Topical microbicides are a broad class of agents designed to block or kill infectious microorganisms directly at the site of transmission. With the AIDS pandemic continuing its unrelenting global march (40 million current infections, 14,000 new infections per day) driven largely by sexual transmission, microbicides have moved steadily toward the front line of preventative strategies. Indeed, many candidate anti-HIV microbicides are currently under development, with several already in clinical trials. A battery of promising protein-based HIV inhibitors can potentially be developed (1), but they face serious challenges of high production costs and instability during transport and storage. In a recent issue of PNAS, Rao et al. (2) presented an intriguing version of a “live microbicide” approach whereby a commensal bacterium is engineered to secrete a potent anti-HIV peptide. When administered orally or as a rectal suppository, the bacteria would colonize the gut mucosa and secrete the peptide in situ, thereby providing protection in advance of exposure hopefully for days, weeks, or even months. This delivery mode would be highly advantageous over others requiring repeated topical application before each act of intercourse; also, the engineered bacteria would be relatively simple and inexpensive to manufacture, transport, and store.
Although the general concept of a live microbicide is not new (as noted by Rao et al.), several aspects of this study are particularly noteworthy. The first is the choice of HIV-1 inhibitor: a 52-aa peptide derived from the C-terminal heptad repeat (HR2) region of gp41, the transmembrane subunit of the HIV-1 envelope glycoprotein (Env). Extensive studies have documented the potent neutralizing activities of various HR2-based peptides (3). They block HIV-1 entry by binding to the N-terminal heptad repeat (HR1) region of the gp41 prehairpin metastable intermediate that forms transiently after interaction of the Env gp120 subunit with target cell receptors (CD4 followed by coreceptor CCR5 or CXCR4). HR2 peptide binding prevents the prehairpin intermediate from collapsing into the “trimer-of-hairpins structure,” the conformational change that drives the membrane fusion mechanism for HIV entry. Advantages of HR2 peptides for the live microbicide approach are many: they are small relative to alternative protein-based inhibitors, they are less sensitive to proteolysis and denaturation, they require no posttranslational modifications, they act extracellularly, they target the highly conserved HR1 region of gp41 and can neutralize genotypically and phenotypically diverse HIV-1 variants, and their efficacy is not compromised by the resistance mutations that accompany standard antiretroviral regimens targeting HIV reverse transcriptase and/or protease. The clinical success of the HR2 peptide enfuvirtide (Fuzeon, formerly T-20) as a salvage therapy attests to its in vivo activity and safety when administered systemically by s.c. injection (3). The other noteworthy features of Rao et al. are the particular organism used and how it was engineered for optimal secretion. The highly colonizing Escherichia coli Nissle 1917 strain, which has been used for decades to prevent inflammatory bowel diseases (4), was obtained from a commercially available probiotic tablet. The bacteria were cotransformed with an expression plasmid containing a genetic construct encoding the 52-aa HR2 sequence grafted onto various lengths of the C-terminal secretion signal derived from hemolysin A, plus a second plasmid containing the transporter genes of the E. coli hemolysin secretion system (5). The former and latter plasmids also contained genes conferring resistance to ampicillin and chloramphenicol, respectively.
A commensal bacterium is engineered to secrete a potent anti-HIV peptide.
Rao et al. (2) demonstrate high-level in vitro secretion of the intact fusion peptides, whose neutralizing activities were comparable to those reported for various unfused HR2 peptides. The engineered bacteria were administered to CD-1 mice orally or rectally, and colonization (as measured by fecal bacteria counts) was observed; it was maintained at high levels for up to 12 days, but only if ampicillin was coadministered for selection. To achieve more durable colonization in the absence of antibiotic, animals were treated with ampicillin for 50 days to minimize competition with the indigenous microflora; significant colonization then persisted for at least 50 more days after antibiotic removal. Tissue examination at 3 days after inoculation indicated that the bacteria preferentially colonized the lower (rectum up to the ileum) or upper (duodenum down to the colon) GI tract when administered by rectal or oral routes, respectively. Peptide expression was readily detected in day-3 colon samples (immunohistochemistry), and there was no evidence of inflammation or necrosis (histopathology).
Rao et al. (2) provide proof-of-concept that a commensal bacterial strain can indeed be genetically engineered to function as a live microbicide factory capable of setting up shop at various regions of the gut mucosa. These promising findings beg the obvious question: Can a useful microbicide based on the Nissle/HR2 peptide system be developed? Studies using the more relevant macaques/SHIV model will be essential to address this question by testing the most critical issues: protective efficacy and safety. This model has been used to test candidate protein microbicides for protection against vaginal (6–8) or rectal (9) challenge, by using conventional delivery modes such as aqueous solutions or gels. Encouraging in vivo protection results have been obtained with proteins that neutralize infection by binding to free virions, e.g., monoclonal antibody b12, which blocks the CD4 binding site on gp120 (6); cyanovirin-N, which binds to oligosaccharide residues on Env (7, 9). Protection has also been achieved with a chemokine derivative that blocks CCR5 (8). However, in each case, the protein dosage required for protection was orders of magnitude higher than predicted based simply on in vitro potency. These sobering results might be explained in part by the extremely high amounts of challenge virus required for monkey studies in which all control animals must be infected; much lower doses may be required in real practice, given the low sexual transmission frequencies in human populations.
A variety of issues must be addressed if the Rao et al. (2) approach is to advance to human trials. For one, the requirement for antibiotics to maintain colonization must be eliminated. Rao et al. suggest that concerns related to horizontal transfer of plasmid-based antibiotic genes could be addressed by integrating the peptide expression cassette into the bacterial chromosome. Nevertheless, repeated purging of the indigenous gut microflora will pose unacceptable medical risks. Rao et al. note that, because Nissle 1917 is a native human strain, it might colonize more effectively in people than in mice; moreover, colonization efficiency might be improved by genetic manipulation, with the goal of eliminating the antibiotic requirement. A second issue relates to the unique challenges associated with rectal transmission. The GI tract is the largest mucosal tissue/immune organ in the body, with many more potential HIV target cells than the vagina. The sheer volume of the colon and its fragile single-cell thickness combine to present formidable challenges for microbicide development. On the issue of safety, Rao et al. detected no inflammation in mice treated with the engineered bacteria. However, the treatment period lasted only 3 days, leaving open the question of inflammatory reactions with long-term use. Of particular concern is that the gp41-based peptides are foreign substances that may trigger immune responses even in topical application mode, especially given the extraordinary immunological activity of gut-associated lymphoid tissue. Antibodies elicited against the HR2 peptides could abrogate their HIV-neutralizing activities. Perhaps of greater concern is that immune-based inflammatory reactions might generate physical breaches in the epithelial barrier, and also could recruit activated CD4 T cells to the inflamed tissue, thereby providing an abundance of new target cells for HIV. The net effect could possibly be an increase rather than a decrease in the rate of HIV sexual transmission. The lessons learned from clinical trials of nonoxynol-9 as a topical microbicide (10) must be kept in mind.
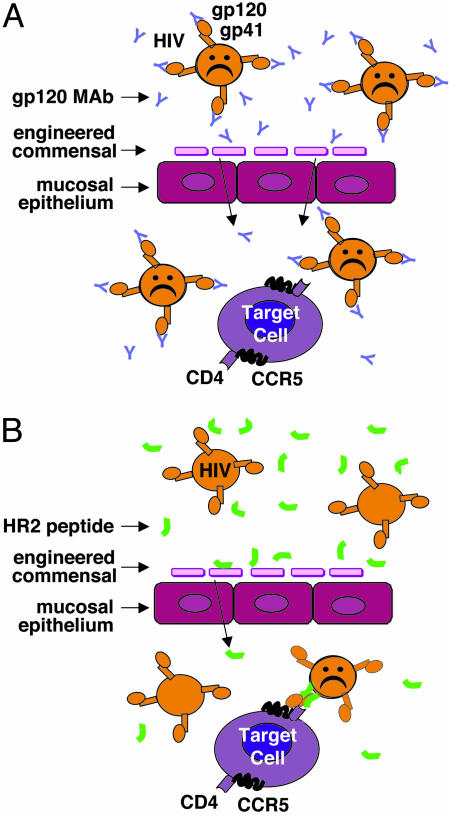
As a final point, it is important to consider factors that differentially influence various classes of candidate protein microbicides. Some act by binding to and neutralizing free virions; e.g., anti-gp120 neutralizing monoclonal antibodies such as b12 (6), cyanovirin-N (7, 9), or soluble CD4 (11) and related CD4-based agents (12). Others, such as chemokine derivatives (8), block specific interactions of virion gp120 with target cell receptors. The HR2 peptides used by Rao et al. (2) represent a sort of hybrid mode: they act by binding to virions, but only after gp120 has engaged the receptors to trigger formation of the gp41 prehairpin intermediate with its HR1 region exposed. Because of their distinct modes of action, these three classes of HIV-inhibitory proteins will likely differ with respect to the anatomical site where they must function (see Fig. 1). Agents such as mAb b12 that bind directly to free virions presumably can intercept their targets directly at the mucosal surface; they therefore should be present in high concentrations at that site (Fig. 1 A). Receptor-binding agents must interact with target cells, and therefore would be most effective when present at submucosal layers. The same is likely to be true for the HR2 peptides, because they function mainly after the specific virion/receptor interactions (Fig. 1B). The present understanding of the critical target cells and anatomical sites for the initial transmitting infections remains unclear; there may be multiple answers depending on individual situations, and they may differ for rectal vs. vaginal transmission. These considerations highlight the critical question for any microbicide approach, including those based on engineered mucosal microorganisms: Will delivery at the mucosal surface be optimal, or must efforts be made to achieve high concentrations at submucosal layers, or perhaps even at more distal sites? The answers will likely influence the potential efficacy for any class of protein microbicide, whether delivered by conventional topical application modes or by live-engineered mucosal organisms. As in so many of life's precarious endeavors, success will depend on being at the right place at the right time.
Fig. 1.
Targeting free (A) vs. receptor-bound (B) virions.
Author contributions: E.A.B. wrote the paper and researched the background.
See companion article on page 11993 in issue 34 of volume 102.
References
- 1.Shattock, R. J. & Moore, J. P. (2003) Nat. Rev. Microbiol. 1, 25–34. [DOI] [PubMed] [Google Scholar]
- 2.Rao, S., Hu, S., McHugh, L., Lueders, K., Henry, K., Zhao, Q., Fekete, R. A., Kar, S., Adhya, S. & Hamer, D. H. (2005) Proc. Natl. Acad. Sci. USA 102, 11993–11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthews, T., Salgo, M., Greenberg, M., Chung, J., DeMasi, R. & Bolognesi, D. (2004) Nat. Rev. Drug Discov. 3, 215–225. [DOI] [PubMed] [Google Scholar]
- 4.Sartor, R. B. (2005) Curr. Opin. Gastroenterol. 21, 44–50. [PubMed] [Google Scholar]
- 5.Blight, M. A. & Holland, I. B. (1994) Trends Biotechnol. 12, 450–455. [DOI] [PubMed] [Google Scholar]
- 6.Veazey, R. S., Shattock, R. J., Pope, M., Kirijan, J. C., Jones, J., Hu, Q., Ketas, T., Marx, P. A., Klasse, P. J., Burton, D. R. & Moore, J. P. (2003) Nat. Med. 9, 343–346. [DOI] [PubMed] [Google Scholar]
- 7.Tsai, C. C., Emau, P., Jiang, Y., Agy, M. B., Shattock, R. J., Schmidt, A., Morton, W. R., Gustafson, K. R. & Boyd, M. R. (2004) AIDS Res. Hum. Retroviruses 20, 11–18. [DOI] [PubMed] [Google Scholar]
- 8.Lederman, M. M., Veazey, R. S., Offord, R., Mosier, D. E., Dufour, J., Mefford, M., Piatak, M., Jr., Lifson, J. D., Salkowitz, J. R., Rodriguez, B., et al. (2004) Science 306, 485–487. [DOI] [PubMed] [Google Scholar]
- 9.Tsai, C. C., Emau, P., Jiang, Y., Tian, B., Morton, W. R., Gustafson, K. R. & Boyd, M. R. (2003) AIDS Res. Hum. Retroviruses 19, 535–541. [DOI] [PubMed] [Google Scholar]
- 10.Hillier, S. L., Moench, T., Shattock, R., Black, R., Reichelderfer, P. & Veronese, F. (2005) J. Acquired Immune Defic. Syndr. 39, 1–8. [DOI] [PubMed] [Google Scholar]
- 11.Chang, T. L., Chang, C. H., Simpson, D. A., Xu, Q., Martin, P. K., Lagenaur, L. A., Schoolnik, G. K., Ho, D. D., Hillier, S. L., Holodniy, M., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 11672–11677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dey, B., Del Castillo, C. S. & Berger, E. A. (2003) J. Virol. 77, 2859–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]