Abstract
In the early development of the central nervous system, neural progenitor cells divide in an asymmetric manner and migrate along the radial glia cells. The radial migration is an important process for the proper lamination of the cerebral cortex. Recently, a new mode of the radial migration was found at the intermediate zone where the neural progenitor cells become multipolar and reduce the migration rate. However, the regulatory signals for the radial migration are unknown. Using the migration assay in vitro, we examined how neural progenitor cell migration is regulated. Neural progenitor cells derived from embryonic mouse telencephalon migrated on laminin-coated dishes. Endothelin (ET)-1 inhibited the neural progenitor cell migration. This ET-1 effect was blocked by BQ788, a specific inhibitor of the ETB receptor, and by the expression of a carboxyl-terminal peptide of Gαq but not Gαi. The expression of constitutively active mutant of Gαq, GαqR183C, inhibited the migration of neural progenitor cells. Moreover, the inhibitory effect of ET-1 was suppressed by the c-Jun N-terminal kinase (JNK) inhibitor SP600125 and the expression of the JNK-binding domain of JNK-interacting protein-1, a specific inhibitor of the JNK pathway. Using the slice culture system of embryonic brain, we demonstrated that ET-1 and the constitutively active mutant of Gαq caused the retention of the neural progenitor cells in the intermediate zone and JNK-binding domain of JNK-interacting protein-1 abrogated the effect of ET-1. These results indicated that G protein-coupled receptor signaling negatively regulates neural progenitor cell migration through Gq and JNK.
Keywords: G protein α-subunit, radial migration, endothelin
Neural stem cells are self-renewing and multipotent cells, which give rise to neurons, astrocytes, and oligodendrocytes in the CNS. In the developing cerebral cortex, neural stem cells differentiate into more committed progenitor cells and migrate from the ventricular zone (VZ) to superficial layers of the cortical plate (CP) along the fibers of radial glial cells (1). This radial migration is an important process for the correct lamination of the cerebral cortex.
Recently, time-lapse imaging studies in slices of the developing neocortex showed that neural progenitor cells migrate by three modes: somal translocation, cellular locomotion, and multipolar migration (2, 3). The migrating cells change migration direction and rate in the intermediate zone (IZ). In this mode, cells change from bipolar to multipolar. The multipolar cells sometimes jump tangentially. Then the cells become bipolar again and progress radially to the CP. The mechanism of multipolar migration is unknown, but the regulatory signals to the migrating cells in the IZ may be physiologically important in the developing cerebral cortex.
The extracellular matrix (ECM) is essential for the developmental process, including the regulation of cell motility, and contributes to the formation of organs (4, 5). ECM and integrins play an important role in the migration of neural crest cells (6) and in the proper lamination in the development of the cerebral cortex (7, 8). Laminin is a major member of the ECM and acts as a permissive migratory substrate for granule cell precursors to migrate from the external granule cell layer into the internal granule cell layer (9).
G protein-coupled receptors (GPCRs) constitute the largest family of seven-transmembrane receptors and are responsible for converting a diverse array of extracellular stimuli into intracellular signaling events. GPCRs are involved in a variety of physiological processes, such as proliferation, differentiation, and migration. Although there are several reports that indicate the involvement of GPCR signaling in neural progenitor cell migration, the mechanism by which GPCR signaling regulates the migration remains to be clarified. Endothelin (ET) is known as vasoactive peptides that comprise three isoforms, ET-1, ET-2, and ET-3. These peptides bind to ETA and ETB receptors with different affinity (10). In the peripheral nervous system, the ETB receptor and ET-1 are important during neural crest development (11). However, the function of ETs and their receptors in the developing CNS is poorly understood. The c-Jun N-terminal kinase (JNK), a member of the mitogen-activated protein kinase (MAPK) family, is generally thought to be involved in inflammation, proliferation, and apoptosis (12). Recently, we (13, 14) and other groups (15–19) demonstrated that JNK and its upstream kinases are implicated in cell migration in a certain type of cells as well as brain.
To investigate the role of GPCR signaling in the developing CNS, the effect of the GPCR and MAPK system on cell migration was examined by using primary neural progenitor cells. We found that the ETB receptor transduced the signal for inhibition of neural progenitor cell migration through the Gq and JNK pathway in vitro and in vivo.
Materials and Methods
Materials. EGF and DNaseI were purchased from Roche Diagnostics. Basic fibroblast growth factor was obtained from Peprotech (London). The B27 supplement and trypsin were purchased from GIBCO/BRL. MAPK cascade inhibitors, U0126, SB202190, and SP600125 were obtained from Calbiochem. ET-1 was purchased from the Peptide Institute (Osaka). Laminin was obtained from Invitrogen.
Preparation and Culture of Neural Progenitor Cells. Neural progenitor cells were prepared from embryonic day (E) 11.5 ICR mice. The telencephalon of the embryo was dissected surgically under the microscope. The dissected telencephalon was treated with 0.05% trypsin for 10 min at 37°C, and this reaction was stopped by 70 μg/ml ovomucoid. Cells were suspended in DMEM/F-12 medium (DF medium). The cell number was counted, and 2 × 106 cells were plated in a 100-mm dish coated with 20 μg/ml poly-2-hydroxyethyl methacrylate. Cells were cultured in DF medium supplemented with a B27 supplement, 20 ng/ml basic fibroblast growth factor, 20 ng/ml EGF, 100 μg/ml BSA, and 2 μg/ml heparin. Cultures were passaged every 3 days.
Migration Assay. A 15-mm cover glass was coated with 200 μg/ml poly-d-lysine (PDL) and then with the indicated concentrations of laminin. The cover glass was placed on a 35-mm plastic dish. Fifty microliters of a cell suspension, including ≈50 neurospheres of similar diameters (100–300 μm), was loaded on the cover glass and incubated at 37°C for 90 min. Two milliliters of DF medium was added to the dish, and, 24 h later, the extent of migration was analyzed under the microscope. Migration was assessed by measurement of the distance from the edge of the sphere to the leading cell of outgrowth. If this distance was more than the diameter of the neurosphere or the neurosphere was completely dispersed, this sphere was evaluated as a migrating neurosphere. At least >40 neurospheres were counted in each experiment.
Recombinant Adenovirus. Adenoviruses expressing GFP, the JNK-binding domain of JNK-interacting protein-1 (JIP1-JBD), Gαq R183C, and C-terminal peptides of Gαq, Gαi2, Gα12, and Gα13 were prepared as described (20). These adenoviruses other than adenovirus expressing the JIP1-JBD express GFP; thus, the infection of adenovirus to cells can be monitored by GFP fluorescence. All adenoviruses were used at a multiplicity of infection of 25 to infect to neural progenitor cells for 3 days.
Immunoblotting. Neural progenitor cells maintained in poly-2-hydroxyethyl methacrylate-coated, 60-mm dishes were lysed and sonicated in 100 μl of an ice-cold cell lysis buffer (20 mM Hepes·NaOH, pH 7.5/3 mM MgCl2/100 mM NaCl/1 mM DTT/1 mM phenylmethane sulfonylfluoride/1 μg/ml leupeptin/1 mM EGTA/1 mM Na3VO4/10 mM NaF/20 mM β-glycerophosphate/0.5% Lubrol PX). Then, the lysates were analyzed by immunoblotting. The blotting membranes were incubated with anti-JNK (rabbit, 1:1,000, Cell Signaling Technology, Beverly, MA) and anti-phospho-JNK (rabbit, 1:2,000, Promega) antibodies for 60 min. After washing, the membrane was incubated with anti-rabbit-IgG conjugated with horseradish peroxidase in 5% skim milk. Immunoblotting was analyzed by using an enhanced chemiluminescence kit according to the manufacturer's instructions (Amersham Pharmacia Biotech). The band intensity of the immunoblotting was quantified with a Lumino Image Analyzer (Fuji).
Cortical Slice Culture. For vibratome sectioning, the whole fetal mouse brain at E16.5 was embedded in 1.5% low-melting agarose in DF medium containing 20 mM Hepes·NaOH (pH 7.5). Sections (150 μm thick) were cut in the coronal plane with a vibratome. The cells were infected with a recombinant adenovirus along the surface of the ventricle with a heat-sharpened glass capillary. Infected sections were incubated for 1 h and then washed out with PBS and DF medium. The sections were placed on Millicell-CM (Millipore) in a 35-mm dish. The slice culture was maintained for 3–4 days in 1 ml of DF medium containing the B27 supplement, EGF, basic fibroblast growth factor, heparin, and BSA. Slices were fixed in 4% paraformaldehyde for 6 h. Fluorescent images of fixed slices were captured by microscopy. Fluorescence intensities of similar-width rectangles in three regions of the cerebral cortex (CP, IZ, and VZ) were measured with Scion (Frederick, MD) image software. Relative intensities to the total fluorescence were calculated as the percentage of GFP-positive cell position.
Results
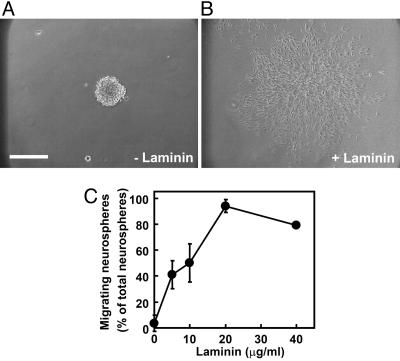
Neural Progenitor Cells Migrated on Laminin. A migration assay was performed as described in Materials and Methods. On a cover glass coated with only PDL, the progenitor cells did not migrate out of the neurosphere for 12 h (Fig. 1A). In contrast, neural progenitor cells on the cover glass coated with PDL plus laminin began to migrate out of the neurosphere within 2 h. Twelve hours after plating, most of the cells migrated from the neurosphere to the monolayer (Fig. 1B). As shown in Fig. 1C, the neural progenitor cells migrated in a concentration-dependent manner. This finding indicates that laminin is an important substrate on the neural progenitor cell migration in vitro.
Fig. 1.
Laminin-dependent migration of neural progenitor cells derived from mouse E11.5 embryonic telencephalon. (A and B) Neurospheres were plated on a cover glass coated with 200 μg/ml PDL (A) or PDL plus 20 μg/ml laminin (B) and cultured for 12 h. (C) Neurospheres were cultured on a cover glass coated with 200 μg/ml PDL and the indicated different concentrations of laminin. Cell migration was assessed as described in Materials and Methods. Data are the mean ± SD of at least three independent experiments. (Bar, 250 μm.)
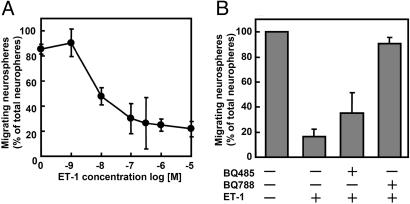
ET-1 Inhibited the Migration of Neural Progenitor Cells Through the ETB Receptor. Previously, we found that ET-1 inhibits the cell migration in human embryonic kidney 293 cells (13). We investigated the effect of ET-1 on neural progenitor cell migration. ET-1 inhibited the migration of neural progenitor cells in a dose-dependent manner (Fig. 2A). Two types of receptors for ETs are known as ETA and ETB receptors. Next, we used specific antagonists for ET receptors. BQ485 is a specific antagonist for the ETA receptor, and BQ788 is specific for the ETB receptor. As shown in Fig. 2B, BQ788 abrogated the ET-1-induced inhibition, but BQ485 had almost no effect on the inhibition. Moreover, ET-3, which activates the ETB receptor but not the ETA receptor, inhibited the neural progenitor cell migration (data not shown). These results indicated that ET negatively regulates the migration of neural progenitor cells through the ETB receptor.
Fig. 2.
ET-1 inhibits the migration of neural progenitor cells on laminin via ETB receptor. (A) Neurospheres were plated on a cover glass coated with PDL and laminin and cultured for 24 h with several concentrations of ET-1. (B) Neurospheres were treated with 10 μM BQ485, an antagonist of the ETA receptor, or 10 μM BQ788, an antagonist of the ETB receptor, for 30 min before treatment with 100 nM ET-1. Data are the mean ± SD of at least three independent experiments.
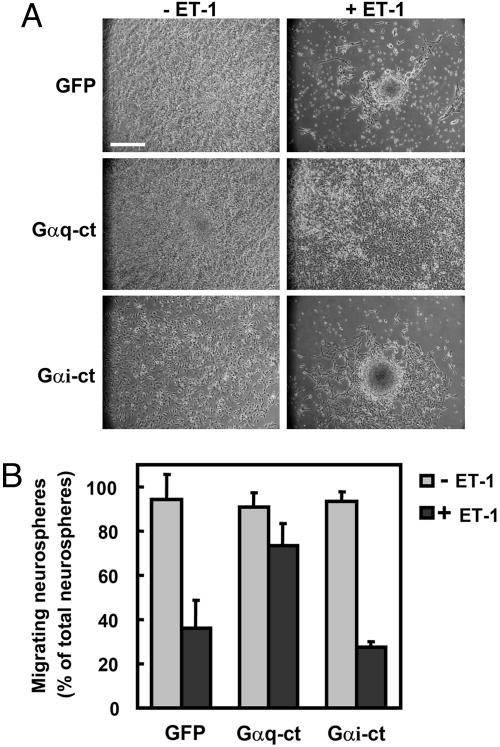
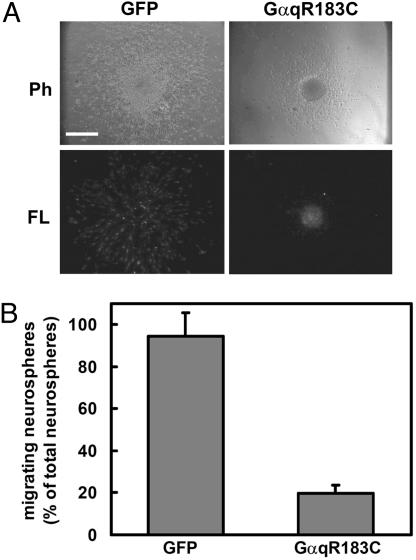
ET-1 Inhibited the Migration of Neural Progenitor Cells Through Gαq. The ETB receptor interacts with Gi (21), Gq, and G12 (22). To determine the role of G proteins in the inhibitory effect of ET-1 on the migration of neural progenitor cells, we examined the effect of the recombinant adenoviruses expressing the carboxyl-terminal peptides of the G protein α-subunit (Gα-ct). Gα-ct can inhibit the receptor–G-protein coupling with selectivity (20). Infection of adenoviruses harboring Gαq-ct and Gαi-ct did not affect the migration of neural progenitor cells (Fig. 3). In contrast, the inhibitory effect of ET-1 on neural progenitor migration was blocked by Gαq-ct but not Gαi-ct. Gα12-ct and Gα13-ct did not inhibit the effect of ET-1 (data not shown). To confirm the involvement of Gαq in the regulation of neural progenitor cell migration, we expressed a constitutively active mutant of Gαq, GαqR183C (Fig. 4). The expression of Gαq R183C inhibited the migration of neural progenitor cells. These results suggested that the inhibitory effect of ET-1 on the migration of neural progenitor cells is mediated through Gq.
Fig. 3.
Gq mediates the inhibitory effect of ET-1 on neural progenitor migration. (A) Neural progenitor cells were infected with adenoviruses harboring GFP, Gαq-ct, and Gαi-ct at a multiplicity of infection of 25. Forty-eight hours after infection, neurospheres were plated on cover glasses coated with PDL and laminin and cultured for 24 h. Representative phase-contrast images are shown. (B) The effect of Gαq-ct and Gαi-ct on the migration of neural progenitor cells is quantitated as a percentage of migrating neurospheres. Data are shown as the mean ± SD of at least three independent experiments. (Bar, 250 μm.)
Fig. 4.
Effect of a constitutively active mutant of Gαq, GαqR183C, on neural progenitor cell migration. (A) Neural progenitor cells were infected with adenoviruses harboring GFP or GαqR183C. Twenty-four hours after plating, the migration of neurospheres was analyzed by phase-contrast microscopy (Ph). The expression of adenoviruses was confirmed by fluorescence microscopy (FL). (B) Effect of GαqR183C on the migration of neural progenitor cells is shown as a percentage of migrating neurospheres. Data are shown as the mean ± SD of at least three independent experiments. (Bar, 250 μm.)
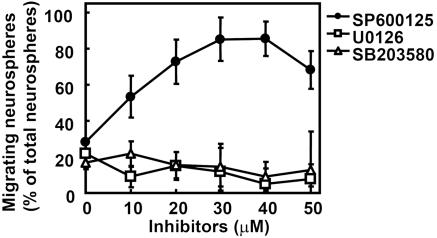
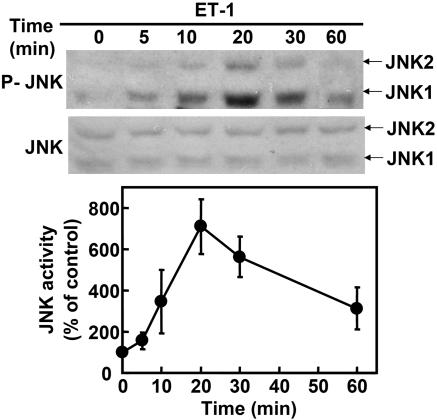
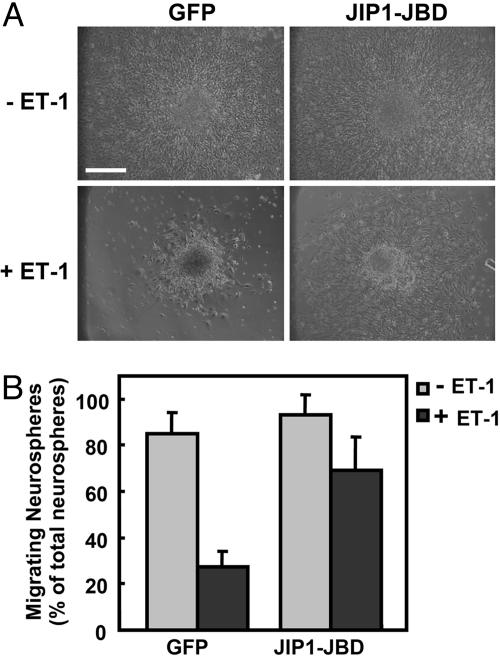
JNK Mediated ET-1-Induced Inhibition of Neural Progenitor Cell Migration. We previously demonstrated that ET-1 inhibits the migration of human embryonic kidney 293 cells via JNK (13). To clarify whether MAPKs were involved in the ET-1-induced inhibition of neural progenitor migration, we used specific inhibitors: U0126, an inhibitor of MEK1/2, which is an activator of extracellular signal-regulated kinase; SB202190, an inhibitor of p38; and SP600125, an inhibitor of JNK. Neural progenitor cells were preincubated with these inhibitors. As shown in Fig. 5, U0126 and SB203580 showed no effect on the inhibition of neural progenitor cell migration by ET-1. In contrast, pretreatment of neurospheres with SP600125, a specific inhibitor of JNK, rescued the inhibitory effect of ET-1. Thus, it was suggested that ET-1 inhibits the migration of neural progenitor cells via the activation of JNK. To confirm the ET-1-induced JNK activation in neural progenitor cells, we investigated the phosphorylation of endogenous JNK by using an antiphosphorylated JNK antibody that recognizes its active state (Fig. 6). Stimulation with ET-1 increased the phosphorylated JNK in neurosphere cells in a time-dependent manner. Moreover, we expressed JIP1-JBD, a specific inhibitor of the JNK pathway (Fig. 7). Although the expression of JIP-JBD did not affect the migration of neural progenitor cells, ET-1-induced inhibition was attenuated in cells expressing the JIP1-JBD. These results indicated that JNK activation is crucial for the inhibition of migration by ET-1.
Fig. 5.
Involvement of JNK in the inhibition of neural progenitor migration by ET-1. Neurospheres were plated on a cover glass coated with PDL and laminin and pretreated with several concentrations of inhibitors for 30 min before the addition of ET-1. U0126 (□), an inhibitor of MEK1/2, and SB203580 (▵), an inhibitor of p38, had no effect on the ET-1-induced inhibition of neural progenitor cell migration. SP600125 (•), a specific inhibitor of JNK, rescued the inhibitory effect of ET-1. Data are shown as the mean ± SD of at least three independent experiments.
Fig. 6.
ET-1 induces JNK activation in neural progenitor cells. Neurospheres were treated with ET-1 for the indicated periods. Activated JNK in neural progenitor cell lysates was detected by immunoblot analysis using antiphospho-JNK (P-JNK) antibody. Data are shown as the mean ± SD of at least three independent experiments.
Fig. 7.
Expression of JIP1-JBD, a specific inhibitor of JNK pathway, attenuates the ET-1-induced inhibition of neural progenitor cells. (A) Neural progenitor cells were infected with the adenovirus harboring GFP (control) or JIP1-JBD. Infected neurospheres were cultured with or without ET-1 for 24 h. (B) Data are shown as the mean ± SD of at least three independent experiments. (Bar, 250 μm.)
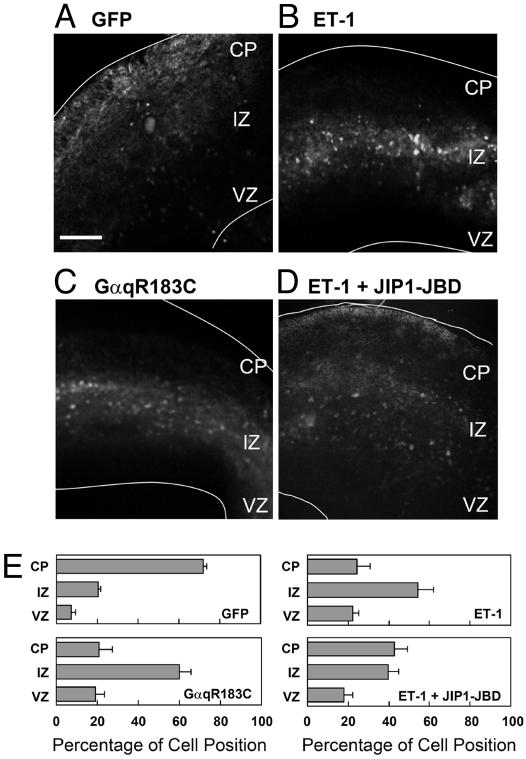
Gq- and JNK-Dependent Signal Caused the Retention of Neural Progenitor Cells at the IZ in a Cortical Slice Culture of E16.5 Cerebral Cortex. Next, we used a slice culture system of E16.5 cerebral cortex to observe the action of the GPCR signaling pathway in developing mouse brain. Cells in the VZ were infected with recombinant adenoviruses expressing GFP to monitor the radial migration. Twenty-four hours after infection, the GFP-expressing cells were distributed from the VZ to the IZ (data not shown). These cells then subsequently migrated to the CP for 3–4 days in culture (Fig. 8A). In the presence of ET-1, 60% of the GFP-expressing cells stopped mainly at the IZ (Fig. 8B). Because the removal of ET-1 from the culture medium rescued the migration to the CP, the inhibitory effect of ET-1 was reversible and not caused by the cytotoxic effect (data not shown). GαqR183C-expressing cells also could not migrate to the layer of the CP and were retained in the IZ (Fig. 8C). Moreover, expression of JIP1-JBD could partially rescue the inhibitory effect of ET-1 (Fig. 8D). These results suggest that the GPCR signal through Gq and JNK can negatively control the radial migration in vivo.
Fig. 8.
Effect of GPCR signaling on the migration of GFP-labeled cells originating from the VZ in a slice culture. Cortical slices were prepared from E16.5 mouse telencephalon. (A–D) Cells in the VZ were infected with adenoviruses harboring GFP alone (A and B), GFP plus GαqR183C (C), or GFP plus JIP1-JBD (D). Slices were cultured for 3 days in vitro without (A and C) or with 1 μM ET-1 (B and D). The white lines represent pial and ventricular surfaces. (E) Quantitative analysis of radial migration in A–D is shown. The slice was subdivided into three regions, indicated as CP, IZ, and VZ. Each score represents the mean percentage of relative intensity ± SD; n = 5. (Bar, 250 μm.)
Discussion
We first showed that neural progenitor cells migrated in a laminin-dependent manner. Jacques et al. (23) analyzed the expression pattern of integrins on neurosphere cells and suggested that neural precursor cell chain migration and division are regulated through different β1 integrins. We examined other ECM proteins, fibronectin, collagen type I, and collagen type IV, but these substrates did not promote the neural progenitor cell migration within 1 day (data not shown). Recently, Kearns et al. (24) reported that laminin and fibronectin significantly increase neurosphere cell migration velocity. They demonstrated that fibronectin affected a maximal velocity after 48 h, whereas maximal velocity on laminin was not reached until 72 h. We used the E11.5 telencephalic neurosphere, whereas they used the neurosphere from postnatal cerebellar-derived neurospheres, suggesting that the sensitivity of neural progenitor cells to ECM may depend on the origin and differentiation.
Although several molecules have been reported to be involved in radial migration during the development of CNS, such as Cdk5, Lis1, Reelin, ApoER2/VLDLR, mDab1, and doublecortin (25–27), the molecular mechanisms that control cell migration remain obscure. G protein is a key regulator of many cellular functions, but the role of G protein signaling in the developing CNS is poorly understood. To determine the role of G proteins in the development of CNS, we examined the effect of agonists that are bound to GPCRs on neuronal migration. Fukushima et al. (28, 29) demonstrated that lysophosphatidic acid (LPA) regulates cortical neuroblast morphology and induces cluster compaction of the neuroblast cell line. They also reported that LPA inhibits neuronal migration in explant cultures and whole-brain cultures. Although we found that LPA as well as ET-1 inhibited the migration of neural progenitor cells with neurospheres, LPA did not strictly control the radial migration in the slice culture system (data not shown). Thus, we investigated the molecular mechanism of regulation of neural progenitor cell migration by ET-1 in this study.
ET-1 binds to two distinct receptors, named ETA and ETB receptors. To reveal which receptor mediated the ET-1 signaling in neural progenitor cells, we used the specific antagonists and found that ET-1 controls the motility of neural progenitor cells via the ETB receptor (Fig. 2). ET plays a critical role in the development of the peripheral nervous system. Mice that are homozygous for a null mutation in the ETB receptor gene are almost completely white and die as juveniles from a ganglionic megacolon (30). Shin et al. (11) exploited the tetracycline-inducible system to generate strains of mice in which the endogenous ETB receptor gene is under the control of tetracycline-dependent transactivators. By using this knockout mouse, they revealed that the ETB receptor is required during neural crest development for the migration of both melanoblasts and enteric neuroblasts. Hirschprung's disease is a common genetic disorder caused by a failure of the neural crest cells to form ganglia in the distal gut, leading to peristaltic misregulation and intestinal obstruction. A number of genes have been linked to Hirschprung's disease, including ET-3 and the ETB receptor. Recently, two groups reported the mechanisms of this disease and the role of ET-3 and its receptor in the development of the enteric nervous system (31, 32). Both groups indicated that ET-3 inhibited the migration of neural crest stem cells induced by glial cell line-derived neurotrophic factor. In contrast, the function of ET in the development of CNS remains largely unknown. Tsaur et al. (33) reported that the ETB receptor gene is abundantly expressed in the VZs and subventricular zones of developing CNS. We also confirmed the expression of the ETB receptor in neurosphere cells by using RT-PCR (data not shown). Moreover, ET-3 and ET-1 inhibited the migration of neural progenitor cell migration (data not shown). Here, we suggested the possibility that ET acts via the ETB receptor in the development of CNS.
To reveal the inhibitory mechanism of ET-1, we analyzed the intracellular signaling cascade downstream of the ETB receptor. It is generally thought that ET-1-mediated responses are mediated by Gαq, Gαi, and Gβγ (34). It was reported that Gα12 and Gα13 are also involved in ET-1-induced JNK activation (22). To analyze the contribution of G proteins to the ET-1-mediated signal transduction pathways, we used a Gα-ct that interferes with receptor–G protein coupling. The carboxyl-terminal region of Gα is thought to be crucial for the coupling of receptors with G proteins. We showed that Gαq-ct attenuated the inhibition of neural progenitor cell migration by ET-1. In contrast, Gαi-ct, Gα12-ct, and Gα13-ct did not affect the inhibitory effect of ET-1. The expression of a constitutively active mutant of Gαq, GαqR183C, inhibited cell migration, as did ET-1. Ehrendreih et al. (35) reported that ETB receptor deficiency increased neuronal apoptosis in the dentate gyrus. However, no prominent phenotype has been reported in ET-1 knockout (36) or inducible ETB knockout (11) in cortical morphogenesis. These results suggested that the Gq-activation signal by other GPCRs negatively regulates neural progenitor cell migration. More recently, Piao et al. (37) reported that mutations in GPR56, which encodes an orphan GPCR, cause a human cortical malformation called bilateral frontical lamination. GPR56 is preferentially expressed in neural progenitor cells of the cerebral cortical VZ and subventricular zone during neurogenesis. Because the association of GPR56 and Gαq/11 has been demonstrated (38), it is possible that unknown ligands for GPR56 negatively regulate the migration of neural progenitor cells through Gq during CNS development.
We investigated whether three MAPKs (extracellular signal-regulated protein kinase 1/2, JNK, and p38) are involved in the inhibition of neural progenitor cell migration by ET-1. Specific inhibitors of the JNK pathway, SP600125 and JIP1-JBD, rescued the inhibition of migration by ET-1. Moreover, we showed that the ET-1/Gq/JNK signaling pathway is involved in the regulation of the radial migration in embryonic brain slices. The migrating cells from the VZ to the CP were retained in the IZ by ET-1 and constitutively active Gαq. Furthermore, JIP1-JBD expression could suppress the ET-1-induced retention of the cell in the IZ. These results suggested the importance of G protein signaling via JNK during cerebral cortex development. Intriguingly, Hirai et al. (17) reported that MAPK-upstream protein kinase (MUK) regulates the radial migration of immature neurons in the developing mouse telencephalon. They demonstrated the expression of MUK and the distribution of active JNK in the IZ. Constitutive expression of MUK using adenovirus injected into the lateral ventricle of an E13 embryo resulted in the arrest of neural progenitor migration. Kawauchi et al. (18) recently reported that Rac1 and its activators, STEF/Tiam1, and JNK regulate cortical neuronal migration. They indicated that dominant negative mutants of Rac1 or STEF/Tiam1 suppress cortical neural migration. They also indicated that immunostaining for activated JNK revealed a strong signal in the IZ of the E15 cerebral cortex. Furthermore, the expression of dominant negative JNK and an inhibitor of JNK, SP600125, suppressed the migration in a slice culture of brain. Our result that the activation of JNK by ET-1 inhibited neural progenitor cell migration in vitro and in a slice culture is consistent with the result of Hirai et al. (17) in vivo. Thus, whether the activation of JNK suppresses or induces neural cell migration may depend on the developmental stage.
The mechanism of cell motility regulated by JNK is not clear. However, JNK pathways should play a pivotal role in the regulation of cell motility. JNK is responsible for cell migration during Drosophila development, specifically during the process of dorsal closure (39, 40). Recently, paxillin, a focal adhesion protein, was identified as a target for JNK in the migration of epithelial cells and keratocytes (19). Phosphorylation of paxillin by JNK may be essential for neural progenitor cell migration. More recently, Gdalyahu et al. (41) demonstrated that microtuble binding protein, doublecortin (DCX), is a substrate of JNK. Mutations in the X-linked gene DCX result in lissencephaly and abnormal neuronal positioning in neuronal migration. It is also reported that DCX is phosphorylated at distinct serine residues and multiple regulated by other kinases, such as PKA/MARK (42) and Cdk5/p35 (43).
Small GTPases play the important role in cellular processes including migration in mammalian cells. Moreover, it has been reported that small GTPases induce JNK activation in many cell types (44). We found that Clostridium difficile toxin B and Clostridium botulinum exoenzyme C3, inhibitors of Rho family GTPase, inhibited the migration of neural progenitor cells (data not shown). Hence, we could not examine the effect of these inhibitors on ET-induced inhibition of the cell migration. Recently, Konno et al. (45) indicated that constitutively active and dominant-negative mutants of Rac1 and Cdc42 significantly inhibited the radial migration in the neocortical slice culture.
Recently, new-mode radial migration has been revealed by time-lapse imaging studies in slices of the developing neocortex (2, 3). This new mode of the radial migration is that migrating cells do not move straight toward the CP, but change the migration rate and their morphology in the IZ. They become highly multipolar, and then become bipolar again as they progress through the IZ and enter the CP. It appears that the multipolar cells with the low migration rate search the signals that will determine whether the cells migrate radially or tangentially (27). Although the regulatory mechanism of the multipolar migration is obscure, the Gq- and JNK-mediated signals may be involved in the multipolar migration.
In summary, we found that ET-1 inhibits the migration of neural progenitor cells through the ETB receptor in vitro. The inhibitory effect of ET-1 was observed in a slice culture and mediated through Gq and JNK. Further research should help reveal details of the regulatory mechanism of CNS development by GPCR signaling.
Acknowledgments
We thank Drs. Motoshi Nagao and Masato Nakafuku for instruction regarding the preparation of neuronal progenitor cells. This work was partially supported by research grants from the Ministry of Education, Science, Sports, and Culture of Japan (Grants 15370057 and 17370051), the Uehara Memorial Foundation, the Yamanouchi Foundation, the Cell Science Foundation, and the Ono Medical Research Foundation.
Author contributions: N.M., J.Y., and H.I. designed research; N.M., H. Kokubu, M.S., and A.N. performed research; H. Kurose contributed new reagents/analytic tools; N.M. and H.I. analyzed data; and N.M., H. Kokubu, M.S., and H.I. wrote the paper.
Abbreviations: ET, endothelin; ECM, extracellular matrix; GPCR, G protein-coupled receptor; JNK, c-Jun N-terminal kinase; JIP1-JBD, JNK-binding domain of JNK-interacting protein-1; MAPK, mitogen-activated protein kinase; VZ, ventricular zone; CP, cortical plate; IZ, intermediate zone; PDL, poly-d-lysine; Gα-ct, carboxyl-terminal peptide of the G protein α-subunit; En, embryonic day n; DF, DMEM/F-12.
References
- 1.Hatten, M. E. (2002) Science 297, 1660–1663. [DOI] [PubMed] [Google Scholar]
- 2.Tabata, H. & Nakajima, K. (2003) J. Neurosci. 23, 9996–10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nadarajah, B., Alifragis, P., Wong, R. O. L. & Parnavelas, G. (2003) Cereb. Cortex 13, 607–611. [DOI] [PubMed] [Google Scholar]
- 4.Miranti, C. K. & Brugge, J. S. (2002) Nat. Cell Biol. 4, 83–90. [DOI] [PubMed] [Google Scholar]
- 5.Bokel, C. & Brown, N. H. (2002) Dev. Cell 3, 311–321. [DOI] [PubMed] [Google Scholar]
- 6.Perris, R. & Perissinotto, D. (2002) Mech. Dev. 95, 3–21. [DOI] [PubMed] [Google Scholar]
- 7.Anton, E. S., Kreidberg, J. A. & Rakic, P. (1999) Neuron 22, 277–289. [DOI] [PubMed] [Google Scholar]
- 8.Dulabon, L., Olson, E. C., Taglienti, M. G., Eisenhuth, S., McGrath, B., Walsh, C. A., Kreidberg, J. A. & Anton, E. S. (2000) Neuron 27, 33–44. [DOI] [PubMed] [Google Scholar]
- 9.Pons, S., Trejo, J. L., Martinez-Morales, J. R. & Marti, E. (2001) Development (Cambridge, U.K.) 128, 1481–1492. [DOI] [PubMed] [Google Scholar]
- 10.Masaki, T. (2004) Trends Pharmacol. Sci. 25, 219–224. [DOI] [PubMed] [Google Scholar]
- 11.Shin, M. K., Levorse, J. M., Ingram, R. S. & Tilghman, S. M. (1999) Nature 402, 496–501. [DOI] [PubMed] [Google Scholar]
- 12.Davis, R. J. (2000) Cell 103, 239–252. [DOI] [PubMed] [Google Scholar]
- 13.Yamauchi, J., Miyamoto, Y., Kokubu, H., Nishii, H., Okamoto, M., Sugawara, Y., Hirasawa, A., Tsujimoto, G. & Itoh, H. (2002) FEBS Lett. 527, 284–288. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto, Y., Yamauchi, J., Mizuno, N. & Itoh, H. (2004) J. Biol. Chem. 279, 34336–34342. [DOI] [PubMed] [Google Scholar]
- 15.Li, J., Molkentin, J. D. & Colbert, M. C. (2001) Dev. Biol. 232, 351–361. [DOI] [PubMed] [Google Scholar]
- 16.Jimenez, B., Volpert, O. V., Reiher, F., Chang, L., Munoz, A., Karin, M. & Bouck, N. (2001) Oncogene 20, 3443–3448. [DOI] [PubMed] [Google Scholar]
- 17.Hirai, S., Kawaguchi, A., Hirasawa, R., Baba, M., Ohnishi, T. & Ohno, S. (2002) Development (Cambridge, U.K.) 129, 4483–4495. [DOI] [PubMed] [Google Scholar]
- 18.Kawauchi, T., Chihama, K., Nabeshima, Y. & Hoshino, M. (2003) EMBO J. 22, 4190–4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang, C., Rajfur, Z., Borchers, C., Schaller, M. D. & Jacobson, K. (2003) Nature 424, 219–223. [DOI] [PubMed] [Google Scholar]
- 20.Maruyama, Y., Nishida, M., Sugimoto, Y., Tanabe, S., Turner, J. H., Kozasa, T., Wada, T., Nagao, T. & Kurose, H. (2002) Circ. Res. 91, 961–969. [DOI] [PubMed] [Google Scholar]
- 21.Takagi, Y., Ninomiya, H., Sakamoto, A., Miwa, S. & Masaki, T. (1995) J. Biol. Chem. 270, 10072–10078. [DOI] [PubMed] [Google Scholar]
- 22.Mao, J., Yuan, H., Xie, W., Simon, M. I. & Wu, D. (1998) J. Biol. Chem. 273, 27118–27123. [DOI] [PubMed] [Google Scholar]
- 23.Jacques, T. S., Relvas, J. B., Nishimura, S., Pytela, R., Edwards, G. M., Streuli, C. H. & ffrench-Constant, C. (1998) Development (Cambridge, U.K.) 125, 3167–3177. [DOI] [PubMed] [Google Scholar]
- 24.Kearns, S. M., Laywell, E. D., Kukekov, V. K. & Steindler, D. A. (2003) Exp. Neurol. 182, 240–244. [DOI] [PubMed] [Google Scholar]
- 25.Gupta, A., Tsai, L. H. & Wynshaw-Boris, A. (2002) Nat. Rev. Genet. 3, 342–355. [DOI] [PubMed] [Google Scholar]
- 26.Olson, E. C. & Walsh, C. A. (2002) Curr. Opin. Genet. Dev. 12, 320–327. [DOI] [PubMed] [Google Scholar]
- 27.LoTurco, J. (2004) Neuron 41, 175–180. [DOI] [PubMed] [Google Scholar]
- 28.Fukushima, N., Weiner, J. A. & Chun, J. (2000) Dev. Biol. 228, 6–18. [DOI] [PubMed] [Google Scholar]
- 29.Fukushima, N., Ishii, I., Habara, Y., Allen, C. B. & Chun, J. (2002) Mol. Biol. Cell 13, 2692–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosoda, K., Hammer, T. E., Richardson, J. A., Baynash, A. G., Cheung, J. C., Giaid, A. & Yanagisawa, M. (1994) Cell 79, 1267–1276. [DOI] [PubMed] [Google Scholar]
- 31.Barlow, A., de Graaff, E. & Pachnis, V. (2003) Neuron 40, 905–916. [DOI] [PubMed] [Google Scholar]
- 32.Kruger, G. M., Mosher, J. T., Tsai, Y. H., Yeager, K. J., Iwashita, T., Gariepy, C. E. & Morrison, S. J. (2003) Neuron 40, 917–929. [DOI] [PubMed] [Google Scholar]
- 33.Tsaur, M. L., Wan, Y. C. & Lai, F. P. (1997) FEBS Lett. 417, 208–212. [DOI] [PubMed] [Google Scholar]
- 34.Miyauchi, T. & Masaki, T. (1999) Annu. Rev. Physiol. 61, 391–415. [DOI] [PubMed] [Google Scholar]
- 35.Ehrenreich, H., Nau, T. R., Dembowski, C., Hasselblatt, M., Barth, M., Hahn, A., Schilling, L., Siren, A.-L. & Bruck, W. (2000) Neuroscience 95, 993–1001. [DOI] [PubMed] [Google Scholar]
- 36.Kurihara, Y., Kurihara, H., Morita, H., Cao, W. H., Ling, G. Y., Kumada, M., Kimura, S., Nagai, R., Yazaki, Y. & Kuwaki, T. (2000) Am. J. Physiol. 279, R515–R521. [DOI] [PubMed] [Google Scholar]
- 37.Piao, X., Hill, R. S., Bodell, A., Chang, B. S., Basel-Vanagaite, L., Straussberg, R., Dobyns, W. B., Qasrawi, B., Winter, R. M., Innes, A. M., et al. (2004) Science 303, 2033–2036. [DOI] [PubMed] [Google Scholar]
- 38.Little, K. D., Hemler, M. E. & Stipp, C. S. (2004) Mol. Biol. Cell 15, 2375–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riesgo-Escovar, J. R., Jenni, M., Fritz, A. & Hafen, E. (1996) Genes Dev. 10, 2759–2768. [DOI] [PubMed] [Google Scholar]
- 40.Sluss, H. K., Han, Z., Barrett, T., Davis, R. J. & Ip, Y. T. (1996) Genes Dev. 10, 2745–2758. [DOI] [PubMed] [Google Scholar]
- 41.Gdalyahu, A., Ghosh, I., Levy, T., Sapir, T., Sapoznik, S., Fishler, Y., Azoulai, D. & Reiner, O. (2004) EMBO J. 23, 823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaar, B. T., Kinoshita, K. & McConnell, S. K. (2004) Neuron 41, 203–213. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka, T., Serneo, F. F., Tseng, H. C., Kulkarni, A. B., Tsai, L. H. & Gleeson, J. G. (2004) Neuron 41, 215–227. [DOI] [PubMed] [Google Scholar]
- 44.Bishop, A. L. & Hall, A. (2000) Biochem. J. 348, 241–255. [PMC free article] [PubMed] [Google Scholar]
- 45.Konno, D., Yoshimura, S., Hori, K., Maruoka, H. & Sobue, K. (2005) J. Biol. Chem. 280, 5082–5088. [DOI] [PubMed] [Google Scholar]