Abstract
IκB kinase (IKK) complex plays a key regulatory role in macrophages for NF-κB activation during both innate and adaptive immune responses. Because IKK1–/– mice died at birth, we differentiated functional macrophages from embryonic day 15.5 IKK1 mutant embryonic liver. The embryonic liver-derived macrophage (ELDM) showed enhanced phagocytotic clearance of bacteria, more efficient antigen-presenting capacity, elevated secretion of several key proinflammatory cytokines and chemokines, and known NFκB target genes. Increased NFκB activity in IKK1 mutant ELDM was the result of prolonged degradation of IκBα in response to infectious pathogens. The delayed restoration of IκBα in pathogen-activated IKK1–/– ELDM was a direct consequence of uncontrolled IKK2 kinase activity. We hypothesize that IKK1 plays a checkpoint role in the proper control of IκBα kinase activity in innate and adaptive immunity.
Keywords: IκBα, LPS
Macrophages constitute a major component of the innate immune system and play a central role in acute inflammatory response. They are normally found in a resting state throughout the body. Upon microbial infection, macrophages are activated and function as producers of cytokines and chemokines to mediate inflammatory responses, and as antigen-presenting cells to activate T helper cells that have been presented with antigen. The activated macrophages also act as potent antimicrobial effector cells to phagocytose invading pathogens. They recognize, bind, and phagocytose pathogen-associated molecular patterns (PAMPs) on infectious pathogens through a number of pattern recognition receptors (PRRs) (1, 2). Innate immune responses of macrophage to PAMPs involve a series of signaling PRR, such as Toll-like receptors (TLRs) (3). Engagement of microbial molecules to these signaling receptors promotes the synthesis and secretion of regulatory cytokines that are crucial to initiate innate and adaptive immunity. Activation of TLR by their ligands initiates a signaling network leading to activation of NF-κB. Transcriptional factor NF-κB plays an important role in the regulation of an inducible nitric oxide synthase, several proinflammatory cytokines and chemokines, and antiapoptotic proteins in response to invading pathogens. TLR-initiated activation of NF-κB is also essential for the induction of adaptive immune responses by regulating surface expression of antigen presenting and costimulatory molecules (4).
NF-κB is a family of transcriptional factors containing five members, including relA (p65), relB, c-Rel, p105/p50, and p100/p52, that function as homo- or heterodimers (5, 6). In most resting cells, NF-κB dimers are retained in the cytosol by association with inhibitory IκB proteins. Upon induction, IκB kinase (IKK) phosphorylates IκB, leading to degradation, thereby releasing NF-κB for nuclear translocation where it mediates expression of various inflammatory and stress response genes. One of the target genes is IκBα. The postinduced resynthesized IκBα goes into nucleus, where it binds NF-κB dimers and attenuates NF-κB activation. This pathway has been referred to “classical pathway” for NF-κB activation. The large 700- to 900-kDa IKK complex is the centerpiece of NF-κB pathway. Most NF-κB stimuli, such as cytokine, bacterial and virus infection, converge on the IKK complex through different upstream signaling (7). This complex consists of the kinase subunits IKK1 and IKK2, which are found in association with the regulatory subunits IKKγ/NEMO and ELKS (7, 8). The function of IKK2 as an essential IKK (9) and NEMO as an essential regulatory protein (10–12) for classical NF-κB activation is well established. In contrast, IKK1 mediates transcription of the p52/relB target genes with an “alternative pathway” by regulating the process of p100 into p52 in response to stimuli such as lymphotoxin, CD40, and BAFF (13, 14). In addition, IKK1 regulates the expression of some NF-κB target genes by phosphorylating histone H3 on Ser-10 (15, 16). Furthermore, IKK1 plays a critical role in the differentiation of epidermis independent of its kinase activity and NF-κB signaling pathway (17–19).
Despite significant advances in our understanding of the function of IKK1, it is still unclear whether IKK1 is required for the activation of the classical pathway of NF-κB in inflammation and innate immunity. This question remains to be addressed in a relevant cell system, such as macrophage. In this study, we used macrophages derived from fetal liver hematopoietic stem cells from IKK1–/– mouse embryos and examined their function. We found that deficiency of IKK1 in macrophages enhances bacterial phagocytosis and antigen-presenting cell function to activate T cells and is accompanied by elevated levels of cytokine and chemokine transcription and secretion. Additionally, elevated transcription of inflammatory mediators is a result of higher NF-κB activity in response to stimuli in IKK1-deficient macrophages. Furthermore, we showed a decreased level of postinduction IκBα protein due to increased kinase activity of IKK complex in IKK1–/– macrophages. Collectively, our results suggest that IKK1 functions as a negative mediator of NF-κB activation in macrophages by modulating IKK activity of the IKK complex.
Materials and Methods
Mice and Cell Culture. Pathogen-free IKK1 heterozygous mice were generated, as described (18) and were backcrossed onto a C57BL/6 genetic background. OVA323–339 (ISQAVHAAHAEINEAGR)-specific TCR-transgenic mice were purchased from The Jackson Laboratory. Mice were bred and housed in the animal care facility at the Salk Institute. Experimental procedures involving mice followed the guidelines from the National Institutes of Health and were approved by the Animal Use Committee at the Salk Institute.
WT and IKK1–/– fetal liver cells from embryonic day 13.5–15.5 mice were obtained as described by removing the cells from the liver. Hematopoietic progenitor cells in fetal liver were grown and enriched in StemSpan SFEM medium (StemCell Technologies, Vancouver) supplemented with 5 ng/ml IL-3, 5 ng/ml colony-stimulating factor (CSF), and 5 ng/ml granulocyte–macrophage CSF (StemCell Technoligies). After 4–5 days of expansion, the progenitor cells were differentiated into macrophages by either growing in DMEM (supplemented with 10% FCS/200 units/ml penicillin/200 mg/ml streptomycin/10 mM glutamine) containing macrophage CSF or by mixing with 30% of macrophage CSF containing L929 conditioned medium for 5–7 days. Mouse embryonic fibroblast cells were cultured in DMEM.
Phagocytosis Assay. Phagocytosis assays using f luorescein-conjugated Escherichia coli bioparticles as targets were performed in 96-well plates following the manufacturer's instructions (Molecular Probes) and described by Ojielo et al. (20) and detailed in Supporting Text, which is published as supporting information on the PNAS web site.
Antigen Presentation. Naïve OVA-specific transgenic T-cells were isolated by glass wood column from OVA323–339-specific TCR transgenic mice (DO11.10) (21). The embryonic liver-derived macrophage (ELDM) was cultured and γ-irradiated (2,000 rad). ELDM were subsequently cocultured with fresh isolated DO11.10 T cells in the presence or absence of OVA323–339 peptide in serum-free medium for 3 days at 37°C, 5% CO2. The ELDM/T cell ratio was used at 5:1, 1:1, and 1:5. The cultures were pulsed with 1 μCi/ml [3H]-thymidine (1 Ci = 37 GBq) per well for 18 h and then harvested onto glass filters. Radioactivity was assessed by liquid scintillation counter. The data were a mean value of six individual wells, expressed as cpm of [3H]-thymidine incorporated into proliferating T cell. The results presented are one representative of three performances.
RNA and Quantitative PCR (Q-PCR). For isolation of RNA for Q-PCR analysis, ELDM were seeded in 2.5-cm2 tissue-culture plates at a density of 85–95% confluency and allowed to settle for 1–2 days. The cultures were stimulated for the indicated periods of time before RNA was extracted. Total RNA was extracted by using TRIzol reagent (Invitrogen) and analyzed by Q-PCR as described (13, 22, 23) and detailed in Supporting Text.
ELISA. ELDMs were seeded in a 24-well plate at 90% confluency. Cells were treated with 100 ng/ml LPS for 6, 12, or 22 h. After the indicated time points, supernatants were collected to measure secretion of TNF-α, macrophage inflammatory protein 1α (MIP1α), and monocyte chemotactic protein 1 (MCP1). ELISA results were normalized by protein concentration. Detailed information is provided in Supporting Text.
Generation of Lentiviral Vector and Lentiviruses and Luciferase Assay.
Lentiviral vector containing five copies of the NF-κB consensus-binding site (GGGACTTTCC) and a minimal promoter linked to the luciferase reporter gene has been described (24). The generation of lentiviruses was conducted by using a four-plasmid transfection system as described (25, 26), and titers were determined by an amount of p24 by using ELISA kit (PerkinElmer). Mouse embryonic fibroblasts and macrophages were infected with lentiviruses at a multiplicity of infection of 50. Cells were lysed in reporter lysis buffer (Promega), and luciferase activity of protein samples was determined by the Steady-Glo Luciferase Assay System (Promega).
Western Analysis and Antibodies. Cells were treated with 100 ng/ml LPS for 30 min, 2 h, and 4 h before proteins were isolated from cells with RIPA buffer. The antibodies used in the Western analysis include anti-IKK2 (BioSource International, Camarillo, CA), anti-phospho-IKK2 (Ser-181) (Cell Signaling Technology, Beverly, MA), anti-IKK1 (Santa Cruz Biotechnology), anti-p65 (Santa Cruz Biotechnology), anti-phospho-p65(Ser-536) (Cell Signaling Technology), anti-IκBα (Santa Cruz Biotechnology), and anti-phospho-IκBα (Ser-32) (Cell Signaling Technology).
Pulse–Chase. Pulse–chase labeling experiments were carried out in IKK1 WT and mutant EDLM, as described in Supporting Text.
Kinase Assay. IKK kinase activity was measured as described in Supporting Text (9, 18, 27). Macrophages were serum-starved for 4 h before addition of LPS (100 ng/ml) for 7, 15, and 60 min, then IKK complexes were immunoprecipitated by using anti-IKKγ antibody (BD PharMingen).
Results
In Vitro Induction of ELDM. Macrophages were derived from IKK1 mutant and WT embryonic mouse livers at embryonic days 13.5–15.5. Liver cells isolated from both genotypes proliferated and expanded normally when cultured in StemSpan SFEM medium (StemCell Technologies, Vancouver) in the presence of 5 ng/ml IL-3. Expanded cells from both genotypes also showed parallel differentiation in response to recombinant macrophage CSF. After 7 days of induction, the majority of IKK1 mutant embryonic liver cells, like WT controls, developed into macrophage populations expressing Mac-1/CD11b markers (data not shown). These differentiated macrophages showed normal maturation response to LPS treatment with characteristics of enhanced surface expression of Mac-1/CD11b (data not shown). The induced IKK mutant ELDM showed normal morphological features (data not shown) and basic phagocytotic activity.
Enhanced Phagocytic and Antigen-Presenting Functions of IKK1–/– Macrophage Derived from Embryonic Liver. Macrophages provide the first line of host defense and play a key role in the innate immune response against bacterial and viral pathogens by binding and phagocytosing invaders. Phagocytosis is a process that involves binding and internalization of pathogens and virus-infected cells. It is essential for host defense in higher eukaryotes, which is mainly achieved by macrophages, neutrophils, and natural killer cells. To evaluate the function of macrophages in the absence of IKK1, we tested the phagocytotic clearance of E. coli K-12 by IKK1–/– ELDM in vitro. Interestingly, IKK1 mutant ELDM uptook bacteria with higher efficiency than their WT control (Fig. 1A). Although a 5-h LPS pretreatment significantly increased the ingestion rate in both groups, the increase was more significant in IKK1 mutant ELDM compared with WT (Fig. 1B).
Fig. 1.
Functional analyses of the IKK1 mutant macrophages derived from embryonic liver. In vitro differentiated IKK1 mutant ELDM showed increased phagocytotic ingestion of E. coli K-12 by IKK1 mutant macrophages at a condition either without LPS pretreatment (A) or with 5-h pretreatment in the presence of 100 ng/ml LPS (B). The data represent five independent experiments, and the results were expressed as the relative fluorescence units over time 0. (C) IKK1 mutant ELDM presented OVA peptide more efficiently to OVA-specific T cells. Data shown are means (±SD) of six replicate wells from each culture condition in each of three independent experiments. *, P < 0.01; **, P < 0.08.
Another major function of macrophages is the presentation of antigens to naïve T helper cells. It plays an important role in integrating innate and adaptive immune responses by uptaking and processing pathogenic antigens and presenting them to T helper cells. Because the NF-κB signaling pathway participates in the regulation of antigen presentation (4, 28), we investigated in vitro proliferation of naïve T cells from OVA peptide-specific TCR transgenic mice (BALB/c DO11.10) in the coculture with IKK1–/– or WT ELDMs loaded with OVA peptide (OVA323–339). The results showed that after 72-h coculture with IKK1 mutant ELDM, the T cells proliferated at a significant higher rate with average 2- to 4-fold increases over the WT control group (Fig. 1C). These findings suggest that macrophage cellular functions are enhanced in IKK1–/– macrophages.
Elevated Production of Proinflammatory Cytokines and Chemokines by IKK1–/– Macrophage Associated with Increased NF-κB Activity. In addition to phagocytotic clearance of pathogens and presentation of antigens to T helper cells, the macrophage also responds to pathogens by producing and secreting cytokines and chemokines that regulate other cellular components of the host's immune response. To examine the role of IKK1 in mediating this process, we examined the LPS-induced transcriptional expression of proinflammatory regulators such as TNF-α, MIP1α, MCP1, and Cox2 in IKK1 mutants and WT ELDM by Q-PCR analysis. LPS treatment resulted in a significant increase in RNA levels of TNF-α, MIP1α, MCP1, and Cox2 in IKK1 mutant ELDM (Fig. 2A). The elevation of mutant over WT was clearly evident at the early time point of 2-h treatment. To substantiate the hyperresponses of IKK1 mutant ELDM to LPS treatment, we further evaluated the protein level of these target genes by performing ELISA to detect secretion of TNF-α, MIP1α, and MCP1 into culture medium by IKK1 mutant ELDM upon LPS treatment. Consistent with results at the transcriptional level, the secreted proteins were also elevated in supernatant collected from LPS-treated IKK1–/– ELDM (Fig. 2B).
Fig. 2.
Increased expressions of cytokines and chemokines and NF-κB activity in IKK1-deficient macrophages. (A) Expression of TNF-α, MIP1α, MCP1, and Cox2 in response to LPS stimulation. IKK1 WT and mutant ELDM were stimulated with LPS (100 ng/ml) for 2, 4, and 6 h, and total RNA was extracted with TRIzol. After cDNA synthesis, Q-PCR was performed. The data were normalized with the expression of cyclophylin A. Data shown are a representative result from one of three independent experiments. (B) Proinflammatory cytokine level in the culture supernatant was determined by quantitative ELISA. Confluent ELDM were treated with LPS (100 ng/ml) for 6, 12, and 22 h, and their supernatants were collected and spun to remove cell debris and stored at –80°C for ELISA analysis. Total proteins were extracted from the ELDM, and their concentrations were determined and used to normalize the ELISA results. Data are the average from triplicates.(C) IKK1 WT and mutant ELDM were infected with lentiviruses containing the κB-luciferase reporter construct. Two days later, cells were treated with various NF-κB stimuli as indicated for 4 h after being serum-starved (DMEM containing 0.5% FCS) for 4 h. The luciferase assay was performed by using protein lysis. Results were normalized by basal activity of cells untreated and averaged from triplicates. (D) A similar analysis as in C was done in mouse embryonic fibroblasts either WT or deficient of IKK1/2, p65, and p50.
Based on the well established role of NF-κB in the regulation of a variety of proinflammatory cytokine expressions, we tested whether the increased production of proinflammatory cytokines was caused by hyperactivation of NF-κB in IKK1 mutant ELDM. We introduced ELDMs with a luciferase reporter driven by five copies of the NF-κB-binding sites on a lentiviral-based vector. After 2 days of transduction, cells were treated for 4 h with either LPS or control medium before lysing. The lysates were further subjected to luciferase assays. As shown in Fig. 2C, LPS treatment led to a 1.5-fold increase in the NF-κB-mediated gene activation. To access the specificity of the luciferase reporter construct, we also introduced this same construct into three cell types; those were deficient in both IKK1 and IKK2, p65, or p50. In each case, the specific luciferase activity was completely unresponsive to LPS stimulation (Fig. 2D). These results indicate that increased production of proinflammatory cytokines results from increased NF-κB signaling pathway to LPS stimulation in IKK1–/– ELDM.
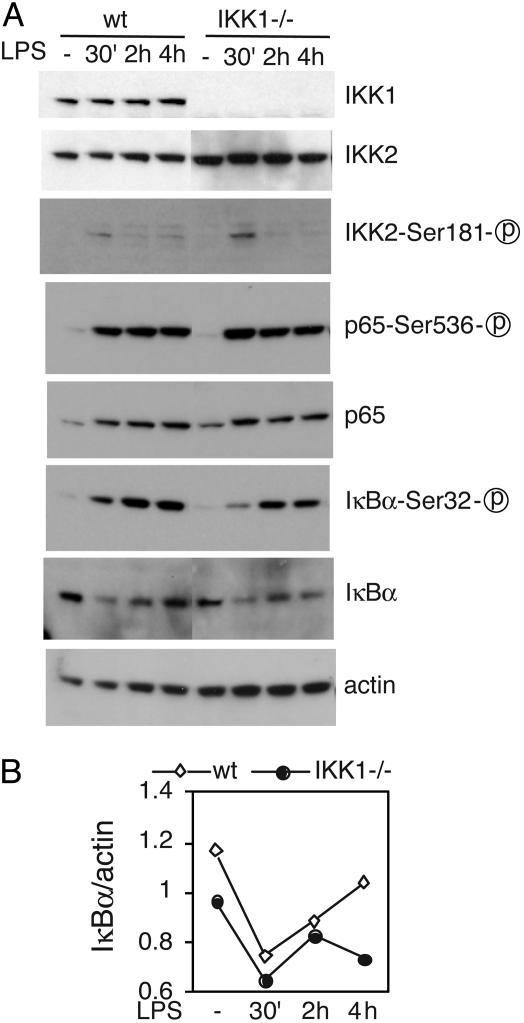
IκBα Protein Level at Postinduction Decreased in IKK1–/– Macrophage. IKK2 is primarily responsible for IKK activity, and activation of NF-κB depends on the induced phosphorylation of Ser-181 in the IKK2 activation loop (29). To test whether the loss of IKK1 impacts IKK2 kinase expression and activity, we first examined IKK2 expression and LPS-induced phosphorylation at Ser-181. We did not detect any change in either IKK2 expression or LPS-induced transient IKK2 phosphorylation at Ser-181 (Fig. 3A).
Fig. 3.
The postinduction level of IκBα is reduced in IKK1–/– macrophages. Ninety percent confluent IKK1 WT and mutant ELDM were serum-starved for 4 h, then treated with 100 ng/ml LPS for 30 min, 1 h, and 2 h. The protein lysis from whole cells was resolved by using 10% SDS/PAGE and probed with the antibodies indicated (A). Data shown are a representative result from one of three independent experiments. Relative amounts of protein were measured by the level of actin. us, unspecific. IκBα levels were quantified from the film shown in A by using nih image 1.62 software and normalized by levels of actin (B).
Several studies have suggested that phosphorylation of Ser-536, located in the transactivation domain of p65 by the IKK complex, controls recruitment of p300/CBP to the nuclear pool of p65 and augments its transcriptional activity (30–32). Using antibodies specific for p65 and Ser-536-phosphorylated p65, we compared the level and kinetics of p65 and the phosphorylation of Ser-536 in WT and mutant ELDMs (Fig. 3A). We did not detect any change in the total level of p65 and in the kinetics and magnitude of p65 phosphorylation at Ser-536. We conclude that IKK1 is not involved in the LPS-induced p65 phosphorylation on Ser-536 in macrophages.
We next examined the phosphorylation and degradation of IκBα, a key step for NF-κB activation. LPS treatment rapidly induced the phosphorylation and degradation of IκBα in both mutant and WT ELDMs after a 30-min treatment (Fig. 3A). It has been well documented that IκBα is quickly degraded upon activation by its kinases and will be resynthesized as a target gene of NF-κB activation. The IκBα protein level in WT macrophages increased gradually 2 h after LPS induction. Strikingly, the IκBα in IKK1–/– macrophage remained at a lower level at 2 and 4 h postinduction (Fig. 3B). Because IκBα negatively regulates NF-κB activation, and NF-κB activity correlates inversely with the levels of IκBα, enhanced NF-κB activity in IKK1 mutant ELDM is likely due to result from decreased level of IκBα after LPS induction.
Decreased IκBα in Postinduction Due to Enhanced IκBα Phosphorylation and Degradation. TNF-α regulation of IκBα transcription is mediated through Ser-10 phosphorylation of histone H3 by IKK1 (15, 16). The lower level of IκBα transcripts in IKKI mutant ELDM could be caused by insufficient phosphorylation of histone H3 by IKK1 for transcriptional regulation of IκBα resynthesis. To test this hypothesis, we measured the IκBα mRNA level after the indicated time points of LPS treatment. To our surprise, in contrast to the protein level, IκBα mRNA induced by LPS displayed a higher level in IKK1–/– ELDM than WT ELDM (Fig. 4A). This result is consistent with our early observation that NF-κB-mediated transcription by LPS is enhanced in the IKK1–/– macrophage (Fig. 2).
Fig. 4.
Enhanced LPS-induced IκBα degradation and phosphorylation by the IKK complex lacking IKK1. (A) Normal transcription of the IκBα gene induced by LPS in IKK1 lacking macrophages. (B) Autoradiogram of IκBα or p65 immunoprecipitated from WT and IKK1–/– macrophages after a 1-h [35S]-methionine- and cysteine-labeling pulse and cold chase of the indicated duration. The chase was conducted in the presence or absence of LPS. IκBα signal intensity was quantified from phosphorimager scan data with or without normalization with a p65 protein level. (C) The IκBα signal intensity in the same assay as shown in B was quantified from phosphorimager scan data with or without normalization with a p65 protein level. (D) Autoradiogram of 32P-labeled IκBα phosphorylated by IKK complexes immunoprecipitated from WT and IKK1–/– macrophages treated with LPS (100 ng/ml) for the indicated periods. (Lower) Western analysis showing the amount of IKK2 protein used in the kinase assays. (E) Quantified results of the kinase assay in D without (Left) and with normalization of the IKK2 level (Right). Data shown are a representative result from one of three independent experiments.
This led us to examine another possibility that the decreased IκBα protein level after LPS induction in IKK1–/– ELDM is possibly caused by enhanced IκBα protein degradation. To evaluate this hypothesis, we performed pulse–chase labeling experiments to track the degradation of IκBα in response to LPS induction. Cells were first pulse-labeled with [35S]methionine and cysteine, then changed to “cold” medium. The cultures were chased in the presence or absence of LPS for the indicated times. Half of cell lysate was immunoprecipitated with anti-IκBα antibody, and the other half was immunoprecipitated with antibody against p65. The immunoprecipitated samples were separated on SDS/PAGE gel, and the isotope-labeled immunoprecipitates were recorded by exposing radioactivity to a phosphorimager plate. As shown in Fig. 4 B and C, S35-labeled IκBα from mutant ELDM was significantly degraded 15 min after LPS treatment. In contrast, degradation of IκBα in WT macrophages occurred 30 min after LPS stimulation. No change in the degradation pattern of p65 protein was observed. We suggest that IκBα degradation is accelerated in IKK1–/– macrophages.
Extended IκBα Degradation Caused by Hyperactive Kinase Activity of the IKK2 Homocomplex. Engagement of LPS with TLR-4 and CD14 on the cell surface triggers a series of signaling kinase activities, which include activation of the IKK kinase complex. Phosphorylation at Ser-32 and -36 on IκBα by the IKK complex is a prerequisite for IκBα degradation. We therefore examined IKK activity of the IKK complex in the absence of IKK1. We immunoprecipitated the IKK complexes using anti-NEMO antibody from ELDM treated with LPS for the indicated time points. The immunoprecipitated IKK complexes were further subjected to an in vitro kinase assay by using GST-IκBα as a substrate. Interestingly, we detected increased IκBα kinase activity from IKK complexes that did not contain IKK1 (Fig. 4 D and E).
Discussion
We have observed in this study that embryonic-derived macrophages lacking IKK1 show greater phagocytotic activity and antigen-presentation capacity to T cells than WT ELDM. Macrophages recognize and bind bacteria through a wide spectrum of receptors for specific components on the bacteria (33). Of those receptors, class A scavenger receptors have been well studied (33). They are able to bind a variety of molecules on the bacterial well and trigger immune response against bacteria by mediating binding and phagocytosis. LPS on the well of the Gram-negative bacteria have been shown to be able not only to bind the scavenger receptors on macrophage and trigger phagocytotic clearance of pathogenic bacteria but also to activate immune responses through the binding to TLR. It has been well documented that binding of LPS, TLR-4 activates NF-κB proteins (3). LPS treatment increases surface expression on scavenger receptors on murine macrophages (34, 35). Macrophages with higher NF-κB activity are hypothesized to be more susceptible to LPS stimulation or more capable of combating infectious bacteria. We have shown in this study that mouse ELDM lacking IKK1 were able to uptake encountered bacteria with expanded capacity when compared with controls. The elevation of phagocytosis was more evident after pretreatment of macrophages with LPS, suggesting that macrophages lacking IKK1 are functionally more competent to counter innate immune responses.
Antigen presentation is a key step in the immune response. There are three major professional antigen-presentation cells in mammals, one of which is macrophages. The IKK-IκB-NF-κB signal pathway has been documented to play an important role in regulating antigen-presentation function, including MHC and costimulatory molecule (CD80, CD86) expression and cytokine (IL-12, TNF-α) production. Blockage of NF-κB activation by overexpression of IκB or inhibition of IκB degradation in dendritic cells inhibits surface expression of T cell receptors (MHC II in murine and HLA-DR, DQ in human), costimulating receptors (CD80 and CD86) and sequentially the T cell proliferative response in vitro (4, 28). We have shown in this study that macrophages with IKK1 deficiency demonstrated elevated antigen-presenting activity toward T cells. It is suggestive that IKK1 has a negative effect in antigen-presentation function, probably through down-regulation of NF-κB activation.
To access the hyperreactivity of IKK1 mutant ELDM to stimuli, we have investigated cytokine production upon LPS or TNF-α treatment. Interestingly, production of proinflammatory regulators increased in IKK1–/– macrophages in response to LPS, enhancing immune response associated with rapid and extended degradation of IκBα protein and increased NF-κB activation. Furthermore, IKK complexes without IKK1 showed enhanced IκBα kinase activity, leading to increased IκBα degradation. Our results are consistent with several previous reports, which showed that purified recombinant protein IKK2 has a much higher level (≈20- to 50-fold) of IκBα kinase activity than IKK1 (27, 31, 36–39), and IKK2 oligomerization can be induced by physiological stimulus in vivo (40). It is conceivable that IKK2 homodimer functions as a better IκBα kinase in IKK1-deficient macrophages.
Lawrence et al. (41) have recently reported the role of IKK1 in inflammation and innate immunity in vivo, using knock-in mice that express kinase-deficient (AA) instead of WT IKK1. Similar to our study, they showed that IKK1 limited macrophage NF-κB activation and contributed to the resolution of inflammation. In addition, they showed that enhancement of NF-κB activity was due to effects on p65 phosphorylation at Ser-536 and p65 turnover by IKK1. However, we found no evidence for the involvement of IKK1 in LPS-induced phosphorylation of Ser-536 on p65 in macrophages (Figs. 3 and 4 B and C). Furthermore, we were not able to detect LPS-induced p65 degradation and the effects of IKK1 on p65 turnover. The reason for this discrepancy is not clear, except that in our studies, IKK1 was completely removed, whereas in the studies reported by Lawrence et al. (41), only kinase activity of IKK1 was compromised. It has been shown (19) that phenotypes caused by IKK1 removal can be complemented by kinase-deficient IKK1. It is thus formally possible that the results observed in “knock-in” mice are due to other domains of IKK1. Our study demonstrated the physiological relevance of IKK1 in regulating IKK function and the NF-κB pathway in macrophages. In contrast to its positive role in the alternative pathway of NF-κB activation (14), IKK1 appears to function as a negative regulator of NF-κB activity in macrophages. Interestingly, zebrafish IKK1 also negatively regulates NF-κB activity, but the mechanism seems to involve sequestration of NEMO, thereby compromising the activity of IKK2 (42). The presence of both IKK1 and IKK2 in the same complex provides a potential mechanism to integrate the diverse signaling pathways in different cell types and channel them toward specific NF-κB responses. It is clear that regulation of NF-κB activity requires rapid induction to combat invading pathogens but then also needs to attenuate to prevent overstimulation of the immune system. IKK1 may provide such a checkpoint.
Supplementary Material
Acknowledgments
We thank N. Droin for the helpful discussion of Q-PCR primer designing. Q. Li is partially supported by a grant from Wyeth Pharmaceutical. I.M.V. is an American Cancer Society Professor of Molecular Biology. He is supported in part by grants from the National Institutes of Health; the Larry L. Hillblom Foundation, Inc.; the Lebensfeld Foundation; the Wayne and Gladys Valley Foundation; the H. N. and Frances C. Berger Foundation; and Merck Research Laboratories.
Author contributions: Q. Li and Q. Lu designed research; Q. Li, Q. Lu, V.B., G.E., and L.M. performed research; Q. Li, Q. Lu, V.B., L.M., F.M., and I.M.V. analyzed data; Q. Li and Q. Lu wrote the paper; V.B., L.M., and F.M. edited the manuscript; and I.M.V. edited the manuscript.
Abbreviations: IKK, IκB kinase; TLR, Toll-like receptor; Q-PCR, quantitative PCR; ELDM, embryonic liver-derived macrophage; MIP1α, macrophage inflammatory protein 1α; MCP1, monocyte chemotactic protein 1.
References
- 1.Janeway, C. A., Jr., & Medzhitov, R. (2002) Annu. Rev. Immunol. 20, 197–216. [DOI] [PubMed] [Google Scholar]
- 2.Taylor, P. R., Martinez-Pomares, L., Stacey, M., Lin, H. H., Brown, G. D. & Gordon, S. (2005) Annu. Rev. Immunol. 23, 901–944. [DOI] [PubMed] [Google Scholar]
- 3.Akira, S. & Takeda, K. (2004) Nat. Rev. Immunol. 4, 499–511. [DOI] [PubMed] [Google Scholar]
- 4.Yoshimura, S., Bondeson, J., Foxwell, B. M., Brennan, F. M. & Feldmann, M. (2001) Int. Immunol. 13, 675–683. [DOI] [PubMed] [Google Scholar]
- 5.Verma, I. M., Stevenson, J. K., Schwarz, E. M., Van Antwerp, D. & Miyamoto, S. (1995) Genes. Dev. 9, 2723–2735. [DOI] [PubMed] [Google Scholar]
- 6.Li, Q. & Verma, I. M. (2002) Nat. Rev. Immunol. 2, 725–734. [DOI] [PubMed] [Google Scholar]
- 7.Karin, M. & Ben-Neriah, Y. (2000) Annu. Rev. Immunol. 18, 621–663. [DOI] [PubMed] [Google Scholar]
- 8.Ducut Sigala, J. L., Bottero, V., Young, D. B., Shevchenko, A., Mercurio, F. & Verma, I. M. (2004) Science 304, 1963–1967. [DOI] [PubMed] [Google Scholar]
- 9.Li, Q., Van Antwerp, D., Mercurio, F., Lee, K. F. & Verma, I. M. (1999) Science 284, 321–325. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt-Supprian, M., Bloch, W., Courtois, G., Addicks, K., Israel, A., Rajewsky, K. & Pasparakis, M. (2000) Mol. Cell 5, 981–992. [DOI] [PubMed] [Google Scholar]
- 11.Rudolph, D., Yeh, W. C., Wakeham, A., Rudolph, B., Nallainathan, D., Potter, J., Elia, A. J. & Mak, T. W. (2000) Genes Dev. 14, 854–862. [PMC free article] [PubMed] [Google Scholar]
- 12.Makris, C., Godfrey, V. L., Krahn-Senftleben, G., Takahashi, T., Roberts, J. L., Schwarz, T., Feng, L., Johnson, R. S. & Karin, M. (2000) Mol. Cell 5, 969–979. [DOI] [PubMed] [Google Scholar]
- 13.Dejardin, E., Droin, N. M., Delhase, M., Haas, E., Cao, Y., Makris, C., Li, Z. W., Karin, M., Ware, C. F. & Green, D. R. (2002) Immunity 17, 525–535. [DOI] [PubMed] [Google Scholar]
- 14.Senftleben, U., Cao, Y., Xiao, G., Greten, F. R., Krahn, G., Bonizzi, G., Chen, Y., Hu, Y., Fong, A., Sun, S. C. & Karin, M. (2001) Science 293, 1495–1499. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto, Y., Verma, U. N., Prajapati, S., Kwak, Y. T. & Gaynor, R. B. (2003) Nature 423, 655–659. [DOI] [PubMed] [Google Scholar]
- 16.Anest, V., Hanson, J. L., Cogswell, P. C., Steinbrecher, K. A., Strahl, B. D. & Baldwin, A. S. (2003) Nature 423, 659–663. [DOI] [PubMed] [Google Scholar]
- 17.Hu, Y., Baud, V., Delhase, M., Zhang, P., Deerinck, T., Ellisman, M., Johnson, R. & Karin, M. (1999) Science 284, 316–320. [DOI] [PubMed] [Google Scholar]
- 18.Li, Q., Lu, Q., Hwang, J. Y., Buscher, D., Lee, K. F., Izpisua-Belmonte, J. C. & Verma, I. M. (1999) Genes Dev. 13, 1322–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu, Y., Baud, V., Oga, T., Kim, K. I., Yoshida, K. & Karin, M. (2001) Nature 410, 710–714. [DOI] [PubMed] [Google Scholar]
- 20.Ojielo, C. I., Cooke, K., Mancuso, P., Standiford, T. J., Olkiewicz, K. M., Clouthier, S., Corrion, L., Ballinger, M. N., Toews, G. B., Paine, R., 3rd, et al. (2003) J. Immunol. 171, 4416–4424. [DOI] [PubMed] [Google Scholar]
- 21.Murphy, K. M., Heimberger, A. B. & Loh, D. Y. (1990) Science 250, 1720–1723. [DOI] [PubMed] [Google Scholar]
- 22.Bottero, V., Imbert, V., Frelin, C., Formento, J. L. & Peyron, J. F. (2003) Mol. Diagn. 7, 187–194. [DOI] [PubMed] [Google Scholar]
- 23.Droin, N. M., Pinkoski, M. J., Dejardin, E. & Green, D. R. (2003) Mol. Cell. Biol. 23, 7638–7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tergaonkar, V., Bottero, V., Ikawa, M., Li, Q. & Verma, I. M. (2003) Mol. Cell. Biol. 23, 8070–8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyoshi, H., Blomer, U., Takahashi, M., Gage, F. H. & Verma, I. M. (1998) J. Virol. 72, 8150–8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dull, T., Zufferey, R., Kelly, M., Mandel, R. J., Nguyen, M., Trono, D. & Naldini, L. (1998) J. Virol. 72, 8463–8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mercurio, F., Zhu, H., Murray, B. W., Shevchenko, A., Bennett, B. L., Li, J., Young, D. B., Barbosa, M., Mann, M., Manning, A. & Rao, A. (1997) Science 278, 860–866. [DOI] [PubMed] [Google Scholar]
- 28.Yoshimura, S., Bondeson, J., Brennan, F. M., Foxwell, B. M. & Feldmann, M. (2001) Eur. J. Immunol. 31, 1883–1893. [DOI] [PubMed] [Google Scholar]
- 29.Delhase, M., Hayakawa, M., Chen, Y. & Karin, M. (1999) Science 284, 309–313. [DOI] [PubMed] [Google Scholar]
- 30.Sakurai, H., Chiba, H., Miyoshi, H., Sugita, T. & Toriumi, W. (1999) J. Biol. Chem. 274, 30353–30356. [DOI] [PubMed] [Google Scholar]
- 31.Chen, L. F. & Greene, W. C. (2003) J. Mol. Med. 81, 549–557. [DOI] [PubMed] [Google Scholar]
- 32.Sakurai, H., Suzuki, S., Kawasaki, N., Nakano, H., Okazaki, T., Chino, A., Doi, T. & Saiki, I. (2003) J. Biol. Chem. 278, 36916–36923. [DOI] [PubMed] [Google Scholar]
- 33.Gordon, S. (2002) Cell 111, 927–930. [DOI] [PubMed] [Google Scholar]
- 34.van der Laan, L. J., Dopp, E. A., Haworth, R., Pikkarainen, T., Kangas, M., Elomaa, O., Dijkstra, C. D., Gordon, S., Tryggvason, K. & Kraal, G. (1999) J. Immunol. 162, 939–947. [PubMed] [Google Scholar]
- 35.Fitzgerald, M. L., Moore, K. J., Freeman, M. W. & Reed, G. L. (2000) J. Immunol. 164, 2692–2700. [DOI] [PubMed] [Google Scholar]
- 36.Zandi, E., Chen, Y. & Karin, M. (1998) Science 281, 1360–1363. [DOI] [PubMed] [Google Scholar]
- 37.Kwak, Y. T., Guo, J., Shen, J. & Gaynor, R. B. (2000) J. Biol. Chem. 275, 14752–14759. [DOI] [PubMed] [Google Scholar]
- 38.Lee, F. S., Peters, R. T., Dang, L. C. & Maniatis, T. (1998) Proc. Natl. Acad. Sci. USA 95, 9319–9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakano, H., Shindo, M., Sakon, S., Nishinaka, S., Mihara, M., Yagita, H. & Okumura, K. (1998) Proc. Natl. Acad. Sci. USA 95, 3537–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang, E. D., Inohara, N., Wang, C. Y., Nunez, G. & Guan, K. L. (2003) J. Biol. Chem. 278, 38566–38570. [DOI] [PubMed] [Google Scholar]
- 41.Lawrence, T., Bebien, M., Liu, G. Y., Nizet, V. & Karin, M. (2005) Nature 434, 1138–1143. [DOI] [PubMed] [Google Scholar]
- 42.Correa, R. G., Matsui, T., Tergaonkar, V., Rodriguez-Esteban, C., Izpisua-Belmonte, J. C. & Verma, I. M. (2005) Curr. Biol. 15, 1291–1295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.