) | ALK Rearrangements (n = 34) |
|---|---|---|---|
| Confirmed ORR, No. (%) | 49 (35.8) | 34 (43.6) | 8 (23.5) |
| 95% CIa | 27.8 to 44.4 | 32.4 to 55.3 | 10.7 to 41.2 |
| CR, No. (%) | 4 (2.9) | 4 (5.1) | 0 |
| PR, No. (%) | 45 (32.8) | 30 (38.5) | 8 (23.5) |
| SD, No. (%) | 56 (40.9) | 27 (34.6) | 17 (50.0) |
| PD, No. (%) | 19 (13.9) | 10 (12.8) | 5 (14.7) |
| Non-CR/non-PD, No. (%) | 3 (2.2) | 3 (3.8) | 0 |
| NE for BOR, No. (%) | 10 (7.3) | 4 (5.1) | 4 (11.8) |
| DCR, No. (%) | 108 (78.8) | 64 (82.1) | 25 (73.5) |
| 95% CIa | 71.0 to 85.3 | 71.7 to 89.8 | 55.6 to 87.1 |
| DOR, months, median | 7.0 | 7.0 | 7.0 |
| 95% CIb | 4.2 to 9.8 | 4.2 to 10.2 | 2.8 to 8.4 |
| CBR, No. (%) | 64 (46.7) | 42 (53.8) | 12 (35.3) |
| 95% CIb | 38.1 to 55.4 | 42.2 to 65.2 | 19.7 to 53.5 |
| Time to response, months, median | 1.5 | 1.5 | 1.4 |
| Range | 1.1-11.3 | 1.2-11.3 | 1.1-4.1 |
| PFS, months, medianb | 5.4 | 5.8 | 4.3 |
| 95% CIb | 4.7 to 7.0 | 5.4 to 8.3 | 2.6 to 6.9 |
NOTE. Confirmed responses require at least two determinations of responses ≥4 weeks apart before progression.
Abbreviations: BOR, best overall response; CBR, clinical benefit rate; CR, complete response; DCR, disease control rate; DOR, duration of response; NE, not evaluable; ORR, objective response rate; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
The two-sided 95% CIs are based on the Clopper-Pearson exact binomial method.
The two-sided 95% CIs are computed using the Brookmeyer-Crowley method.
FIG 2.
Antitumor activity of Dato-DXd in the overall cohort. (A) Waterfall plot of best percentage change in the SOD from baseline by BICR (n = 126). Snapshot data cutoff, December 14, 2022. Below the waterfall plot, categories of genomic alterations before treatment with Dato-DXd are identified for each patient. (B) Percentage change from baseline in SOD in target lesions in patients with a confirmed CR/PR (upper panel) or SD (lower panel) by BICR. aExon 19 deletion (Ex19Del) or Exon 21 L858R (Ex21 L858R). bIncludes Exon 18 G719 (Ex18 G719), Exon 19 insertion (Ex19 Ins), Exon 20 insertion (Ex20 Ins), Exon 20 S768I (Ex 20 S768I), Exon 21 L861G (Ex 21 L861G), V323I p.1759M, G724S, and unknown EGFRm. BICR, blinded independent central review; CR, complete response; Dato-DXd, datopotamab deruxtecan; EGFRm, EGFR mutation; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease; SOD, sum of the diameters.
FIG 3.
Kaplan-Meier estimates of survival. (A) PFS by BICR per RECIST v1.1. (B) OS. BICR, blinded independent central review; NE, not evaluable; OS, overall survival; PFS, progression-free survival.
Preplanned subset analyses showed that the confirmed ORR was 43.6% (95% CI, 32.4 to 55.3) in 78 patients with EGFR mutations, including all four complete responders seen on study (Table 2). With regard to the heterogeneity of EGFR mutational status, 80.9% of patients with sensitizing or T790M mutations had previously received osimertinib, and the ORR in this subgroup was 49.1% (95% CI, 35.4 to 62.9). The median DOR for patients with any EGFR mutations was 7.0 months (95% CI, 4.2 to 10.2) and the DCR was 82.1% (95% CI, 71.7 to 89.8). In 34 patients harboring ALK rearrangements, the ORR was 23.5% (95% CI, 10.7 to 41.2), comprising eight patients who achieved a PR, with a median DOR of 7.0 months (95% CI, 2.8 to 8.4) and DCR of 73.5% (95% CI, 55.6 to 87.1).
Median PFS was 5.8 months (95% CI, 5.4 to 8.3) in patients with EGFR mutations and 4.3 months (95% CI, 2.6 to 6.9) in patients with ALK rearrangements (Table 2). Median OS was 18.3 (95% CI, 12.4 to NE) and 9.3 (95% CI, 5.8 to 18.3) months for patients with EGFR mutations and ALK rearrangements, respectively.
Safety
All 137 patients were included in the safety population. Overall, 129 (94.2%) and 39 (28.5%) patients had any grade and grade ≥3 treatment-related adverse events (TRAEs; as assessed by investigator), respectively (Table 3). TRAEs led to dose reductions in 27 (19.7%) patients, dose delays in 29 (21.2%) patients, and discontinuations in seven (5.1%) patients.
TABLE 3.
Safety Summary
| Adverse Event | N = 137, No. (%) | |||
|---|---|---|---|---|
| Any Grade | Grade 3 | Grade 4 | Grade 5 | |
| TRAEs | 129 (94.2) | 38 (27.7) | 1 (0.7) | 0 |
| Dose adjustments because of TRAEs | ||||
| Dose reductions | 27 (19.7) | 10 (7.3) | 0 | 0 |
| Dose delay | 29 (21.2) | 11 (8.0) | 0 | 0 |
| Treatment discontinuation | 7 (5.1) | 1 (0.7) | 1 (0.7) | 0 |
| Serious TRAEs | 11 (8.0) | 6 (4.4) | 1 (0.7) | 0 |
| AESIs | ||||
| Oral mucositis/stomatitis | 90 (65.7) | 15 (10.9) | 0 | 0 |
| Mucosal inflammation | 1 (0.7)a | 0 | 0 | 0 |
| Ocular surface events | 36 (26.3) | 3 (2.2) | 0 | 0 |
| Infusion-related reactions | 22 (16.1) | 0 | 0 | 0 |
| Adjudicated ILD/pneumonitis | 5 (3.6) | 0 | 0 | 1 (0.7) |
| Dose adjustments because of AESIs | ||||
| Dose reductions | 17 (12.4) | 6 (4.4) | 0 | 0 |
| Dose delay | 16 (11.7) | 5 (3.6) | 0 | 1 (0.7) |
| Treatment discontinuations | 5 (3.6) | 0 | 0 | 1 (0.7) |
| Serious AESIs | 5 (3.6) | 3 (2.2) | 0 | 1 (0.7) |
| AESI-associated death | 1 (0.7) | 0 | 0 | 1 (0.7) |
Abbreviations: AESI, adverse event of special interest; ILD, interstitial lung disease; TRAE, treatment-related adverse event.
The verbatim term of the mucosal inflammation event was nasal mucositis.
The most common TRAEs were stomatitis (56.2%), nausea (54.7%), and alopecia (49.6%), with most events being grade 1-2 (Table 4). The most common grade ≥3 TRAEs were stomatitis (preferred term; 9.5%), amylase increase (5.1%), anemia (2.9%), nausea (2.2%), and decreased appetite (2.2%). Anemia was the most common hematologic event, with any grade and grade ≥3 TRAEs occurring in 9.5% and 2.9% of patients, respectively. Other hematologic TRAEs (any grade, grade ≥3, respectively) included neutropenia (3.6%, 2.2%), thrombocytopenia (1.5%, 1.5%), and lymphopenia (0.7%, 0.0%). Two AEs associated with death were observed during the study; one was attributed to a serious event of NSCLC (dyspnea) and the other to a serious event of NSCLC disease progression; neither was deemed by the investigator to be related to the study drug.
TABLE 4.
Treatment-Related Adverse Events (≥10%)
| TRAE | N = 137, No. (%) | |||
|---|---|---|---|---|
| Any Grade | Grade 1 | Grade 2 | Grade ≥3 | |
| Stomatitis (PT) | 77 (56.2) | 37 (27.0) | 27 (19.7) | 13 (9.5) |
| Nausea | 75 (54.7) | 44 (32.1) | 28 (20.4) | 3 (2.2) |
| Alopecia | 68 (49.6) | 48 (35.0) | 19 (13.9) | 1 (0.7) |
| Decreased appetite | 28 (20.4) | 11 (8.0) | 14 (10.2) | 3 (2.2) |
| Fatigue | 26 (19.0) | 14 (10.2) | 10 (7.3) | 2 (1.5) |
| Constipation | 21 (15.3) | 16 (11.7) | 5 (3.6) | 0 |
| Rash | 19 (13.9) | 14 (10.2) | 5 (3.6) | 0 |
| Vomiting | 19 (13.9) | 10 (7.3) | 8 (5.8) | 1 (0.7) |
| Asthenia | 15 (10.9) | 8 (5.8) | 5 (3.6) | 2 (1.5) |
Abbreviations: PT, preferred term; TRAE, treatment-related adverse event.
The most common AESI was oral mucositis/stomatitis, with any grade and grade ≥3 events occurring in 90 (65.7%; grade 1: 32.8%; grade 2: 21.9%) and 15 (10.9%) patients, respectively (Table 3). One patient (0.7%) discontinued because of oral mucositis/stomatitis (Data Supplement, Table S2). One patient (0.7%) had a grade 2 event of mucosal inflammation. Of 36 (26.3%) patients who experienced ocular surface events, dry eye (10.9%), blurred vision (8.8%), and keratitis (5.1%) were the most frequent. There were three grade 3 ocular surface events (corneal disorder, cornea verticillate, and punctate keratitis occurred in one patient each). IRRs occurred in 22 (16.1%) patients, all of which were grade 1-2. No patients discontinued treatment because of ocular surface events or IRRs. Adjudicated treatment-related ILD/pneumonitis occurred in five (3.6%) patients (grade 1: n = 1; grade 2: n = 3; grade 5: n = 1), all of which were coded under the preferred term of pneumonitis. Four patients discontinued study treatment per management guidelines because of ILD/pneumonitis. At data cutoff, two had not recovered: the first had an adjudicated grade 2 event and the second had a grade 1 event that progressed to grade 2.
DISCUSSION
Dato-DXd demonstrated encouraging antitumor activity in heavily pretreated patients with advanced/metastatic NSCLC and various actionable genomic alterations. The primary end point of ORR was 35.8% in the overall population, including four patients who achieved a CR. Moreover, responses were durable with a median DOR of 7.0 months, median PFS of 5.4 months, and median OS of 13.6 months. Dato-DXd also exhibited a manageable safety profile, consistent with previously reported data in patients with NSCLC,11 and no new safety signals were identified.
There remains an unmet need for novel treatments of these patients who progress on current standard-of-care therapies. For patients harboring EGFR mutations, osimertinib is the preferred first-line treatment and has demonstrated improved OS compared with first-generation epidermal growth factor receptor (EGFR) TKIs14; however, resistance invariably occurs and disease progression is observed after a median of 19 months.15,16 After progression on osimertinib, treatment options are complex and depend on the nature of the resistance mechanism; a recent study revealed that both EGFR-dependent and independent mechanisms are common, including the emergence of EGFR C797X mutation, MET alterations, small cell lung cancer transformation, and oncogenic fusions.17 In a study looking at postprogression on osimertinib for EGFR-mutated lung adenocarcinoma, the chemotherapy arm demonstrated a median PFS of only 4.2 months.18 TROPION-Lung05 enrolled patients who had also received chemotherapy after progression on osimertinib and demonstrated a relatively favorable median PFS of 5.8 months in this subpopulation.18 However, there are limited data postprogression on separate lines of osimertinib and chemotherapy, complicating efforts at a comparative assessment of efficacy outcomes representative of this study cohort. Two ongoing phase III randomized trials—TROPION-Lung14 (ClinicalTrials.gov identifier: NCT06350097; Dato-DXd + osimertinib v osimertinib as a first-line treatment of advanced/metastatic NSCLC) and TROPION-Lung15 (ClinicalTrials.gov identifier: NCT06417814; Dato-DXd with or without osimertinib v platinum-based doublet chemotherapy in patients with advanced/metastatic NSCLC progressing after up to two prior EGFR TKIs including osimertinib)—will inform the potential role for Dato-DXd in patients with EGFR-mutated disease.
In this study, among heavily pretreated patients in the advanced/metastatic setting, the ORR was 43.6% in patients with EGFR mutations and 49.1% in those with sensitizing or T790M mutations who had previously received osimertinib. Additionally, all four complete responders seen in the study had EGFR mutations. These findings are consistent with prior experience for Dato-DXd in patients with NSCLC and actionable genomic alterations. In the phase I TROPION-PanTumor01 study, the ORR was 44.1% in patients with actionable genomic alterations.11 In the phase III TROPION-Lung01 trial (including patients with and without actionable genomic alterations), ORRs of 37.5% for Dato-DXd and 8.0% for docetaxel were seen in the subgroup of patients with nonsquamous histology and actionable genomic alterations.19 Recently, the results from the phase II ICARUS-Lung01 study evaluating Dato-DXd in patients with previously treated NSCLC reported an ORR of 50% in a subset of 12 patients with EGFR or BRAF mutations.20 Similar results in patients with and without actionable genomic alterations have been seen with other TROP2-directed ADCs.21,22
Similar to EGFR TKIs, third-generation anaplastic lymphoma kinase (ALK) TKIs have also improved upon first-generation therapies and are the preferred treatment option for patients with ALK rearrangements; however, resistance ultimately develops.6 In our study, Dato-DXd monotherapy elicited an ORR of 23.5% in a heavily pretreated population of patients with ALK rearrangements, including eight who achieved a PR. Compared with patients with EGFR mutations, a higher proportion of patients with ALK rearrangements had stage IVB disease, had current or a history of liver metastases, and had received more prior therapies in the advanced/metastatic setting, which may be contributing factors to the lower ORR seen in this patient group.
Although targeting driver mutations has led to the development of effective therapies for patients with NSCLC, the complexity and heterogeneity of acquired resistance4 creates enormous challenges for creating novel drugs that can successfully overcome resistance. Resistance mechanisms to osimertinib and ALK TKIs are varied, involving both EGFR- or ALK-dependent and independent mechanisms, and a considerable proportion of mechanisms are yet to be defined.6,23,24 This represents a substantial need for novel therapeutic approaches beyond specific resistance mechanisms. The broad expression of TROP2 in NSCLC, even after anticancer treatment, provides an opportunity for treatment of resistance post-targeted therapy.8,25 Baseline TROP2 tumor membrane expression by immunohistochemistry was moderate to high, regardless of genomic alteration status, and not associated with response or PFS. Overall, these findings show that Dato-DXd provides a potential therapeutic approach with meaningful activity in heavily pretreated patients with varied actionable genomic alterations, with encouraging PFS, OS, ORR, and DOR.
The safety profile of Dato-DXd was manageable, characterized by a low incidence of hematologic or treatment-related grade ≥3 toxicities, consistent with the earlier phase I clinical experience.11 Oral mucositis/stomatitis was seen at a relatively high frequency in TROPION-Lung05; however, most patient cases were low-grade and resulted in only 1 treatment discontinuation (0.7%). Oral mucositis/stomatitis is an identified risk for Dato-DXd. While grade 1 events may be associated with only mild symptoms, grade 2 events are often associated with considerable discomfort; hence, even low-grade events can be burdensome to patients. Recommendations for prophylaxis and management of oral mucositis/stomatitis were implemented during the study.13
ILD is an important identified risk for deruxtecan-containing ADCs and other ADCs with different payloads.26-30 Grade 1 events are typically asymptomatic, underscoring the need for early detection.31 There were a total of five patients with adjudicated treatment-related ILD/pneumonitis in this study, four of whom discontinued treatment per management guidelines and 1 who died. The overall low incidence, particularly of severe events, of adjudicated treatment-related ILD/pneumonitis is consistent with previous studies on Dato-DXd.13,32 However, the occurrence of some severe events highlights the critical importance of careful monitoring and adherence to ILD/pneumonitis management guidelines established at the beginning of Dato-DXd clinical development.13 These guidelines recommend that Dato-DXd is immediately withheld if ILD/pneumonitis is suspected and permanently withdrawn once confirmed, with the exception of grade 1 events that resolve within 28 days (dose maintained) or between 28 and 84 days (dose reduction).13
Ocular surface events have also been reported for other ADCs that target different molecular tumor-associated antigens.29,30,33,34 Treatment-emergent ocular surface events were seen with Dato-DXd, with the vast majority of events being grade 1 or 2.13 Such events, particularly keratitis, are an important identified risk of Dato-DXd that requires careful monitoring, and management guidelines are in place.13
Currently, trastuzumab deruxtecan is the only approved ADC in NSCLC, indicated for patients with activating HER2 mutations.26 Several other ADCs are undergoing clinical investigation. Sacituzumab govitecan and SKB-264 are two other TROP2-directed ADCs that are currently being evaluated in patients with NSCLC with and without actionable genomic alterations.35-38 Patritumab deruxtecan (human epidermal growth factor receptor 3 [HER3]-DXd) is a HER3-directed ADC being investigated in patients with EGFR-mutated NSCLC; recent findings from the phase II HERTHENA-Lung01 trial (ClinicalTrials.gov identifier: NCT04619004) in patients who had received prior EGFR TKI therapy and platinum-based chemotherapy showed that patients receiving HER3-DXd achieved an ORR of 29.8% and median PFS of 5.5 months.39 A pivotal phase III study (HERTHENA-Lung02; ClinicalTrials.gov identifier: NCT05338970) is in progress.40 Furthermore, the EGFR × HER3 bispecific ADC BL-B01D1 is under phase I investigation in locally advanced/metastatic tumors, and an ORR of 61.8% was observed in patients with EGFR-mutated NSCLC.41
In summary, Dato-DXd elicited promising ORRs, durable responses, and an acceptable safety profile in heavily pretreated patients with advanced NSCLC and actionable genomic alterations, including in the two predominant EGFR-mutation and ALK-rearrangement subgroups. These findings suggest that this novel TROP2-directed ADC may provide clinically meaningful benefit in a difficult-to-treat population with poor prognosis and lack of effective therapies. These results support recent findings from the pivotal TROPION-Lung01 trial (ClinicalTrials.gov identifier: NCT04656652), comparing Dato-DXd with docetaxel in patients with pretreated advanced/metastatic NSCLC, that demonstrated clinically meaningful benefit for patients with nonsquamous NSCLC both with and without actionable genomic alterations.
ACKNOWLEDGMENT
The authors would like to thank the patients, their families, and all investigators involved in this study. Medical writing support was provided by Katie Webster, BSc, and editorial support was provided by Isobel Markham, MSc, and Jess Fawcett, BSc, all of Core (a division of Prime, London, United Kingdom) supported by Daiichi Sankyo, Inc, according to Good Publication Practice guidelines (https://www.acpjournals.org/doi/10.7326/M22-1460). The authors also thank Dr Satoru Kitazono for his invaluable contributions to the conduct of the study while at The Cancer Institute Hospital of JFCR, Tokyo, Japan.
Jacob Sands
Honoraria: Pfizer
Consulting or Advisory Role: AstraZeneca, Medtronic, Daiichi Sankyo/UCB Japan, Sanofi, Boehringer Ingelheim, PharmaMar, Guardant Health, AbbVie, Gilead Sciences, Lilly, G1 Therapeutics
Research Funding: Amgen, Harpoon
Travel, Accommodations, Expenses: AstraZeneca
Myung-Ju Ahn
Honoraria: AstraZeneca, Lilly, MSD, Takeda, Amgen, Merck Serono, Yuhan, Daiichi Sankyo/AstraZeneca
Consulting or Advisory Role: AstraZeneca, Lilly, MSD, Takeda, Alpha Pharmaceutical, Amgen, Merck Serono, Pfizer, Yuhan, Arcus Ventures, Daiichi Sankyo/AstraZeneca
Research Funding: Yuhan
Aaron Lisberg
Employment: Boston Scientific
Stock and Other Ownership Interests: Boston Scientific
Consulting or Advisory Role: AstraZeneca, Leica Biosystems, Bristol Myers Squibb, Novocure, Pfizer, Jazz Pharmaceuticals, MorphoSys, Lilly, Oncocyte, Novartis, Sanofi/Regeneron, Janssen Oncology, Sanofi, G1 Therapeutics, Molecular Axiom, Amgen, Daiichi Sankyo Nordics, Bayer, IQVIA, Gilead Sciences
Research Funding: Daiichi Sankyo, AstraZeneca, Calithera Biosciences, Dracen, WindMIL, Duality Biologics, eFFECTOR Therapeutics
Patents, Royalties, Other Intellectual Property: Pending Patents U.S. Provisional Patent Application No. 63/527,899
Byoung Chul Cho
Employment: Yonsei University Health System
Leadership: J Ints Bio
Stock and Other Ownership Interests: Theravance, Gencurix, Bridgebio, Kanaph Therapeutics, Cyrus Therapeutics, Interpark Bio, J Ints Bio
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Roche, Yuhan, Pfizer, Janssen, Takeda, MSD, Lilly, Medpacto, Blueprint Medicines, Cyrus Therapeutics, Guardant Health, Novartis, CJ Bioscience, Abion, BeiGene, CureLogen, GI Cell, inno.N, Imnewrun, Hanmi, RandBio, Kanaph Therapeutics, Bridgebio, Oscotec, BMS, Ono Pharmaceutical, Onegene Biotechnology, J Ints Bio, Therapex Co, Ltd, Gilead Sciences, Amgen
Research Funding: Novartis, Bayer, AstraZeneca, Dong-A ST, Champions Oncology, Janssen, Yuhan, Ono, Dizal Pharma, MSD, AbbVie, GI Innovation, Blueprint Medicines, Interpark Bio, LG Chem, Oscotec, GI Cell, Abion, Boehringer Ingelheim, CJ Bioscience, CJ Blossom Park, Cyrus Therapeutics, Genexine, Nuvalent, Inc, Oncternal Therapeutics, Regeneron, Bridgebio, ImmuneOncia, Illumina, Kanaph Therapeutics, Therapex, J Ints Bio, Hanmi, CHA Bundang Medical Center, Mogam Biotechnology Research Institue, Lilly, Vertical Bio AG
Patents, Royalties, Other Intellectual Property: Champions Oncology, Crown Bioscience, Imagen, PearlRiver Bio GmbH
Other Relationship: DAAN Biotherapeutics
George Blumenschein
Employment: Janssen, Johnon & Johnson
Stock and Other Ownership Interests: Virogin Biotech
Consulting or Advisory Role: Bristol Myers Squibb, Bayer, Celgene, Clovis Oncology, AbbVie, ARIAD, Merck, Genentech, Novartis, Xcovery, Adicet Bio, Amgen, AstraZeneca, Roche, MedImmune, Maverick Therapeutics, Johnson & Johnson, Virogin Biotech, Gilead Sciences, Daiichi Sankyo, Inc, Tyme, Janssen Oncology, Lilly, Instil Bio, BeiGene, CytomX Therapeutics, InterVenn Biosciences, Onconova Therapeutics, Regeneron, Sanofi, Seagen, Genzyme, Scorpion Therapeutics, Immunocore
Research Funding: Merck, Celgene, Genentech, Xcovery, Novartis, Bristol Myers Squibb, GlaxoSmithKline, Adaptimmune, Macrogenics, Kite, a Gilead company, Immatics, Torque, Incyte, MedImmune, Exelixis, Immunocore, Roche, AstraZeneca, Bayer, Tmunity Therapeutics, Inc, Regeneron, BeiGene, Repertoire Immune Medicines, Daiichi Sankyo Inc, Verastem, Amgen, CytomX Therapeutics, Duality Biologics, Mythic Therapeutics, Takeda, Aulos Bioscience, Nuvalent, Inc, Turning Point Therapeutics, Seagen, Sanofi
Elaine Shum
Consulting or Advisory Role: AstraZeneca, Janssen, Genentech, Blueprint Medicines, Boehringer Ingelheim, Regeneron, Gilead Sciences
Research Funding: Delfi Diagnostics (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Boehringer Ingelheim
Elvire Pons-Tostivint
Consulting or Advisory Role: AstraZeneca, Sanofi Pasteur, Takeda, Daiichi Sankyo/AstraZeneca, Roche, Bristol Myers Squibb Foundation, Janssen
Research Funding: AstraZeneca (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Takeda, Pfizer, Sanofi, Daiichi Sankyo/AstraZeneca
Yasushi Goto
Honoraria: AstraZeneca, Boehringer Ingelheim, Lilly Japan, Chugai Pharma, Taiho Pharmaceutical, Ono Pharmaceutical, MSD, Pfizer, Bristol Myers Squibb Japan, Novartis, Thermo Fisher Scientific, Merck, Guardant Health AMEA, Takeda, Daiichi Sankyo/AstraZeneca, Daiichi Sankyo/UCB Japan, Amgen, Janssen, Sandoz, Nichiiko
Consulting or Advisory Role: Lilly, Boehringer Ingelheim, Taiho Pharmaceutical, Pfizer, Novartis, AstraZeneca, Chugai Pharma, Guardant Health AMEA, Daiichi Sankyo/UCB Japan, Janssen, Ono Pharmaceutical
Research Funding: AbbVie (Inst), Lilly Japan (Inst), Pfizer (Inst), Ono Pharmaceutical (Inst), Daiichi Sankyo (Inst), Bristol Myers Squibb Japan (Inst), Chugai Pharma (Inst), Taiho Pharmaceutical (Inst), MSD (Inst), AstraZeneca (Inst), Novartis (Inst), Merck Serono (Inst), Genomic Health (Inst), CMIC (Inst), Takeda (Inst), EPS Holdings (Inst), IQvia (Inst), Daiichi Sankyo/UCB Japan (Inst), Janssen (Inst), Amgen (Inst), EP Croit Co (Inst), Astellas Amgen BioPharama (Inst), Bayer (Inst), Preferred Network (Inst), Medpace (Inst), Sysmex (Inst)
Uncompensated Relationships: Cancer Net Japan, JAMT
Kiyotaka Yoh
Honoraria: Chugai Pharma, AstraZeneca, Lilly Japan, Bristol Myers Squibb Japan, Takeda, Amgen, Ono Pharmaceutical, MSD, Daiichi Sankyo, Kyowa Kirin
Consulting or Advisory Role: Boehringer Ingelheim, AbbVie
Research Funding: Lilly Japan (Inst), AstraZeneca (Inst), Taiho Pharmaceutical (Inst), Chugai Pharma (Inst), MSD (Inst), Takeda (Inst), Daiichi Sankyo (Inst), AbbVie (Inst), ArriVent Biopharma (Inst), Amgen (Inst), Boehringer Ingelheim (Inst)
Rebecca Heist
Consulting or Advisory Role: Novartis, Daichii Sankyo, AbbVie, Sanofi, Lilly, Regeneron, Claim Therapeutics, AstraZeneca, Merck, Biohaven Pharmaceuticals
Research Funding: AbbVie (Inst), Novartis (Inst), Roche (Inst), Mirati Therapeutics (Inst), Exelixis (Inst), Corvus Pharmaceuticals (Inst), Daiichi Sankyo (Inst), Agios (Inst), Pfizer (Inst), Lilly (Inst), Turning Point Therapeutics (Inst), Erasca, Inc (Inst), Mythic Therapeutics (Inst)
Junichi Shimizu
Consulting or Advisory Role: Daiichi Sankyo/UCB Japan
Speakers' Bureau: Ono Pharmaceutical, MSD Oncology, Novartis, AstraZeneca, Taiho Pharmaceutical, Bristol Myers Squibb, Chugai Pharma, Takeda, Merck, Amgen
Paul Baas
Honoraria: Bristol Myers Squibb, MSD (Inst)
Consulting or Advisory Role: Merck Sharp & Dohme (Inst), Bristol Myers Squibb (Inst)
Research Funding: Bristol Myers Squibb (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme, BMS (Inst)
Other Relationship: Bristol Myers Squibb
David Planchard
Honoraria: Prime Oncology, PeerVoice, Medscape
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Novartis, Roche, Pfizer, MSD Oncology, Celgene, BeiGene, Samsung, AbbVie, Janssen, Daiichi Sankyo/AstraZeneca, Pierre Fabre, Seagen, Gilead Sciences, Anheart Therapeutics, ArriVent Biopharma, Ellipses Pharma
Research Funding: AstraZeneca/MedImmune (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), Lilly (Inst), Merck (Inst), Novartis (Inst), Pfizer (Inst), Roche (Inst), Sanofi/Aventis (Inst), Taiho Pharmaceutical (Inst), Daiichi Sankyo (Inst), AbbVie (Inst), Janssen (Inst), Pierre Fabre (Inst), Seagen (Inst), ArriVent Biopharma (Inst), Ellipses Pharma (Inst)
Maurice Pérol
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Lilly, Roche/Genentech, Pfizer, AstraZeneca, Merck Sharp & Dohme, Bristol Myers Squibb, Novartis, Amgen, Takeda, Sanofi, GlaxoSmithKline, Janssen Oncology, Ipsen, Eisai, Novocure, Daiichi Sankyo, Gilead Sciences
Research Funding: Takeda (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Roche, Bristol Myers Squibb, Merck Sharp & Dohme, Pfizer, Takeda, Chugai Pharma
Enriqueta Felip
Consulting or Advisory Role: AbbVie, Amgen, AstraZeneca, Bayer, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, Roche, Gilead Sciences, GlaxoSmithKline, Janssen, Merck Serono, Merck Sharp & Dohme, Novartis, Peptomyc, Pfizer, Regeneron, Sanofi, Takeda, Genmab
Speakers' Bureau: Amgen, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Lilly, Roche, Genentech, Janssen, Medical Trends, Medscape, Merck Serono, Merck Sharp & Dohme, Peervoice, Pfizer, Sanofi, Takeda, Touch Oncology
Travel, Accommodations, Expenses: AstraZeneca, Janssen, Roche
Other Relationship: Grifols
Uncompensated Relationships: Member of the Scientific Advisory Committee-Hospital Universitari Parc Taulí-, SEOM (Sociedad Española de Oncología Médica), President from 2021 to 2023, “ETOP IBCSG Partners” Member of the Scientific Committee
Wu-Chou Su
Consulting or Advisory Role: Bayer Schering Pharma, MSD Oncology, Lilly, Merck
Hong Zebger-Gong
Employment: Daiichi Sankyo Europe GmbH
Stock and Other Ownership Interests: Bayer, Daiichi Sankyo
Lan Lan
Employment: Daiichi Sankyo Inc
Stock and Other Ownership Interests: Daiichi Sankyo Inc
Travel, Accommodations, Expenses: Daiichi Sankyo Inc
Chelsea Liu
Employment: Daiichi Sankyo Inc
Stock and Other Ownership Interests: Daiichi Sankyo Inc
Paul Howarth
Employment: Daiichi Sankyo
Rachel Chiaverelli
Employment: Daiichi Sankyo/AstraZeneca
Stock and Other Ownership Interests: Daiichi Sankyo/AstraZeneca
Luis Paz-Ares
Leadership: Altum Sequencing, Stab Therapeutics
Stock and Other Ownership Interests: Altum Sequencing, Stab Therapeutics
Honoraria: Roche/Genentech, Lilly, Pfizer, Bristol Myers Squibb, MSD, AstraZeneca, Merck Serono, PharmaMar, Novartis, Amgen, Sanofi, Bayer, Takeda, Mirati Therapeutics, Daiichi Sankyo, BeiGene, GlaxoSmithKline, Janssen, Medscape, Regeneron, Boehringer Ingelheim
Consulting or Advisory Role: Lilly, MSD, Roche, Pharmamar, Merck, AstraZeneca, Novartis, Amgen, Pfizer, Sanofi, Bayer, BMS, Mirati Therapeutics, GlaxoSmithKline, Janssen, Takeda, Regeneron, AbbVie
Speakers' Bureau: MSD Oncology, BMS, Roche/Genentech, Pfizer, Lilly, AstraZeneca, Merck Serono
Research Funding: BMS (Inst), AstraZeneca (Inst), PharmaMar (Inst), MSD (Inst), Pfizer (Inst)
Other Relationship: Novartis, Ipsen, Pfizer, Servier, Sanofi, Roche, Amgen, Merck
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the European Society for Medical Oncology, Madrid, Spain, October 20-24, 2023.
SUPPORT
Supported by Daiichi Sankyo, Inc. In July 2020, AstraZeneca entered into a global development and commercialization collaboration agreement with Daiichi Sankyo, Inc for Dato-DXd. The study was designed by the funder in collaboration with the study investigators.
CLINICAL TRIAL INFORMATION
NCT04484142 (TROPION-Lung05)
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO-24-01349. Deidentified individual participant data and applicable supporting clinical trial documents may be available on request at Vivli-Center for Global Clinical Research Data. In cases where trial data and supporting documents are provided pursuant to our company policies and procedures, Daiichi Sankyo, Inc will continue to protect the privacy of our clinical trial participants. Details on data sharing criteria and the procedure for requesting access can be found online at https://vivli.org/ourmember/daiichi-sankyo/.
AUTHOR CONTRIBUTIONS
Conception and design: Jacob Sands, Aaron Lisberg, Byoung Chul Cho, David Planchard, Hong Zebger-Gong, Lan Lan, Chelsea Liu, Luis Paz-Ares
Provision of study materials or patients: Myung-Ju Ahn, Aaron Lisberg, Elaine Shum, Kiyotaka Yoh, Rebecca Heist, Junichi Shimizu, Paul Baas, Enriqueta Felip, Wu-Chou Su, Hong Zebger-Gong, Luis Paz-Ares
Collection and assembly of data: Jacob Sands, Aaron Lisberg, Byoung Chul Cho, Elaine Shum, Rebecca Heist, Jong-Seok Lee, Paul Baas, David Planchard, Maurice Pérol, Enriqueta Felip, Wu-Chou Su, Hong Zebger-Gong, Lan Lan, Chelsea Liu, Paul Howarth, Luis Paz-Ares
Data analysis and interpretation: Myung-Ju Ahn, Byoung Chul Cho, Elvire Pons Tostivint, Yasushi Goto, Kiyotaka Yoh, Rebecca Heist, Junichi Shimizu, David Planchard, Maurice Pérol, Enriqueta Felip, Hong Zebger-Gong, Lan Lan, Chelsea Liu, Paul Howarth, Rachel Chiaverelli
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Datopotamab Deruxtecan in Advanced or Metastatic Non–Small Cell Lung Cancer With Actionable Genomic Alterations: Results From the Phase II TROPION-Lung05 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jacob Sands
Honoraria: Pfizer
Consulting or Advisory Role: AstraZeneca, Medtronic, Daiichi Sankyo/UCB Japan, Sanofi, Boehringer Ingelheim, PharmaMar, Guardant Health, AbbVie, Gilead Sciences, Lilly, G1 Therapeutics
Research Funding: Amgen, Harpoon
Travel, Accommodations, Expenses: AstraZeneca
Myung-Ju Ahn
Honoraria: AstraZeneca, Lilly, MSD, Takeda, Amgen, Merck Serono, Yuhan, Daiichi Sankyo/AstraZeneca
Consulting or Advisory Role: AstraZeneca, Lilly, MSD, Takeda, Alpha Pharmaceutical, Amgen, Merck Serono, Pfizer, Yuhan, Arcus Ventures, Daiichi Sankyo/AstraZeneca
Research Funding: Yuhan
Aaron Lisberg
Employment: Boston Scientific
Stock and Other Ownership Interests: Boston Scientific
Consulting or Advisory Role: AstraZeneca, Leica Biosystems, Bristol Myers Squibb, Novocure, Pfizer, Jazz Pharmaceuticals, MorphoSys, Lilly, Oncocyte, Novartis, Sanofi/Regeneron, Janssen Oncology, Sanofi, G1 Therapeutics, Molecular Axiom, Amgen, Daiichi Sankyo Nordics, Bayer, IQVIA, Gilead Sciences
Research Funding: Daiichi Sankyo, AstraZeneca, Calithera Biosciences, Dracen, WindMIL, Duality Biologics, eFFECTOR Therapeutics
Patents, Royalties, Other Intellectual Property: Pending Patents U.S. Provisional Patent Application No. 63/527,899
Byoung Chul Cho
Employment: Yonsei University Health System
Leadership: J Ints Bio
Stock and Other Ownership Interests: Theravance, Gencurix, Bridgebio, Kanaph Therapeutics, Cyrus Therapeutics, Interpark Bio, J Ints Bio
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Roche, Yuhan, Pfizer, Janssen, Takeda, MSD, Lilly, Medpacto, Blueprint Medicines, Cyrus Therapeutics, Guardant Health, Novartis, CJ Bioscience, Abion, BeiGene, CureLogen, GI Cell, inno.N, Imnewrun, Hanmi, RandBio, Kanaph Therapeutics, Bridgebio, Oscotec, BMS, Ono Pharmaceutical, Onegene Biotechnology, J Ints Bio, Therapex Co, Ltd, Gilead Sciences, Amgen
Research Funding: Novartis, Bayer, AstraZeneca, Dong-A ST, Champions Oncology, Janssen, Yuhan, Ono, Dizal Pharma, MSD, AbbVie, GI Innovation, Blueprint Medicines, Interpark Bio, LG Chem, Oscotec, GI Cell, Abion, Boehringer Ingelheim, CJ Bioscience, CJ Blossom Park, Cyrus Therapeutics, Genexine, Nuvalent, Inc, Oncternal Therapeutics, Regeneron, Bridgebio, ImmuneOncia, Illumina, Kanaph Therapeutics, Therapex, J Ints Bio, Hanmi, CHA Bundang Medical Center, Mogam Biotechnology Research Institue, Lilly, Vertical Bio AG
Patents, Royalties, Other Intellectual Property: Champions Oncology, Crown Bioscience, Imagen, PearlRiver Bio GmbH
Other Relationship: DAAN Biotherapeutics
George Blumenschein
Employment: Janssen, Johnon & Johnson
Stock and Other Ownership Interests: Virogin Biotech
Consulting or Advisory Role: Bristol Myers Squibb, Bayer, Celgene, Clovis Oncology, AbbVie, ARIAD, Merck, Genentech, Novartis, Xcovery, Adicet Bio, Amgen, AstraZeneca, Roche, MedImmune, Maverick Therapeutics, Johnson & Johnson, Virogin Biotech, Gilead Sciences, Daiichi Sankyo, Inc, Tyme, Janssen Oncology, Lilly, Instil Bio, BeiGene, CytomX Therapeutics, InterVenn Biosciences, Onconova Therapeutics, Regeneron, Sanofi, Seagen, Genzyme, Scorpion Therapeutics, Immunocore
Research Funding: Merck, Celgene, Genentech, Xcovery, Novartis, Bristol Myers Squibb, GlaxoSmithKline, Adaptimmune, Macrogenics, Kite, a Gilead company, Immatics, Torque, Incyte, MedImmune, Exelixis, Immunocore, Roche, AstraZeneca, Bayer, Tmunity Therapeutics, Inc, Regeneron, BeiGene, Repertoire Immune Medicines, Daiichi Sankyo Inc, Verastem, Amgen, CytomX Therapeutics, Duality Biologics, Mythic Therapeutics, Takeda, Aulos Bioscience, Nuvalent, Inc, Turning Point Therapeutics, Seagen, Sanofi
Elaine Shum
Consulting or Advisory Role: AstraZeneca, Janssen, Genentech, Blueprint Medicines, Boehringer Ingelheim, Regeneron, Gilead Sciences
Research Funding: Delfi Diagnostics (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Boehringer Ingelheim
Elvire Pons-Tostivint
Consulting or Advisory Role: AstraZeneca, Sanofi Pasteur, Takeda, Daiichi Sankyo/AstraZeneca, Roche, Bristol Myers Squibb Foundation, Janssen
Research Funding: AstraZeneca (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Takeda, Pfizer, Sanofi, Daiichi Sankyo/AstraZeneca
Yasushi Goto
Honoraria: AstraZeneca, Boehringer Ingelheim, Lilly Japan, Chugai Pharma, Taiho Pharmaceutical, Ono Pharmaceutical, MSD, Pfizer, Bristol Myers Squibb Japan, Novartis, Thermo Fisher Scientific, Merck, Guardant Health AMEA, Takeda, Daiichi Sankyo/AstraZeneca, Daiichi Sankyo/UCB Japan, Amgen, Janssen, Sandoz, Nichiiko
Consulting or Advisory Role: Lilly, Boehringer Ingelheim, Taiho Pharmaceutical, Pfizer, Novartis, AstraZeneca, Chugai Pharma, Guardant Health AMEA, Daiichi Sankyo/UCB Japan, Janssen, Ono Pharmaceutical
Research Funding: AbbVie (Inst), Lilly Japan (Inst), Pfizer (Inst), Ono Pharmaceutical (Inst), Daiichi Sankyo (Inst), Bristol Myers Squibb Japan (Inst), Chugai Pharma (Inst), Taiho Pharmaceutical (Inst), MSD (Inst), AstraZeneca (Inst), Novartis (Inst), Merck Serono (Inst), Genomic Health (Inst), CMIC (Inst), Takeda (Inst), EPS Holdings (Inst), IQvia (Inst), Daiichi Sankyo/UCB Japan (Inst), Janssen (Inst), Amgen (Inst), EP Croit Co (Inst), Astellas Amgen BioPharama (Inst), Bayer (Inst), Preferred Network (Inst), Medpace (Inst), Sysmex (Inst)
Uncompensated Relationships: Cancer Net Japan, JAMT
Kiyotaka Yoh
Honoraria: Chugai Pharma, AstraZeneca, Lilly Japan, Bristol Myers Squibb Japan, Takeda, Amgen, Ono Pharmaceutical, MSD, Daiichi Sankyo, Kyowa Kirin
Consulting or Advisory Role: Boehringer Ingelheim, AbbVie
Research Funding: Lilly Japan (Inst), AstraZeneca (Inst), Taiho Pharmaceutical (Inst), Chugai Pharma (Inst), MSD (Inst), Takeda (Inst), Daiichi Sankyo (Inst), AbbVie (Inst), ArriVent Biopharma (Inst), Amgen (Inst), Boehringer Ingelheim (Inst)
Rebecca Heist
Consulting or Advisory Role: Novartis, Daichii Sankyo, AbbVie, Sanofi, Lilly, Regeneron, Claim Therapeutics, AstraZeneca, Merck, Biohaven Pharmaceuticals
Research Funding: AbbVie (Inst), Novartis (Inst), Roche (Inst), Mirati Therapeutics (Inst), Exelixis (Inst), Corvus Pharmaceuticals (Inst), Daiichi Sankyo (Inst), Agios (Inst), Pfizer (Inst), Lilly (Inst), Turning Point Therapeutics (Inst), Erasca, Inc (Inst), Mythic Therapeutics (Inst)
Junichi Shimizu
Consulting or Advisory Role: Daiichi Sankyo/UCB Japan
Speakers' Bureau: Ono Pharmaceutical, MSD Oncology, Novartis, AstraZeneca, Taiho Pharmaceutical, Bristol Myers Squibb, Chugai Pharma, Takeda, Merck, Amgen
Paul Baas
Honoraria: Bristol Myers Squibb, MSD (Inst)
Consulting or Advisory Role: Merck Sharp & Dohme (Inst), Bristol Myers Squibb (Inst)
Research Funding: Bristol Myers Squibb (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme, BMS (Inst)
Other Relationship: Bristol Myers Squibb
David Planchard
Honoraria: Prime Oncology, PeerVoice, Medscape
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Novartis, Roche, Pfizer, MSD Oncology, Celgene, BeiGene, Samsung, AbbVie, Janssen, Daiichi Sankyo/AstraZeneca, Pierre Fabre, Seagen, Gilead Sciences, Anheart Therapeutics, ArriVent Biopharma, Ellipses Pharma
Research Funding: AstraZeneca/MedImmune (Inst), Bristol Myers Squibb (Inst), Boehringer Ingelheim (Inst), Lilly (Inst), Merck (Inst), Novartis (Inst), Pfizer (Inst), Roche (Inst), Sanofi/Aventis (Inst), Taiho Pharmaceutical (Inst), Daiichi Sankyo (Inst), AbbVie (Inst), Janssen (Inst), Pierre Fabre (Inst), Seagen (Inst), ArriVent Biopharma (Inst), Ellipses Pharma (Inst)
Maurice Pérol
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Lilly, Roche/Genentech, Pfizer, AstraZeneca, Merck Sharp & Dohme, Bristol Myers Squibb, Novartis, Amgen, Takeda, Sanofi, GlaxoSmithKline, Janssen Oncology, Ipsen, Eisai, Novocure, Daiichi Sankyo, Gilead Sciences
Research Funding: Takeda (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Roche, Bristol Myers Squibb, Merck Sharp & Dohme, Pfizer, Takeda, Chugai Pharma
Enriqueta Felip
Consulting or Advisory Role: AbbVie, Amgen, AstraZeneca, Bayer, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, Roche, Gilead Sciences, GlaxoSmithKline, Janssen, Merck Serono, Merck Sharp & Dohme, Novartis, Peptomyc, Pfizer, Regeneron, Sanofi, Takeda, Genmab
Speakers' Bureau: Amgen, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Lilly, Roche, Genentech, Janssen, Medical Trends, Medscape, Merck Serono, Merck Sharp & Dohme, Peervoice, Pfizer, Sanofi, Takeda, Touch Oncology
Travel, Accommodations, Expenses: AstraZeneca, Janssen, Roche
Other Relationship: Grifols
Uncompensated Relationships: Member of the Scientific Advisory Committee-Hospital Universitari Parc Taulí-, SEOM (Sociedad Española de Oncología Médica), President from 2021 to 2023, “ETOP IBCSG Partners” Member of the Scientific Committee
Wu-Chou Su
Consulting or Advisory Role: Bayer Schering Pharma, MSD Oncology, Lilly, Merck
Hong Zebger-Gong
Employment: Daiichi Sankyo Europe GmbH
Stock and Other Ownership Interests: Bayer, Daiichi Sankyo
Lan Lan
Employment: Daiichi Sankyo Inc
Stock and Other Ownership Interests: Daiichi Sankyo Inc
Travel, Accommodations, Expenses: Daiichi Sankyo Inc
Chelsea Liu
Employment: Daiichi Sankyo Inc
Stock and Other Ownership Interests: Daiichi Sankyo Inc
Paul Howarth
Employment: Daiichi Sankyo
Rachel Chiaverelli
Employment: Daiichi Sankyo/AstraZeneca
Stock and Other Ownership Interests: Daiichi Sankyo/AstraZeneca
Luis Paz-Ares
Leadership: Altum Sequencing, Stab Therapeutics
Stock and Other Ownership Interests: Altum Sequencing, Stab Therapeutics
Honoraria: Roche/Genentech, Lilly, Pfizer, Bristol Myers Squibb, MSD, AstraZeneca, Merck Serono, PharmaMar, Novartis, Amgen, Sanofi, Bayer, Takeda, Mirati Therapeutics, Daiichi Sankyo, BeiGene, GlaxoSmithKline, Janssen, Medscape, Regeneron, Boehringer Ingelheim
Consulting or Advisory Role: Lilly, MSD, Roche, Pharmamar, Merck, AstraZeneca, Novartis, Amgen, Pfizer, Sanofi, Bayer, BMS, Mirati Therapeutics, GlaxoSmithKline, Janssen, Takeda, Regeneron, AbbVie
Speakers' Bureau: MSD Oncology, BMS, Roche/Genentech, Pfizer, Lilly, AstraZeneca, Merck Serono
Research Funding: BMS (Inst), AstraZeneca (Inst), PharmaMar (Inst), MSD (Inst), Pfizer (Inst)
Other Relationship: Novartis, Ipsen, Pfizer, Servier, Sanofi, Roche, Amgen, Merck
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, et al. : Cancer statistics, 2021. CA Cancer J Clin 71:7-33, 2021 [DOI] [PubMed] [Google Scholar]
- 2.Guo H, Zhang J, Qin C, et al. : Biomarker-targeted therapies in non-small cell lung cancer: Current status and perspectives. Cells 11:3200, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrella F, Rizzo S, Attili I, et al. : Stage III non-small-cell lung cancer: An overview of treatment options. Curr Oncol 30:3160-3175, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendriks LE, Kerr KM, Menis J, et al. : Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 34:339-357, 2023 [DOI] [PubMed] [Google Scholar]
- 5.Zhang YL, Yuan JQ, Wang KF, et al. : The prevalence of EGFR mutation in patients with non-small cell lung cancer: A systematic review and meta-analysis. Oncotarget 7:78985-78993, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itchins M, Pavlakis N: The quantum leap in therapeutics for advanced ALK+ non-small cell lung cancer and pursuit to cure with precision medicine. Front Oncol 12:959637, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeng P, Chen M-B, Zhou L-N, et al. : Impact of TROP2 expression on prognosis in solid tumors: A systematic review and meta-analysis. Sci Rep 6:33658, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inamura K, Yokouchi Y, Kobayashi M, et al. : Association of tumor TROP2 expression with prognosis varies among lung cancer subtypes. Oncotarget 8:28725-28735, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakach E, Sacks R, Kalinsky K: Trop-2 as a therapeutic target in breast cancer. Cancers 14:5936, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okajima D, Yasuda S, Maejima T, et al. : Datopotamab deruxtecan, a novel TROP2-directed antibody-drug conjugate, demonstrates potent antitumor activity by efficient drug delivery to tumor cells. Mol Cancer Ther 20:2329-2340, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimizu T, Sands J, Yoh K, et al. : First-in-human, phase I dose-escalation and dose-expansion study of trophoblast cell-surface antigen 2-directed antibody-drug conjugate datopotamab deruxtecan in non-small-cell lung cancer: TROPION-PanTumor01. J Clin Oncol 41:4678-4687, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Heist RS, Sands J, Bardia A, et al. : Clinical management, monitoring, and prophylaxis of adverse events of special interest associated with datopotamab deruxtecan. Cancer Treat Rev 125:102720, 2024 [DOI] [PubMed] [Google Scholar]
- 14.Ramalingam SS, Vansteenkiste J, Planchard D, et al. : Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 382:41-50, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Soria JC, Ohe Y, Vansteenkiste J, et al. : Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 378:113-125, 2018 [DOI] [PubMed] [Google Scholar]
- 16.Di Noia V, D'Aveni A, D'Argento E, et al. : Treating disease progression with osimertinib in EGFR-mutated non-small-cell lung cancer: Novel targeted agents and combination strategies. ESMO Open 6:100280, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piper-Vallillo AJ, Sequist LV, Piotrowska Z: Emerging treatment paradigms for EGFR-mutant lung cancers progressing on osimertinib: A review. J Clin Oncol 38:2926-2936, 2020 [DOI] [PubMed] [Google Scholar]
- 18.Passaro A, Wang J, Wang Y, et al. : Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: Primary results from the phase III MARIPOSA-2 study. Ann Oncol 35:77-90, 2024 [DOI] [PubMed] [Google Scholar]
- 19.Ahn MJ, Tanaka K, Paz-Ares L, et al. : Datopotamab deruxtecan versus docetaxel for previously treated advanced or metastatic non–small cell lung cancer: The randomized, open-label phase III TROPIONLung01 study. J Clin Oncol 43:260-272, 2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Planchard D, Cozic N, Wislez M, et al. : ICARUS-LUNG01: A phase 2 study of Dato-DXd in patients with previously treated advanced NSCLC, with sequential tissue biopsies and biomarkers analysis to predict treatment outcome. J Clin Oncol 42, 2024. (suppl 16; abstr 8501) [Google Scholar]
- 21.Fang W, Wang Q, Cheng Y, et al. : Sacituzumab tirumotecan (SKB264/MK-2870) in combination with KL-A167 (anti-PD-L1) as first-line treatment for patients with advanced NSCLC from the phase II OptiTROP-Lung01 study. Presented at the 2024 ASCO Annual Meeting, Chicago, IL, May 31-June 4, 2024
- 22.Paz-Ares LG, Juan-Vidal O, Mountzios GS, et al. : Sacituzumab govitecan (SG) vs docetaxel (doc) in patients (pts) with metastatic non-small cell lung cancer (mNSCLC) previously treated with platinum (PT)-based chemotherapy (chemo) and PD(L)-1inhibitors (IO): Primary results from the phase 3 EVOKE-01 study. Presented at the 2024 ASCO Annual Meeting, Chicago, IL, May 31-June 4, 2024
- 23.Schoenfeld AJ, Chan JM, Kubota D, et al. : Tumor analyses reveal squamous transformation and off-target alterations as early resistance mechanisms to first-line osimertinib in EGFR-mutant lung cancer. Clin Cancer Res 26:2654-2663, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin JJ, Riely GJ, Shaw AT: Targeting ALK: Precision medicine takes on drug resistance. Cancer Discov 7:137-155, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omori S, Muramatsu K, Kawata T, et al. : Trophoblast cell-surface antigen 2 expression in lung cancer patients and the effects of anti-cancer treatments. J Cancer Res Clin Oncol 148:2455-2463, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.United States Food and Drug Administration : Full prescribing information ENHERTU, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761139s021lbl.pdf
- 27.United States Food and Drug Administration : Full prescribing information: KADCYLA, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125427lbl.pdf
- 28.United States Food and Drug Administration : Highlights of prescribing information: ADCETRIS, 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125388s099lbl.pdf
- 29.United States Food and Drug Administration : Full prescribing information: TIVDAK, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761208Orig1s000lbledt.pdf
- 30.United States Food and Drug Administration : Full prescribing information: ELAHERE, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761310s000lbl.pdf
- 31.Henning JW, Brezden-Masley C, Gelmon K, et al. : Managing the risk of lung toxicity with trastuzumab deruxtecan (T-DXd): A Canadian perspective. Curr Oncol 30:8019-8038, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sands J, Lisberg A, Bardia A, et al. : Analysis of drug-related interstitial lung disease (ILD) in patients treated with datopotamab deruxtecan (Dato-DXd). J Clin Oncol 42, 2024. (suppl 16; abstr 8623) [Google Scholar]
- 33.United States Food and Drug Administration : Highlights of prescribing information: BLENREP, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761158s000lbl.pdf
- 34.United States Food and Drug Administration : Highlights of prescribing information: ENHERTU, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761139s021lbl.pdf
- 35.Garon EB, Liu SV, Owen SP, et al. : EVOKE-02: A phase 2 study of sacituzumab govitecan (SG) plus pembrolizumab (pembro) with or without platinum chemotherapy in first-line metastatic non–small cell lung cancer (NSCLC). J Clin Oncol 40, 2022. (suppl 16; abstr TPS9146) [Google Scholar]
- 36.Paz-Ares LG, Juan-Vidal O, Mountzios GS, et al. : Sacituzumab govitecan versus docetaxel for previously treated advanced or metastatic non-small cell lung cancer: The randomized, open-label phase III EVOKE-01 study. J Clin Oncol 42:2860-2872, 2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang W, Cheng Y, Chen Z, et al. : SKB264 (TROP2-ADC) for the treatment of patients with advanced NSCLC: Efficacy and safety data from a phase 2 study. J Clin Oncol 41, 2023. (suppl 16; abstr 9114) [Google Scholar]
- 38.United States Food and Drug Administration : Full prescribing information: TRODELVY, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761115s035lbl.pdf
- 39.Yu HA, Goto Y, Hayashi H, et al. : HERTHENA-Lung01, a phase II trial of Patritumab deruxtecan (HER3-DXd) in epidermal growth factor receptor-mutated non-small-cell lung cancer after epidermal growth factor receptor tyrosine kinase inhibitor therapy and platinum-based chemotherapy. J Clin Oncol 41:5363-5375, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ClinicalTrials.gov : HERTHENA-Lung02: A study of patritumab deruxtecan versus platinum-based chemotherapy in metastatic or locally advanced EGFRm NSCLC after failure of EGFR TKI therapy, 2023. https://clinicaltrials.gov/study/NCT05338970?term=herthena%20lung02&rank=1
- 41.Zhang L, Ma Y, Zhao Y, et al. : BL-B01D1, a first-in-class EGFRxHER3 bispecific antibody-drug conjugate (ADC), in patients with locally advanced or metastatic solid tumor: Results from a first-in-human phase 1 study. J Clin Oncol 41, 2023. (suppl 16; abstr 3001) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO-24-01349. Deidentified individual participant data and applicable supporting clinical trial documents may be available on request at Vivli-Center for Global Clinical Research Data. In cases where trial data and supporting documents are provided pursuant to our company policies and procedures, Daiichi Sankyo, Inc will continue to protect the privacy of our clinical trial participants. Details on data sharing criteria and the procedure for requesting access can be found online at https://vivli.org/ourmember/daiichi-sankyo/.