Abstract
Understanding modulation of memory, as well as the mechanisms underlying memory formation, has become a key issue in neuroscience research. Previously, we found that the formation of long-term, but not short-term, memory for a nonassociative form of learning, sensitization, was modulated by the circadian clock in the diurnal Aplysia californica. To define the scope of circadian modulation of memory, we examined an associative operant learning paradigm, learning that food is inedible (LFI). Significantly greater long-term memory of LFI occurred when A. californica were trained and tested during the subjective day, compared with animals trained and tested in the subjective night. In contrast, animals displayed similar levels of short-term memory for LFI when trained in either the subjective day or night. Circadian modulation of long-term memory for LFI was dependent on the time of training, rather than the time of testing. To broaden our investigation of circadian modulation of memory, we extended our studies to a nocturnal species, Aplysia fasciata. Contrary to the significant memory observed during the day with the diurnal A. californica, A. fasciata showed no long-term memory for LFI when trained during the day. However, A. fasciata demonstrated significant long-term memory when trained and tested during the night. Thus, the circadian clock modulates memory formation in phase with the animals' activity period. The results from our studies of circadian modulation of long-term sensitization and LFI suggest that circadian modulation of memory formation may be a general phenomenon with potentially widespread implications for many types of long-term learning.
Keywords: biological rhythms, circadian clock, long-term memory
The ability to remember an experience allows an organism to use information gained in planning its response to future events. A variety of factors can modulate the formation and recall of memory. In fact, very few of the events occurring in the environment are remembered. Health, age, motivation, previous experience, stress, and many other factors may modulate learning and memory. Modulation of memory formation can affect how input information is processed, how memories are stored, the length of time that memories last, or even the mechanisms by which memories are recalled. Understanding the modulation of memory will provide insight into the cellular and molecular processes underlying memory formation per se. The circadian clock allows animals to predict when important events occur in the environment and to anticipate changes in the environment based on time of day. Given the enormous and widespread impact that the circadian clock has on an animal's physiology and behavior, we investigated modulation of memory formation by the circadian clock.
The time of day can impact learning by becoming part of the context in which the learning occurs (time-stamping), or the time of day can affect the amount of memory that is formed or recalled. Time-stamping has been demonstrated in invertebrates (1) and in mammals (2-5). However, in time-stamping, the circadian clock is not actually modulating learning itself.
Varying results have been obtained by using mammalian models to assess the modulatory role of the circadian clock in memory formation. Researchers using fear conditioning found that mice exhibit a diurnal rhythm in contextual fear conditioning, with animals demonstrating greater memory during the night. However, no rhythm existed for cued fear conditioning (6). In other research, also using fear conditioning in mice, a circadian rhythm of recall was observed for context and cued fear conditioning, with peaks in recall seen in the early day regardless of the time of training (7). In rats, no time-of-day modulation has been observed with several different learning paradigms (8), although recent research suggests that strain differences may affect circadian modulation (9). Studies in humans also have yielded mixed results. Thus, using complex model systems, it has been difficult to consistently demonstrate a modulatory role of the circadian clock on the formation of long-term memory.
Aplysia provides a superb opportunity to investigate circadian modulation of long-term memory, because considerable information on long-term memory formation is available at the molecular, cellular, and behavioral levels (10). As a first step, we demonstrated that the circadian clock modulates long-term sensitization (LTS), a nonassociative form of learning (11). In circadian modulation of LTS, animals demonstrate robust long-term memory when trained during the day but little long-term memory when trained during the night. Circadian modulation of LTS appears to occur during the induction and formation of long-term memory, rather than during recall. Interestingly, short-term memory for sensitization is not modulated by the circadian clock (11).
How broadly does the circadian clock affect long-term memory? Are more complex forms of learning modulated? Does the neural or molecular circuitry involved in a particular type of learning limit circadian modulation of memory? To address these questions, we investigated circadian modulation of learning that food is inedible (LFI), an associative form of operant conditioning. In LFI, netted seaweed is presented to the animal. The animal responds by head-waving, biting, food entry into the mouth, attempts to swallow, and rejection of the food. Food entry into the mouth and continued failed swallowing represent negative reinforcement and are necessary for memory formation. Learning for this paradigm is specific to the food presented during training (12).
We found that the circadian clock modulates long-term memory of LFI in diurnal Aplysia californica, with significantly greater long-term memory formed during the subjective day. In contrast, the circadian clock did not modulate short-term memory of LFI. Moreover, we found that circadian modulation of long-term LFI depended on the time of training and not the time of testing. We studied circadian modulation of long-term memory across species by investigating a nocturnal species Aplysia fasciata (13). In A. fasciata, we also found that significantly greater long-term memory occurred when animals were trained during their active phase, such that the nocturnal species exhibited robust long-term memory only when trained during the night. Thus, our research indicates that the circadian clock is a strong modulator of long-term memory for both associative and nonassociative learning paradigms and that this type of modulation extends across species such that memory formation is attenuated during an animal's inactive phase.
Materials and Methods
Animal Maintenance. A. californica (100-175 g) were housed in boxes in artificial seawater (15°C) in 12:12-h light-dark (LD) cycles. Animals were fed romaine lettuce (Lactuca sativa) every second day until they were fed to satiation. For circadian experiments, animals were entrained and then transferred to constant darkness (DD). Manipulations in the dark used dim red light. As a control for non-LD-cycle cues controlling the phase of the rhythm, some animals were entrained to a reversed-phase LD cycle. Experiments were performed at 15°C.
A. fasciata (50-150 g) were collected along the Mediterranean coast of Israel during the early summer and housed five to six to a cage in Mediterranean seawater at 18°C (12:12-h LD). The animals were fed one to two times weekly with Ulva lactuca (sea lettuce, a green alga) and used 1 week to 1 month after collection.
Behavioral Training and Testing. For A. californica, animals were fed to satiation with laver seaweed and then starved for 5-7 days before training. LFI training protocols were established by A.J.S. (12, 14-16). Animals were presented with a small piece of laver seaweed wrapped in netting that they could not swallow. Animals received a one-trial training paradigm that continued until the animal stopped responding to the netted seaweed for 3 min. Two parameters were recorded: (i) the total response time and (ii) the cumulative time that the animal retained the netted seaweed in its mouth. Memory was represented as a decrease in these times. Testing occurred using the same procedure either 30 min later for short-term memory or 24 h later for long-term memory.
A. fasciata were transferred to individual cages and food-deprived 7 days before the start of experiments. At 24 h before an experiment, they were transferred to 10-liter aquaria maintained at 19-20°C. Because in A. fasciata the presence of a conspecific is needed for animals to learn that a food is inedible (17), a second Aplysia was added to the aquarium. The second animal was maintained behind a partition that allowed the flow of seawater but prevented contact between animals. Animals were trained and tested as previously described (12, 14-16, 18, 19). For A. fasciata, the time the food was retained in the mouth was measured only for the first 5 min.
In experiments with A. californica, the person who tested the animals was not the person who trained that batch of animals. For both A. californica and A. fasciata, naïve animals were included during long-term testing, and the individual testing the animals did not know which animals were previously trained. Statistical analysis of the data were by ANOVA with Tukey's posthoc analyses (P < 0.05 was considered significant). Experiments with A. fasciata were done at Bar Ilan University; experiments with A. californica were done at the University of Houston.
Results
Diurnal Modulation of Long-Term LFI in A. californica. To investigate circadian modulation of more complex forms of learning, we examined an associative form of learning, LFI, in A. californica. Using this paradigm, the animals learn an association between netted seaweed and failed attempts at swallowing. Failure to consume the food acts as a negative reinforcer. To examine diurnal modulation of long-term LFI, animals entrained to LD cycles were trained by using the LFI paradigm at either zeitgeber time (ZT) 3 or ZT 9 (during the day with lights on; ZT 0 is lights on) or at ZT 15 or ZT 21 (during the night using dim red light; ZT 12 is lights off). Animals were tested 24 h after training. Memory was expressed as a decrease in the amount of time required to stop responding and a decrease in the amount of time that the netted food remained in the mouth. Animals trained during the day and tested 24 h later showed significant reductions in both total response time and the amount of time that the food was retained in the mouth (Fig. 1). However, when animals were trained and tested during the night (at ZT 15 or ZT 21), no decreases were seen for either parameter, indicating the absence of long-term memory for LFI. Thus, similar to our previous results examining modulation of LTS (11), diurnal modulation of LFI occurred with greater long-term memory formed during the animals' active phase.
Fig. 1.
Diurnal modulation of long-term LFI in A. californica. A. californica were entrained to 12:12-h LD cycles, trained with netted food at ZT 3, 9, 15, or 21 (n = 10, 9, 9, and 10, respectively), and tested 24 h after training. Memory was expressed as a decrease in the total response time and a decrease in the amount of time that the netted food remained in the mouth. White bars represent data obtained in the light, whereas gray bars represent data obtained in the dark. Error bars are SEM. (A) Animals demonstrated significantly greater decreases in total response time when trained and tested during the day, compared with animals trained and tested during the night [F(3, 34) = 14.98, P < 0.001; Tukey's posthoc analyses, P < 0.01 for ZT 3 vs. 15, ZT 3 vs. 21, ZT 9 vs. 15, and ZT 9 vs. 21; raw data (min), ZT 3 train = 17.2 ± 1.4, ZT 3 test = 8.0 ± 0.9; ZT 9 train = 17.9 ± 2.0, ZT 9 test = 10.7 ± 0.9; ZT 15 train = 22.2 ± 4.5, ZT 15 test = 24.5 ± 3.5; ZT 21 train = 20.3 ± 1.8, and ZT 21 test = 20.7 ± 1.7]. (B) Animals displayed significantly greater decreases in the amount of time that the netted food remained in the mouth when animals were trained and tested during the day than when trained and tested at night [F(3, 33) = 9.277; P < 0.001; Tukey's posthoc analyses, P < 0.05 for ZT 3 vs. 15, ZT 3 vs. 21, ZT 9 vs. 15, and ZT 9 vs. 21; raw data (min), ZT 3 train = 7.7 ± 1.1, ZT 3 test = 2.8 ± 0.3; ZT 9 train = 7.1 ± 0.7, ZT 9 test = 3.9 ± 0.3; ZT 15 train = 10.6 ± 2.2, ZT 15 test = 12.8 ± 1.3; ZT 21 train = 8.1 ± 1.1, and ZT 21 test = 9.7 ± 1.3].
Circadian Modulation of Long-Term LFI. To test whether the modulation of LFI was due to the circadian clock or direct effects of light in the LD cycle, animals were trained at various times on day 2 of DD and tested 24 h after training. Animals that were trained and tested during the subjective (projected) day showed significantly greater reductions in both total response time and the amount of time that food was retained in the mouth than did animals that were trained and tested during the subjective night (Fig. 2). These results demonstrate that the circadian clock modulates an associative-learning paradigm, with robust long-term memory formed in the subjective day and little or no memory formed during the subjective night.
Fig. 2.
The circadian clock modulated long-term LFI. Animals were entrained to 12:12-h LD cycles, switched to DD, and then subjected to LFI training at CT 3, 9, 15, or 21 (n = 6 at each CT 3, 9, and 15, n = 5 at CT 21) on day 2 of DD. Animals were tested 24 h later. Gray bars represent subjective day (projected time in DD), whereas black bars represent subjective night. (A) Changes in total response time varied significantly depending on when LFI training and testing occurred during the circadian cycle [F(3, 19) = 15.37; P < 0.001; Tukey's posthoc analyses, P < 0.01 for CT 3 vs. 15, CT 3 vs. 21, CT 9 vs. 15, and CT 9 vs. 21; raw data (min), CT 3 train = 27.2 ± 3.4, CT 3 test = 13.8 ± 2.1; CT 9 train = 31.4 ± 3.6, CT 9 test = 14.6 ± 2.3; CT 15 train = 26.7 ± 2.0, CT 15 test = 25.9 ± 2.1; CT 21 train = 31.2 ± 2.6, and CT 21 test = 27.6 ± 1.7]. Animals demonstrated significantly greater memory when trained and tested during the subjective day, compared with animals trained and tested during the subjective night. (B) Animals displayed significantly greater decreases in the time the food was retained in the mouth when animals were trained and tested during the subjective day than during the subjective night [F(3, 19) = 6.18; P < 0.005; Tukey's posthoc analyses, P < 0.05 for CT 9 vs. 15 and CT 9 vs. 21; raw data (min), CT 3 train = 10.9 ± 1.9, CT 3 test = 4.6 ± 0.3; CT 9 train = 12.7 ± 1.7, CT 9 test = 5.1 ± 0.9; CT 15 train = 13.6 ± 1.9, CT 15 test = 9.4 ± 1.6; CT 21 train = 12.3 ± 1.8, CT 21 test = 9.1 ± 1.1].
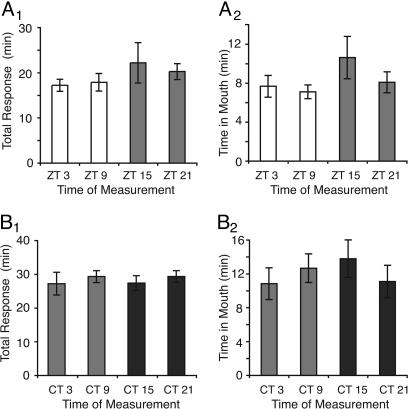
No Diurnal or Circadian Modulation of Behavior During Training. It is possible that the circadian modulation of long-term LFI was caused by modulation of feeding behavior per se during training or testing. To test this possibility, we examined several parameters of feeding during training. In LD conditions, there were no significant differences between animals trained in the light or dark either in the total response time or in the amount of time that food was retained in the mouth (Fig. 3 A1 and A2). Furthermore, under constant conditions, analysis of baseline behavioral responses during training revealed no circadian differences (Fig. 3 B1 and B2). Although diurnal and circadian rhythms in feeding behaviors have been found in Aplysia (20, 21), no apparent diurnal or circadian rhythm exists in the training responses for LFI.
Fig. 3.
No diurnal or circadian modulation of training responses. (A1) No diurnal rhythm in total response time occurred when animals were trained at different times of day [F(3, 34) = 0.769; P = 0.52; n = 10, 9, 9, and 10, respectively]. (A2) No diurnal rhythm in the amount of time that the food was retained in the mouth occurred when animals were trained at different times of day [F(3, 34) = 1.26; P = 0.30]. (B1) No circadian rhythm in total response time occurred when animals were trained on the day 2 of DD [F(3, 20) = 0.3487; P = 0.79; n = 6 at each point]. (B2) No circadian rhythm in time the food was retained in the mouth occurred when animals were trained on day 2 of DD [F(3, 20) = 0.51; P = 0.68].
Although no time-of-day differences were observed in training responses on day 2 of DD, it is possible that the observed rhythms in long-term LFI were due to changes in the animals' responses during testing on day 3 of DD due to a longer time in DD or a longer time isolated from food. To investigate these possibilities, we trained naïve animals on day 3 of DD. No time-of-day differences were observed in the responses on day 3 of DD between animals trained during the subjective day and animals trained at night [circadian time (CT) 3 and CT 9; 30.6 ± 3.1 min total response time, compared with CT 15 and CT 21; 29.4 ± 1.5 min total response time, t = 0.127, P = 0.72]. Thus, circadian modulation of long-term LFI appears to be due to the circadian modulation of long-term memory rather than any circadian differences in baseline feeding behaviors during training or testing.
No Circadian Modulation of Short-Term Memory. Mechanistic differences exist in the formation of short-term and long-term memory. Consequently, we tested whether the circadian clock modulated short-term as well as long-term memory formation. A full-length single training paradigm (training continues until the animal stops responding for 3 min) is sufficient to induce both short-term and long-term memory formation of LFI (14). We trained groups of animals at different times on day 2 of DD and tested the animals 30 min after the end of training. No circadian modulation of short-term LFI was seen for either the total response time (Fig. 4A; ANOVA, P = 0.70) or the amount of time that the food was retained in the mouth (Fig. 4B; ANOVA, P = 0.94). Thus, animals can form short-term memory at night, whereas long-term memory for LFI cannot be formed at night. These results are similar to our previous results for sensitization (11).
Fig. 4.
The circadian clock did not modulate short-term memory. Animals were entrained to 12:12-h LD cycles, switched to DD, and trained at CT 3, 9, 15, or 21 (n = 4 at CT 3 and CT 9, n = 6 at CT 15 and CT 21) on day 2 of DD. Animals were tested 30 min after training. Animals demonstrated robust short-term learning during both the subjective day and subjective night. No significant differences were seen with respect to time of day for either the percent change in total response time [ANOVA, P = 0.70; raw data (min): CT 3 train = 22.1 ± 4.6, CT 3 test = 7.6 ± 0.3; CT 9 train = 24.0 ± 3.7, CT 9 test = 5.4 ± 0.9; CT 15 train = 28.8 ± 5.9, CT 15 test = 6.8 ± 0.8; CT 21 train = 26.5 ± 2.5, CT 21 test = 7.1 ± 1.4] (A) or the percent change in the amount of time that food was retained in the mouth [ANOVA, P = 0.94; raw data (min): CT 3 train = 11.9 ± 3.1, CT 3 test = 1.8 ± 0.4; CT 9 train = 12.6 ± 3.0, CT 9 test = 2.0 ± 0.4; CT 15 train = 13.3 ± 3.5, CT 15 test = 1.7 ± 0.4; CT 21 train = 10.7 ± 2.4, CT 21 test = 1.6 ± 0.5] (B).
Circadian Modulation of Long-Term Learning Depended on the Time of Training, Not the Time of Testing. The observed circadian modulation of long-term LFI could be due to the modulation of either memory consolidation or memory recall or both. To determine whether time of training determined the circadian modulation of long-term LFI, animals were trained at CT 9 on day 2 of DD (a time when significant learning is observed) and then tested either 24 h later at CT 9, 36 h later at CT 21 (a time when learning was not previously observed) or 48 h after training at CT 9 on the day 4 of DD (Fig. 5A). Animals trained at CT 9 and tested 36 h later at CT 21 showed robust learning with significant decreases observed in both total response times and the amount of time that food was retained in the mouth (data not shown). This memory at CT 21 when animals were trained at CT 9 differed significantly from the responses observed when animals were trained and tested at CT 21 (Figs. 2 and 5A). Thus, recall of long-term memory can occur during the subjective night when animals are trained during the subjective day. These results demonstrate that the circadian clock modulates the formation of long-term memory, rather than the recall of memory.
Fig. 5.
Time of training, rather than time of testing (recall), determined the circadian modulation of long-term LFI. (A) To determine the contribution of the time of training to the modulation in long-term LFI, animals were trained at CT 9 on day 2 of DD and then tested either 24 h later at CT 9 (day 3 of DD), 36 h later at CT 21, or 48 h later at CT 9 (day 4 of DD). Column shading indicates circadian time of testing as shown. Animals displayed robust learning when tested at either CT 9 or CT 21. No significant differences existed between animals tested at 24 h, 36 h, or 48 h after training. Results were similar for the time that food was retained in the mouth (data not shown). Thus, time of training determined whether long-term memory was formed. (B) To determine whether the time of testing contributed to the modulation in LFI, animals were trained at CT 21 and tested 24 h later at CT 21, 36 h later at CT 9, or 48 h later at CT 21. As shown in Fig. 3, a small amount of memory was observed when animals were tested 24 h after training at CT 21, but animals failed to exhibit any learning when tested 36 h after training at CT 9 (a time when LFI normally occurs) or when tested 48 h later. Results were similar when the amount of time that food was retained in the mouth was analyzed (data not shown).
A complementary set of experiments was done to determine whether circadian modulation of recall also existed. In these experiments, animals were trained at CT 21 on the day 2 of DD and then tested 24 h later at CT 21, 36 h later at CT 9, or 48 h later at CT 21. When animals were trained at CT 21 and tested 36 h later at CT 9, no significant decreases were seen in either total response time (Fig. 5B) or the amount of time that the food was retained in the mouth (data not shown). Thus, even though animals were tested at a time (CT 9) when recall can occur (Fig. 2), no long-term memory was evident when animals were trained at CT 21. These experiments demonstrate that circadian modulation occurs at the time of training, presumably during the induction and formation of long-term memory, rather than modulation of recall at the time of testing.
Diurnal and Nocturnal Species of Aplysia Exhibit Opposite Phases of Modulation of Long-Term LFI. To date, our experiments examining circadian modulation of both LFI and LTS have shown that diurnal A. californica display significantly greater long-term memory when they are trained during the day or the subjective day. These experiments raise the question of whether circadian modulation of long-term memory occurs with respect to particular times within the LD cycle (e.g., light phase or projected light phase) or whether circadian modulation occurs such that the phase of learning is coordinated with the animal's inherent activity pattern. Analyzing circadian modulation of long-term memory in a nocturnal species, A. fasciata, provided the answer to this question. Nocturnal A. fasciata demonstrated significantly greater long-term LFI when trained and tested during the night than when trained and tested during the day (Fig. 6 A1 and A2). The phase of the modulation of long-term LFI for nocturnally active A. fasciata appeared to be reversed from that seen for the diurnally active A. californica (compare Figs. 1 and 6). Thus, rhythmic modulation of long-term memory formation occurs such that formation of long-term memory is significantly greater when animals are trained during their active periods irrespective of the phase of the LD cycle or the time of occurrence of specific environmental events.
Fig. 6.
A nocturnal and a diurnal species of Aplysia exhibited opposite phases of diurnal modulation of long-term LFI. (A1) Nocturnal A. fasciata demonstrated significantly greater decreases in total response time when trained and tested during the night, compared with animals trained and tested during the day [t = 31.66; P < 0.001; ZT 4, n = 8; ZT 16, n = 5; raw data (min), ZT 4 train = 14.4 ± 2.3, ZT 4 test = 14.3 ± 1.4, ZT 16 train = 14.9 ± 1.0, ZT 16 test = 4.1 ± 0.7]. Thus, the phase of the circadian modulation of LFI appears reversed for A. fasciata, compared with A. californica (compare Figs. 1 and 6). (A2) The time that food was retained in the mouth during the first 5 min of the training and testing sessions was modulated [t = 9.25; P < 0.025; raw data (s), ZT 4 train = 110.0 ± 20.2, ZT 4 test = 113.8 ± 19.7, ZT 16 train = 113.5 ± 15.5, ZT 16 test = 47.2 ± 11.6]. (B1) As with A. californica, no diurnal rhythm in total response time was seen during training (t = 0.26; P = 0.876; n = 8, 5). (B2) Similarly, no diurnal modulation was apparent for the amount of time that the animals retained food in the mouth during training (t = 0.15; P = 0.905).
The initial training of A. fasciata also was examined to determine whether rhythms in baseline behavior might underlie the observed diurnal modulation of long-term LFI. As with A. californica, there were no time-of-day differences in total response time or the amount of time that the food was retained in the mouth (Fig. 6 B1 and B2). Additionally, naïve animals trained 1 day later also displayed no time-of-day differences in either training parameter (n = 5 each time point; total response time, ZT 4 = 16.9 ± 1.1 min, ZT 16 = 16.4 ± 1.9 min, t = 0.032, P = 0.86; time in mouth, ZT 4 = 96.8 ± 20.7 sec, ZT 16 = 107.8 ± 10.9 sec, t = 0.22, P = 0.65). These results indicate that the formation of long-term memory in A. fasciata, rather than the feeding behavior, was modulated by time of day.
Discussion
Our research is aimed at understanding modulation of the formation of long-term memory. We have importantly expanded our previous findings demonstrating circadian modulation of a nonassociative form of long-term learning in Aplysia, LTS, by showing that the circadian clock also modulates a more complex associative form of learning, LFI (Figs. 1 and 2). Moreover, as with LTS, modulation of long-term LFI was not subtle, but rather circadian modulation resulted in a major suppression of the formation of memory during the animals' inactive phase. In contrast, the circadian clock did not modulate short-term memory for either sensitization (11) or LFI (Fig. 4).
The circadian modulation seen in long-term LFI could be due to circadian rhythms in activity or feeding behaviors that might affect the animal during training or the circadian clock could directly modulate the signaling cascades that lead to the consolidation of memory. In LFI, the experimenter elicits the feeding behaviors of the animal, so rhythms in the animal's activity or feeding behaviors should not affect learning. In our experiments, no time-of-day differences occurred in the training responses for either A. californica or A. fasciata (Figs. 3 and 6). Additionally, A. californica exhibited long-term memory when trained during the day and tested at night (Fig. 5), demonstrating that animals are capable of displaying previous learning at night. These results suggest that modulation of LFI occurs through either modulation of the formation or recall of long-term memory, rather than through the modulation of the animal's behavior during training or testing. Disturbing the animals at night by training during their inactive period also could be responsible for the lack of long-term memory formation at night. If uninterrupted inactive time is necessary for memory consolidation, we would expect animals trained during the early night at CT (ZT) 15 to show long-term memory formation, because these animals still have 8 h of uninterrupted inactive time (night time) after training. However, no long-term memory formation appears to occur for animals trained either early in the night or late in the night. We still cannot rule out the possibility that circadian modulation of activity or other behaviors led to the observed circadian modulation of long-term memory formation. The molecular mechanisms underlying the formation and modulation of long-term memory for LFI need to be understood before the contribution of activity-dependent modulation of learning can be assessed.
Circadian modulation of long-term LFI appears to occur during the induction of memory or consolidation, rather than the recall of memory. A. californica trained during the subjective day demonstrated significant long-term LFI when tested during either the day or the night (Fig. 5A). However, animals trained at night failed to demonstrate significant long-term memory when tested during either the day or the night (Fig. 5B). Thus, it appears that for associative (LFI) and nonassociative forms of long-term learning (LTS), as well as appetitive (feeding) and defensive (withdrawal) forms of behavior, the circadian clock modulates events during the induction and consolidation of memory, rather than events during recall. Additionally, the fact that there was no difference in the amount of memory observed when animals were trained during the subjective day, regardless of whether the animals were tested 24, 36, or 48 h later (Fig. 5A), demonstrates that time-stamping or contextual association was not involved in these memories.
The question of how the circadian clock modulates the formation of long-term memory can be addressed at multiple levels, from the molecular level to the location of the circadian clock responsible for the circadian modulation of memory. In Aplysia, researchers have documented only one circadian oscillator, located in the eye (22, 23). The eye contains all of the necessary components of a complete circadian system and anatomically projects through the optic nerve to the cerebral, pleural, and pedal ganglia (23, 24). Consequently, the eye may well be responsible for the circadian modulation of memory through optic nerve projections. Circadian modulation of long-term memory also could occur through humoral factors as the eye has been shown to be neurosecretory (25). Alternatively, because invertebrates, such as Drosophila (26), have independent peripheral oscillators located throughout the organism, previously undetected peripheral oscillators may be responsible for circadian modulation of LFI.
How does the circadian clock modulate the very different neural circuitry responsible for LTS and LFI? Sensory and motor neurons involved in feeding behaviors are located primarily within the buccal and cerebral ganglia (27, 28), although the neurons specifically responsible for long-term LFI are undetermined as of yet. The neural circuit underlying LTS resides mainly in the pleural and pedal ganglia, although some facilitatory neurons in the cerebral ganglia may be involved. One interesting possibility is that the cerebral ganglion may serve as a central point of modulation by the circadian clock, given the involvement of the cerebral ganglion in both feeding behaviors and LTS. Finding additional types of memory with different neural circuitry that are modulated by the circadian clock might point to humoral control of modulation by a central clock or perhaps the existence of some common neuronal elements involved in the formation of memory for different behaviors.
How does circadian modulation of long-term LFI occur at the cellular level? The circadian clock could modulate the formation of long-term memory by (i) “gating” sensory input, (ii) modulating events involved in the induction or consolidation of long-term memory, or (iii) modulating the activity of motor neurons. We find the first and third of these possibilities unlikely, given that animals display short-term learning equally well during the day and the night, and the fact that no time-of-day differences are seen in the animals' responses during the training protocol. It appears most likely, as a conserved mechanism across behaviors, that the circadian clock modulates events within sensory neurons involved in the induction and consolidation of long-term, but not short-term, memory. However, it also is possible that the clock modulates events in other neurons involved in the circuit for LFI.
What are the possible mechanisms for the circadian modulation of long-term learning at the molecular level? In both LTS and long-term LFI, circadian modulation of long-term learning was dependent on the time of training, indicating that the circadian clock modulates the formation of long-term memory. Consequently, the circadian clock could modulate “core steps” common to the induction or formation of multiple types of long-term memory. Thus, we hypothesize that, although the anatomical locations of the neuronal circuits for different types of long-term learning may vary, similar mechanisms for the formation of long-term memory will be found at the molecular level and that these shared processes will be putative targets for circadian modulation. Indeed, recent research has shown that the induction and formation of long-term memory for both LTS and LFI may share common features at the molecular level. Changes in chromatin structure are presumed necessary for the initiation of transcription. Poly(ADP-ribosyl)ation [by poly(ADP-ribose) polymerase 1 (PARP 1)] is one posttranslational modification of nuclear proteins that can regulate the ability of transcription factors to bind DNA. In Aplysia, PARP 1 is activated in pleural-pedal ganglia by protocols (five pulses of 5-hydroxytryptamine) that induce long-term facilitation in isolated ganglia as well as by electrical stimulation that induces LTS in the intact animal (29). Moreover, PARP 1 also is activated in buccal and cerebral ganglia after LFI training (29). Inhibition of PARP 1 during training specifically blocked long-term, but not short-term, memory. Thus, it appears that both simple and complex forms of long-term learning and memory formation may have common avenues of modulation and regulation.
One of the unique aspects of the current research is the ability to directly compare rhythmic modulation of learning between the diurnal A. californica and the nocturnal A. fasciata. Previously, we suggested that circadian modulation of long-term learning had adaptive significance such that long-term learning occurred to a significantly greater extent during the animals' active period (11). Consequently, the prediction was made that nocturnal animals would display greater long-term learning during their active phase at night than during the day. As predicted, the nocturnal A. fasciata demonstrated significantly greater LFI (seen in both total response time and the amount of time that food was retained in the mouth) when trained and tested during the night, compared with animals trained and tested during the day (Fig. 6A). Thus, the timing of circadian modulation of memory formation is determined by the animal's internal clock, with the largest amount of memory formation occurring during the animal's active phase rather than during some specific set of events in the environment. This research directly compares the effect of the circadian clock on learning in both a diurnal and a nocturnal species. Researchers studying conditioned taste aversion (operant conditioning) in snails also have found that higher rates of voluntary activity are linked to higher levels of learning (30), and one study of fear conditioning in mice (nocturnal) demonstrated increased learning in animals trained during the early night (6). Future experiments extending our studies on circadian modulation of learning in additional species as well as with other learning paradigms will provide more insight into how phase is regulated by the circadian oscillator as well as affording us the chance to study the function of circadian modulation of long-term memory. Comparisons of mechanisms modulating the different phases of circadian modulation of learning between nocturnal and diurnal species will be particularly interesting.
Acknowledgments
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS050589 (to A.E.), Israel Science Foundation Grant 357/02-17.2 (to A.J.S.), and an Israel Institute for Psychobiology Postdoctoral Fellowship (to A.K.).
Author contributions: L.C.L., A.J.S., and A.E. designed research; L.C.L., O.R., and A.K. performed research; L.C.L. analyzed data; L.C.L. wrote the first draft of the paper; and A.E., A.K., O.R., and A.J.S. contributed to subsequent revisions of the manuscript.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CT, circadian time; DD, constant darkness; LD, light-dark; LFI, learning that food is inedible; LTS, long-term sensitization; ZT, zeitgeber time.
References
- 1.Pereyra, P., De La Iglesia, H. O. & Maldonado, H. (1996) Physiol. Behav. 59, 19-25. [DOI] [PubMed] [Google Scholar]
- 2.Manrique, T., Molero, A., Ballesteros, M. A., Moron, I., Gallo, M. & Fenton, A. A. (2004) Neurobiol. Learn. Mem. 82, 77-80. [DOI] [PubMed] [Google Scholar]
- 3.Ralph, M. R., Ko, C. H., Antoniadis, E. A., Seco, P., Irani, F., Presta, C. & McDonald, R. J. (2002) Behav. Brain Res. 136, 179-184. [DOI] [PubMed] [Google Scholar]
- 4.Cain, S. W., Chou, T. & Ralph, M. R. (2004) Behav. Brain Res. 150, 201-205. [DOI] [PubMed] [Google Scholar]
- 5.Holloway, F. A. & Wansley, R. A. (1973) Behav. Biol. 9, 1-14. [DOI] [PubMed] [Google Scholar]
- 6.Valentinuzzi, V. S., Kolker, D. E., Vitaterna, M. H., Ferrari, E., Takahashi, J. S. & Turek, F. W. (2001) Anim. Learn. Behav. 29, 133-142. [Google Scholar]
- 7.Chaudhury, D. & Colwell, C. S. (2002) Behav. Brain Res. 133, 95-108. [DOI] [PubMed] [Google Scholar]
- 8.McDonald, R. J., Hong, N. S., Ray, C. & Martin, R. R. (2002) Learn. Motiv. 33, 230-252. [Google Scholar]
- 9.Cain, S. W., Ko, C. H., Chalmers, J. A. & Ralph, M. R. (2004) Neurobiol. Learn. Mem. 81, 217-220. [DOI] [PubMed] [Google Scholar]
- 10.Kandel, E. R. (2001) Science 294, 1030-1038. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez, R. I., Lyons, L. C., Levenson, J., Khabour, O. & Eskin, A. (2003) Proc. Natl. Acad. Sci. USA 100, 14415-14420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Susswein, A. J., Schwarz, M. & Feldman, E. (1986) J. Neurosci. 6, 1513-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziv, I., Lustig, C., Ben-Zion, M. & Susswein, A. J. (1991) Behav. Neural Biol. 55, 86-107. [DOI] [PubMed] [Google Scholar]
- 14.Botzer, D., Markovich, S. & Susswein, A. J. (1998) Learn. Mem. 5, 204-219. [PMC free article] [PubMed] [Google Scholar]
- 15.Katzoff, A., Ben-Gedalya, T. & Susswein, A. J. (2002) J. Neurosci. 22, 9581-9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwarz, M. & Susswein, A. J. (1986) J. Neurosci. 6, 1528-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwarz, M. & Susswein, A. J. (1992) Behav. Neurosci. 106, 250-261. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz, M., Blumberg, S. & Susswein, A. J. (1998) Behav. Neurosci. 112, 942-951. [PubMed] [Google Scholar]
- 19.Schwarz, M., Feldman, E. & Susswein, A. J. (1991) Behav. Neurosci. 105, 193-201. [DOI] [PubMed] [Google Scholar]
- 20.Kupfermann, I. (1974) Behav. Biol. 10, 1-26. [DOI] [PubMed] [Google Scholar]
- 21.Levenson, J., Byrne, J. H. & Eskin, A. (1999) J. Neurosci. 19, 8094-8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacklet, J. W. (1969) Science 164, 563-564. [Google Scholar]
- 23.Eskin, A. (1971) Z. Vergl. Physiol. 74, 353-371. [Google Scholar]
- 24.Olson, L. M. & Jacklet, J. W. (1985) J. Neurosci. 5, 3214-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harf, L., Arch, S. & Eskin, A. (1976) Brain Res. 111, 295-299. [DOI] [PubMed] [Google Scholar]
- 26.Plautz, J. D., Kaneko, M., Hall, J. C. & Kay, S. A. (1997) Science 278, 1632-1635. [DOI] [PubMed] [Google Scholar]
- 27.Elliott, C. J. & Susswein, A. J. (2002) J. Exp. Biol. 205, 877-896. [DOI] [PubMed] [Google Scholar]
- 28.Cropper, E. C., Evans, C. G., Hurwitz, I., Jing, J., Proekt, A., Romero, A. & Rosen, S. C. (2004) Neurosignals 13, 70-86. [DOI] [PubMed] [Google Scholar]
- 29.Cohen-Armon, M., Visochek, L., Katzoff, A., Levitan, D., Susswein, A. J., Klein, R., Valbrun, M. & Schwartz, J. H. (2004) Science 304, 1820-1822. [DOI] [PubMed] [Google Scholar]
- 30.Wagatsuma, A., Sugai, R., Chono, K., Azami, S., Hatakeyama, D., Sadamoto, H. & Itoi, E. (2004) Acta Biol. Hung. 55, 149-155. [DOI] [PubMed] [Google Scholar]