Abstract
Whether nonhuman primates exhibit population-level handedness remains a topic of considerabe theoretical and empirical debate. One continued subject of discussion is whether evidence of population-level handedness in primates is confined to studies in captive animals or whether it is in both captive and wild subjects. Here, we report evidence of population-level handedness in wild chimpanzees for a tool-use task known as “termite-fishing.” We subsequently compared the handedness for termite-fishing with other published reports on handedness for nut-cracking and wadge-dipping and found task-specific differences in handedness. Last, when combing all of the published data on tool use in wild chimpanzees, we show that hand preferences are heritable. Contrary to previous claims, our results demonstrate that populationlevel handedness is evident in wild chimpanzees and suggest that the antecedents of lateralization of function associated with hand use were present at least 5 million years ago, before the Pan-Homo split.
Keywords: hemisphere specialization, laterality, primates
Right-handedness is a universal trait of humans (1), and some have argued that population-level handedness is unique to hominid evolution (2-4). This argument rests primarily on the lack of evidence for population-level handedness in nonhuman primates, particularly in our closest living relative, the chimpanzee. However, recent studies in a host of vertebrate species have demonstrated evidence of population-level behavioral asymmetries (5). For example, studies in chickens and pigeons have shown different abilities of the left and right hemispheres for different visual discrimination tasks (6). Other studies have shown that frogs show right paw preferences for certain motor actions such as removing substrates from their body (7), and fish show preferential looking biases with one eye or the other when viewing a predator (8). Collectively, these findings raise questions about the assumption that language is a necessary condition for the expression of laterality. Notwithstanding the positive findings in lower vertebrates, one area that continues to be a source of considerable empirical and theoretical debate is whether nonhuman primates, particularly great apes, show population-level handedness (9-12).
Recent studies in captive chimpanzees have reported evidence of population-level right-handedness for several measures, including simple reaching (13, 14), bimanual feeding (15), coordinated bimanual actions (16, 17), throwing (18), and manual gestures (19). These findings are consistent with reports in other captive great apes including gorillas (refs. 20-22; but see ref. 23) and bonobos (refs. 24 and 25; but see ref. 26). In contrast to findings in captive great apes, evidence of population-level handedness in wild apes is extremely rare (27-29) or virtually absent in the case of chimpanzees (30-37). The discrepancy in findings between captive and wild apes, notably chimpanzees, has prompted some to argue that the findings from captive apes are due to the individuals being reared in a human, right-handed environment and that population-level asymmetry in hand use is not a species-typical trait (10, 12). In contrast, others have suggested that the absence of population-level handedness in wild chimpanzees reflects a lack of sophistication of the behaviors measured and limited sample sizes within a given study (11). For example, nearly all of the studies in wild chimpanzees have relatively small sample sizes compared with reports about captive apes (11, 38). Studies of captive chimpanzees have significantly more statistical power and therefore are more sensitive to detecting effects compared with studies of wild chimpanzees. Moreover, the types of handedness measured in captive and wild chimpanzees vary dramatically and make comparison of the findings in these two settings very difficult. For example, in studies with captive chimpanzees, the importance of measuring coordinated bimanual actions has been emphasized by a number of investigators (16, 17, 27), and rarely have coordinated bimanual actions been studied in wild apes. In fact, the most compelling evidence of population-level handedness in wild apes has been for measures of bimanual feeding in wild gorillas and chimpanzees (27, 28). In the case of wild chimpanzees (28), significant sex differences were found for bimanual feeding, with males showing left-handedness and females showing right-handedness; therefore, characterizing population-level handedness was sex dependent.
In the current study, we report evidence of population-level handedness for termite-fishing in wild chimpanzees. Termitefishing involves precision movements that require the chimpanzees to insert small sticks into holes in dirt mounds that contain the termites. Hand preferences during the termite-fishing probing actions were obtained in a sample of wild chimpanzees in the current study. We subsequently compared these results to previous reports of handedness for other forms of tool use in wild chimpanzees, including nut-cracking and wadge-dipping and show that directional biases in hand use vary depending on the type of tool use.
In addition to the data on the distribution of hand preferences in wild chimpanzees, we also report evidence that hand preferences run in families of wild chimpanzees, at least in terms of the association between offspring and their mothers' and siblings' hand preferences. Human handedness runs in families, and both genetic (4, 39, 40) and nongenetic (41-43) models have been proposed to explain the preponderance of human righthandedness. In contrast to human models of handedness, many have argued that nonhuman primate handedness is a result of random, nongenetic factors, and this argument explains the previous claims for a lack of significant population-level handedness in these species (44, 45). Our aim was not to test any specific genetic or nongenetic models of handedness, but rather to simply evaluate whether handedness occurred systematically in offspring based on the handedness of their mother. Although evidence of heritability in hand preferences does not necessarily demonstrate a genetic basis of handedness, it would argue against explanations proposing that hand preferences are determined by random factors.
Methods
Subjects and Setting. Hand preference data were initially collected for termite-fishing in a sample of 17 chimpanzees living in the Gombe National Park, Tanzania. Gombe is a small (35-km2) park, located on the western border of Tanzania and is home to three communities of chimpanzees. Individuals from the Kasekela community, which has been studied for >40 years, were observed for this study (Table 1). The chimpanzees termite-fish year-round, but their efforts become intensely concentrated at the start of the rainy season, from October to December (46). For this study, E.V.L. and a Tanzanian research assistant (K. John) collected data during four periods of field work at Gombe National Park, from October through December in 1998 (35 days), 1999 (41 days), 2000 (43 days), and 2001 (44 days) on a total of 5 mothers and 14 offspring (8 males, 6 females) over the 4-year period. Seventeen of these individuals (5 mothers and 12 offspring) were analyzed for this study.
Table 1. Subject and hand use descriptive statistics for termite fishing.
| No. of responses
|
|||||
|---|---|---|---|---|---|
| Subject | Sex | Left | Right | HI | Hand preference |
| FF | F | 199 | 65 | −0.507 | L |
| FLR | F | 82 | 45 | −0.291 | L |
| FE | M | 159 | 178 | 0.056 | A |
| FO | M | 54 | 1 | −0.964 | L |
| FN | F | 0 | 601 | 1.00 | R |
| FU | M | 6 | 25 | 0.613 | R |
| GM | F | 400 | 225 | −0.280 | L |
| GLD* | F | 108 | 62 | −0.271 | L |
| GLT* | F | 150 | 272 | 0.289 | R |
| GA | F | 1,106 | 109 | −0.821 | L |
| GD | M | 93 | 197 | 0.359 | R |
| PI | F | 804 | 0 | −1.00 | L |
| TN | M | 1,109 | 116 | −0.811 | L |
| SA | F | 351 | 196 | −0.283 | L |
| SM | M | 390 | 0 | −1.00 | L |
| SR | F | 530 | 33 | −0.883 | L |
| SI | F | 76 | 25 | −0.505 | L |
HI, handedness index; A, ambiguously handed.
Individuals are fraternal twins.
Procedure. All-day focal animal follows (47) were performed over four consecutive termite-fishing seasons on the females who had offspring <11 years of age. When a termite-fishing session occurred, a focal target was selected from a randomized sequence generated for each family (mother and offspring) and was videotaped for a 15-min bout before moving on to the next individual in the sequence. Unfinished 15-min bouts (e.g., only 9 min of data were collected before the session terminated when the family left the mound) were continued during the next session. By using this methodology, >67 h of video footage from termite-fishing sessions was collected.
Videotaped data were transferred to a digital format and copied onto compact diskettes to facilitate analyses by using the observer video-pro (Noldus Information Technology, Wageningen, The Netherlands), a software package for behavioral analysis. During termite-fishing bouts, each individual sequence of insertion and withdrawal was scored as one “dip.” Each dip was also scored as one of the following: (i)LL, inserted and withdrawn with left hand; (ii) LR, inserted with left and withdrawn with right hand; (iii) RL, inserted with right and withdrawn with left; (iv) RR, inserted and withdrawn with right hand. Dips that were not completed (e.g. the individual inserted the tool and then left without withdrawing it) or that were not visible for the complete sequence of insert/withdraw were excluded in analyses.
For each chimpanzee, a handedness index (HI) was derived for each subject by subtracting the number of left-hand responses from the number of right-hand responses and dividing by the total number of responses. We combined the data from the different years because a split-half correlation coefficient between HI scores revealed a significantly positive association (r = 0.834, P < 0.01), indicating consistent hand use across observation periods. RL and LR responses were rare (<1% of dips); thus, HI scores were based only on the LL or RR response because they were the most frequent. Positive values reflected right-hand biases and negative values reflected left-hand biases. In addition, subjects were classified as left-, right- or ambiguously handed based on binomial z scores calculated on the basis of the frequency of left- and right-hand use. Chimpanzees with z scores above or below 1.96 were classified as right- or left-handed. All others were classified as ambiguously handed.
Results
Population-Level Handedness. A one-sample t test on the HI scores revealed significant population-level left-handedness in chimpanzees [t(16) = 2.32, P < 0.01] (see Fig. 1a). An independent sample t test failed to reveal sex differences in hand use. Based on the classification data, there were significantly more lefthanded (n = 12) than right-handed (n = 4) (z = 2.00, P < 0.05) and ambiguously handed (n = 1) subjects (z = 3.05, P < 0.01).
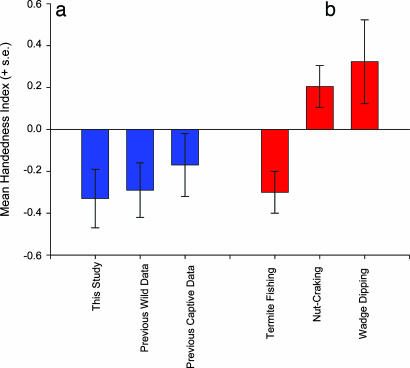
Fig. 1.
Mean HI scores for different chimpanzee cohorts. (a) HI data from this study (n = 17), compared with previous wild data (n = 37) (33, 34, 48) and previous captive data (n = 29) (49-52). (b) All termite- and ant-fishing data (n = 54), compared with nut-cracking (n = 63) (31, 32) and wadge-dipping (n = 16) (32). Within each study, only subjects that made five or more responses were included in the analysis.
Comparison with Previous Studies on Hand Use for Termite Fishing. Hand preferences for termite- or ant-fishing has been reported previously in 52 wild chimpanzees from three separate studies, 37 of which had 5 or more observations of hand use (33, 34, 48). Although no evidence of population-level handedness has been claimed in any of these reports, we compared the HI scores from previous findings on handedness for termite-fishing to those from our sample of wild chimpanzees (see Fig. 1a). Because the raw frequencies in hand use were provided and the previous studies and the methods of data collection were comparable, comparing the data between studies was straightforward. HI scores for the previous studies were derived by using the above referenced formula based on the frequencies of left- and righthand use reported in the papers for individual subjects. An analysis of variance between HI scores from our data and the previous studies on hand use for termite- and ant-fishing failed to reveal significant differences. Combining all of the data on hand use for termite-fishing (n = 54; 17 from this study, 37 from the previous studies) confirms the presence of population-level left-handedness [t(53) = 2.34, P < 0.001]. Of the 54 subjects, there were 29 left-handed, 15 right-handed, and 10 ambiguously handed. The number of left-handed chimpanzees was significantly greater than the number of right- (z = 2.11, P < 0.05) and ambiguously (z = 2.72, P < 0.01) handed individuals.
As noted, some have suggested that the expression of handedness differs between wild and captive chimpanzees because of the influence of human rearing. Rearing chimpanzees by righthanded humans presumably causes captive-born chimpanzees to be more right-handed than wild-born individuals. As a means of testing this hypothesis, the HI scores from the combined wild chimpanzee data were compared with the HI scores provided in three previously published reports of hand use for tool-use tasks in captive chimpanzees (n = 29) that mimic the demands of termite-fishing in wild chimpanzees (49-52). In the captive studies, the chimpanzees needed to probe with sticks to extract food (yogurt or honey) from an artificial termite mound. The mean HI scores for captive chimpanzees did not differ significantly from the data for wild chimpanzees [t(81) = 0.02, not significant] (see Fig. 1a).
Comparison to Previous Studies on Hand Use for Other Forms of Tool Use. Besides termite-fishing, hand use for other forms of tool use have been described in wild chimpanzees, notably during hammer use when cracking open nuts (31, 32, 37) and wadge-dipping (sometimes referred to as leaf-sponging) (32). In contrast to the fine sensorimotor demands of termite-fishing, hammer use by chimpanzees during nut-cracking involves the forceful execution of ballistic actions. Wadge-dipping involves the chimpanzees wadging up leaves to form a sponge that they dip into the bases of tree trunks to extract water. Given the differences in motor and haptic demands of these two tasks relative to termite-fishing, it might be argued that different preferences might be observed in these tool-use tasks. To test this hypothesis, we calculated HI scores from the individual data presented in the studies on nut-cracking and wadge-dipping. The nut-cracking (n = 63) and wadge-dipping (n = 16) HI scores were compared with the termite-fishing data in two separate analyses of variance. Sex and task were the independent variables, whereas the HI scores served as the dependent variable. Although no sex differences were found, significant differences in HI scores were found between termite-fishing and nut-cracking [F(1, 114) = 7.52, P < 0.007] and termite-fishing and wadge-dipping [F(1, 67) = 6.54, P < 0.002] (Fig. 1b). Wild chimpanzees are significantly more right-handed for nut-cracking and wadge-dipping compared with termite-fishing. Moreover, when considering the HI scores for each specific form of tool use, wild chimpanzees show population-level right-handedness for nut-cracking [t(62) = 1.97, P < 0.05], and population-level left-handedness is found for termite-fishing [t(53) = 2.44, P < 0.01]. Chimpanzees were borderline significantly right-handed for wadge-dipping [t(15) = 1.69, P < 0.10].
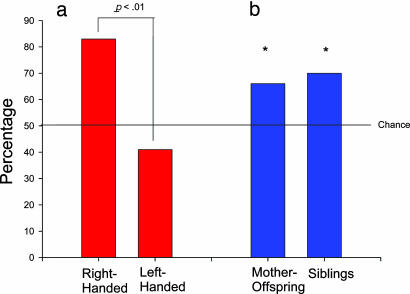
Heritability of Hand Preferences in Wild Chimpanzees. To evaluate whether hand preferences were heritable for all of the abovereferenced studies in wild chimpanzees, correlations between the HI scores of offspring and their mothers (n = 64 pairs) as well as between maternal half-siblings (n = 41 pairs) were performed. In addition, concordance rates in hand use between mothers and offspring, as well as siblings, were calculated. Both mother-offspring (r = 0.39, df = 62, P < 0.04) and maternal half-siblings (r = 0.54, df = 39, P < 0.01) correlation coefficients were found to be positive and significant. A χ2 test of independence between maternal and offspring hand preference classifications revealed a significant association [χ2 (1, n = 64) = 9.18, P < 0.01] (see Fig. 2a). Right- and left-handed females tended to produce offspring exhibiting the same hand preference. Similarly, concordance rates in hand use between mothers and offspring (z = 2.50, P < 0.01) and between maternal half-siblings (z = 2.81, P < 0.05) were significantly different from chance values (see Fig. 2b). Because multiple offspring that had the same mother were assessed for hand use, we took a more conservative approach in evaluating the heritability in hand use. For females that produced >1 offspring, we calculated an average HI score for all of the offspring and correlated this value with their individual mothers HI score. Although the total number of pairs of HI scores was reduced in this analysis (n = 43), the correlation remained essentially unchanged and remained significant (r = 0.395, df = 41, P < 0.05).
Fig. 2.
Heritability of hand preferences in all wild chimpanzees. (a) Values indicate the percentage of offspring classified as right-handed in right- and left-handed females. (b) Values indicate the percentage of mother-offspring dyads and maternal half-siblings that were concordant in hand preference. *, P < 0.05.
Discussion
There are three significant findings revealed in this study. First, contrary to previous claims, wild chimpanzees show population-level handedness in tool use. Second, directional biases in hand use vary depending on the type of tool use. Termite-fishing elicits left-handedness, whereas nut-cracking, and to a lesser degree wadge-dipping, elicits right-handedness. Third, handedness runs in families of wild chimpanzees, with offspring hand use resembling the hand preferences of their mother.
With respect to the evidence of population-level handedness, our findings suggest that the lack of significant findings reported in previous studies was because of limited statistical power due to the relatively small sample sizes. Hopkins and Cantalupo (11) recently demonstrated, based on data from captive chimpanzees, that asymmetries in hand use have a small to moderate effect size. Based on the estimated effect sizes from a number of studies of hand use in chimpanzees, Hopkins and Cantalupo (11) estimated that at least 59 subjects were needed to detect significant effects with 80% confidence. Few individual studies of hand use in wild chimpanzees have samples that exceed 59 individuals, but combining data across studies provides adequate power to detect these effects. It should also be emphasized that in a number of the studies of wild chimpanzees that have measured hand preferences for tool use, the subjects show exclusive or near exclusive hand use (e.g., ref. 32). The estimates of variability in HI scores therefore become larger because there is much greater range in scores between left- and right-handed individuals. The greater range in HI scores creates larger estimates in nonsystematic variability (or error) and also makes detecting population-level effects more difficult.
The results of our study indicate that wild chimpanzees exhibit task-specific population-level handedness. Chimpanzees that nut-crack are predominantly right-handed, whereas the chimpanzees that termite-fish are left-handed. Wild chimpanzees are borderline significantly right-handed for wadge-dipping. There are several possible explanations for the task-specific variation in hand preferences. First, variation in the cognitive, sensory, and motor demands of termite-fishing compared with nut-cracking and wadge-dipping might explain the differences found for hand preferences in each community. For example, nut-cracking involves ballistic movements of the tool on the substrate, whereas termite-fishing might be considered a task requiring fine motor skill because the chimpanzees must insert a small stick into a hole. Unfortunately, evaluating this explanation in this sample of wild chimpanzees is difficult because none of the communities studied show all three behaviors. Second, task-specific variation in hand use may be because of the social or cultural learning traditions of these different chimpanzee societies, as has been suggested to explain the different patterns of tool use observed in African chimpanzees (53, 54). In this scenario, in addition to learning different types of tool-use behavior, the chimpanzees also learn to use a specific hand based on the motor demands of the different tool tasks and the models of hand use seen within each distinct chimpanzee community. However, this conclusion is at odds with the lack of evidence of rearing effects on handedness in captive chimpanzees. If social learning were a strong factor influencing hand preference then, presumably, captive chimpanzees raised by humans would show more pronounced right-handedness. Moreover, in captivity and in the wild, concordance rates in handedness are stronger between siblings than between mothers and their offspring (ref. 33; see also Fig. 2b). Presumably the mother would be a stronger “model” of hand use than siblings (53). Last, many of the chimpanzees in these different communities are genetically related and what may be seen as task-specific differences may reflect the expression of divergent genetic lines of right- and left-handed chimpanzee families. The evidence of heritability in hand preference for tool use in this study supports either the genetic or social learning explanations. Previous studies in captive chimpanzees have also demonstrated heritability in hand preferences and, in these studies, genetics played a stronger role than nongenetic factors (55-57). Whether this observation is the case in wild chimpanzees is not clear because genetically related individuals have not been raised apart, as has been the case in studies in captive chimpanzees (57). Additional studies in captive and wild apes will be needed to isolate the role of these two factors on the development of hand preferences in chimpanzees.
In terms of the distribution of hand use, there is a 2:1 ratio of dominant- to non-dominant-handed individuals within each community of chimpanzees, and these results are consistent with findings in captive chimpanzees (38) but are substantially lower than the typical 8:1 or 9:1 ratio reported in human societies (1, 58). For instance, in three traditional societies, Marchant et al. (59) reported that between 78 and 90% of individuals showed predominantly right-hand preferences for tool use. The origin of the difference in the ratio of dominant- to non-dominant-handed individuals between humans and chimpanzees remains unclear, but one possibility is that there was a genetic mutation in hominid evolution that enhanced preferential use of the right hand and is now seen in modern humans (4). Alternatively, the 2:1 ratio may reflect the true biological expression of hemispheric specialization in primates, and the larger ratios seen in modern humans reflect the additive effects of social, pedagogical, and cultural mechanisms (59, 60).
In sum, our results are evidence of population-level handedness in wild chimpanzees that is not sex dependent (see ref. 28). The results further indicate that directional biases in hand use are task-specific when comparing the distribution of handedness for different tool-use measures. These findings reinforce the view that the motor and cognitive demands of different tasks can have a significant influence on handedness in human and nonhuman primates. Last, hand preferences in wild chimpanzees are heritable. Whether heritability in hand use is because of social learning or genetic factors is not clear and will require additional behavioral-genetic analyses to isolate the mechanism of intra-familial transfer. Additional research, particularly studies focusing on candidate genes for handedness and cerebral dominance, should clarify the difference in distribution of hand use between Pan and Homo.
Acknowledgments
E.V.L. thanks the Gombe National Park staff, the Government of Tanzania, Tanzania National Parks, Tanzania Commission for Science and Technology, and Tanzania Wildlife Research Institute for support and permission while carrying out this research. E.V.L. was funded by the National Science Foundation, the L. S. B. Leakey Foundation, the Wenner-Gren Foundation, and the University of Minnesota Graduate School. W.D.H. was supported by National Institutes of Health Grants NS-36605 and NS-42867.
Author contributions: E.V.L. designed research and performed research; W.D.H. analyzed data; and E.V.L. and W.D.H. wrote the paper.
Abbreviation: HI, handedness index.
References
- 1.Raymond, M. & Pontier, D. (2004) Laterality 9, 35-51. [DOI] [PubMed] [Google Scholar]
- 2.Bradshaw, B. & Rogers, L. (1993) The Evolution of Lateral Asymmetries, Language, Tool-Use, and Intellect (Academic, San Diego).
- 3.Annett, M. (2002) Handedness and Brain Asymmetry: The Right Shift Theory (Psychology Press, London).
- 4.Corballis, M. C. (1997) Psychol. Rev. 104, 714-727. [DOI] [PubMed] [Google Scholar]
- 5.Rogers, L. J. & Andrew, J. R. (2002) Comparative Vertebrate Lateralization (Cambridge Univ. Press, Cambridge, U.K.).
- 6.Vallortigara, G. (1992) Neuropsychologia 30, 761-768. [DOI] [PubMed] [Google Scholar]
- 7.Bisazza, A., Cantalupo, C., Robins, A., Rogers, L. J. & Vallortigara, G. (1996) Nature 379, 408 (lett.). [Google Scholar]
- 8.Bisazza, A., Cantalupo, C., Capocchiano, M. & Vallortigara, G. (2000) Laterality 5, 269-284. [DOI] [PubMed] [Google Scholar]
- 9.Crow, T. (2004) Cortex 40, 120-134. [Google Scholar]
- 10.McGrew, W. C. & Marchant, L. F. (1997) Yearb. Phys. Anthropol. 40, 201-232. [Google Scholar]
- 11.Hopkins, W. D. & Cantalupo, C. (2005) Laterality 10, 65-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmer, A. R. (2002) Am. J. Phys. Anthropol. 118, 191-199. [DOI] [PubMed] [Google Scholar]
- 13.Colell, M., Segarra, M. D. & Sabater-Pi, J. (1995) Int. J. Primatol. 16, 413-434. [Google Scholar]
- 14.Hopkins, W. D., Russell, J., Hook, M., Braccini, S. & Schapiro, S. J. (2005) Int. J. Primatol. 26, 259-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hopkins, W. D. (1994) Dev. Psychobiol. 27, 395-407. [DOI] [PubMed] [Google Scholar]
- 16.Colell, M., Segarra, M. D., Sabater-Pi, J. (1995) J. Comp. Psychol. 109, 298-307. [DOI] [PubMed] [Google Scholar]
- 17.Hopkins, W. D., Wesley, M. J., Izard, M. K., Hook, M. & Schapiro, S. J. (2004) Behav. Neurosci. 118, 659-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopkins, W. D., Russell, J., Cantalupo, C., Freeman, H. & Schapiro, S. J. (2005) J. Comp. Psychol., in press. [DOI] [PMC free article] [PubMed]
- 19.Hopkins, W. D., Russell, J., Freeman, H., Buehler, N., Reynolds, E. & Schapiro, S. J. (2005) Psychol. Sci. 16, 487-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fagot, J. & Vauclair, J. (1988) Brain Behav. Evol. 32, 89-95. [DOI] [PubMed] [Google Scholar]
- 21.Olson, D. A., Ellis, J. E. & Nadler, R. D. (1990) Am. J. Primatol. 20, 83-94. [DOI] [PubMed] [Google Scholar]
- 22.Shafer, D. D. (1993) in Primate Laterality: Current Behavioral Evidence of Primate Asymmetries, eds. Ward, J. P. & Hopkins, W. D. (Springer, New York), pp. 267-283.
- 23.Annett, M. & Annett, J. (1991) Cortex 27, 269-285. [DOI] [PubMed] [Google Scholar]
- 24.Hopkins, W. D. & de Waal, F. D. (1995) Int. J. Primatol. 16, 261-276. [Google Scholar]
- 25.Shafer, D. D. (1997) Primates 38, 303-313. [Google Scholar]
- 26.Vleeschouwer, K. D., Van Elsacker, L. & Verheyen, R. E. (1995) J. Comp. Psychol. 109, 203-207. [DOI] [PubMed] [Google Scholar]
- 27.Byrne, R. W. & Byrne, J. M. (1991) Cortex 27, 521-536. [DOI] [PubMed] [Google Scholar]
- 28.Corp, N. & Byrne, R. W. (2004) Am. J. Phys. Anthropol. 123, 62-68. [DOI] [PubMed] [Google Scholar]
- 29.Parnell, R. J. (2001) J. Comp. Psychol. 115, 365-375. [PubMed] [Google Scholar]
- 30.Albrecht, H. & Dunnett, S. C. (1971) Chimpanzees in Western Africa (Piper, Munich).
- 31.Biro, D., Inoue-Nakamura, N., Tonooka, R., Yamakoshi, G., Sousa, C. & Matsuzawa, T. (2003) Anim. Cogn. 6, 213-223. [DOI] [PubMed] [Google Scholar]
- 32.Boesch, C. (1991) Int. J. Primatol. 6, 541-558. [Google Scholar]
- 33.McGrew, W. C. & Marchant, L. F. (1992) Curr. Anthropol. 33, 114-119. [Google Scholar]
- 34.McGrew, W. C. & Marchant, L. F. (1996) in Great Ape Societies, eds., McGrew, W. C., Marchant, L. F. & Nishida, T. (Cambridge Univ. Press, Cambridge, U.K.), pp. 255-272.
- 35.Marchant, L. F. & McGrew, W. C. (1996) J. Hum. Evol. 30, 427-443. [Google Scholar]
- 36.McGrew, W. C. & Marchant, L. F. (2001) Behaviour 138, 329-358. [Google Scholar]
- 37.Sugiyama, Y., Fushimi, T., Sakura, O. & Matsuzawa, T. (1993) Primates 34, 151-159. [Google Scholar]
- 38.Hopkins, W. D. (2005) Cortex, in press.
- 39.Annett, M. (1985) Left, Right, Hand, and Brain: The Right-Shift Theory (Erlbaum, London).
- 40.McManus, C. (1985) Psychol. Med. 18, 347-355. [Google Scholar]
- 41.Michel, G. F. (1981) Science 212, 685-687. [DOI] [PubMed] [Google Scholar]
- 42.Previc, F. H. (1991) Psychol. Rev. 98, 299-334. [DOI] [PubMed] [Google Scholar]
- 43.Provins, K. A. (1997) Psychol. Rev. 104, 554-571. [DOI] [PubMed] [Google Scholar]
- 44.Ettlinger, G. F. (1988) Cortex 24, 389-398. [DOI] [PubMed] [Google Scholar]
- 45.Warren, J. M. (1980) Physiol. Psychol. 8, 351-359. [Google Scholar]
- 46.Goodall, J. (1986) The Chimpanzees of Gombe: Patterns in Adaptation (Harvard Univ. Press, Cambridge, MA).
- 47.Altman, J. (1974) Behaviour 47, 227-267. [DOI] [PubMed] [Google Scholar]
- 48.Nishida, T. & Hiraiwa, M. (1982) J. Hum. Evol. 14, 73-99. [Google Scholar]
- 49.Fletcher, A. W. & Weghorst, J. A. (2005) Laterality 10, 219-242. [DOI] [PubMed] [Google Scholar]
- 50.Hopkins, W. D. (1999) Int. J. Primatol. 20, 851-866. [Google Scholar]
- 51.Morange, F. (1994) in Behavioral Neuroscience, Physiology, and Reproduction, Current Primatology, eds., Anderson, J. R., Roeder, J. J., Thierry, B. & Herrenschmidt, N. (Univ. Louis Pasteur, Strasburg, France), Vol. 3, pp. 61-67. [Google Scholar]
- 52.Steiner, S. M. (1990) Friends of Washoe 9 (1), 9-19. [Google Scholar]
- 53.Lonsdorf, E. V. (2005) Anim. Behav., in press.
- 54.Whiten, A., Goodall, J., McGrew, W. C., Nishida, T., Reynolds, V., Sugiyama, Y., Tutin, C. E. G., Wrangham, R. W. & Boesch, C. (2001) Behaviour 138, 1489-1525. [DOI] [PubMed] [Google Scholar]
- 55.Hopkins, W. D. (1999) J. Comp. Psychol. 113, 307-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hopkins, W. D., Bales, S. & Bennett, A. J. (1994) Int. J. Neurosci. 74, 17-26. [DOI] [PubMed] [Google Scholar]
- 57.Hopkins, W. D., Dahl, J. F. & Pilcher, D. (2001) Psychol. Sci. 12, 299-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Corballis, M. C. (2002) From Hand to Mouth: The Origins of Language (Princeton Univ. Press, Princeton).
- 59.Marchant, L. F., McGrew, W. C. & Eibl-Eibesfeldt, I. (1995) Ethology 101, 239-258. [Google Scholar]
- 60.Hopkins, W. D. (2004) Int. J. Primatol. 25, 1243-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]