Abstract
Purpose
Gnathia tridens Menzies & Barnard, 1959, is redescribed from material collected from San Diego, California and compared to the original description, as well as material held at the Natural History Museum of Los Angeles County and the Santa Barbara Museum of Natural History.
Materials and Methods
A full redescription is given based on both morphological and molecular characteristics of the male using light and scanning electron microscopy, and COI mtDNA and ITS2 rDNA genes, respectively.
Results
The key distinguishing characters that set G. tridens apart from other congeners are the equally trifid mediofrontal process, the mandible with a large incisor; the mesioventral margin anterior tip dorsally visible; pereonite 4 with distinct visible anterior constriction; and the three proximal tubercles on the antenna articles. Based on the molecular data for COI, the closest congener differs with 122 base pairs.
Conclusion
Together, the combined morphological and molecular characterisation will provide a foundation for future, taxonomic, phylogenetic and biogeographical studies within the genus Gnathia and the Gnathiidae.
Keywords: Marine parasitic isopod, California, Systematic taxonomy, Temperate Northern Pacific Realm, Temporary fish parasite
Introduction
Gnathia Leach, 1814 [1] is the most species-rich genus within the family Gnathiidae with at least 141 known species [2]. According to Kim et al. [3], Ota et al. [4] and [5], 21 species within the genus Gnathia have previously been described and reported from the Temperate Northern Pacific (TNP) realm (see [6] for classification of various realms) (Table 1). Juvenile gnathiid isopods are well-known temporary fish parasites, while adults are free-living and can be identified by the distinct morphology of the adult male mandibles and frontal margin. Host records have been reported for some species, mostly those infesting chondrichthyans, while other species have been reported from specific habitats within brackish and marine waters. These include sandy and muddy substrata, as well as various forms of coral, algae, seagrass and kelp. Of the 21 species reported from the TNP, only Gnathia trimaculata Coetzee, Smit, Grutter & Davies, 2009 [7] from Japan, has been molecularly characterised.
Table 1.
Summary of currently known species of Gnathia Leach, 1814 from the Temperate Northern Pacific (TNP) [6]
| Species | Total length (mm) | Type locality | Depth (m) | Habitat/Substratum Host (if known) |
|---|---|---|---|---|
|
Gnathia bungoensis Nunomura, 1982 [16] (see also [17]) |
3.5* |
Bansho River, Oita Prefecture, eastern coast of Kyusyu, southern Japan; 32° 58′ N, 131° 55′ E |
2 | Muddy colonies of green algae, Ulva spp.; near the sandy bottom of the estuary of Bansho-River; near the rocky shore, in colonies of brown algae, Sargassum spp. |
|
Gnathia capillata Nunomura & Honma, 2004 [18] |
7.6* | Sado Island, western Honshu, Japan | Unknown |
Gill chamber wall Sting ray, Dasyatis akajei; several other chondrichthyan fishes |
|
Gnathia capitellum Ota, Kohtsuka & Tanaka, 2021 [4] |
2.1–2.5 |
Nabeta Bay, Izu Peninsula, Miura Peninsula, Japan; 34° 66′ 45.4″ N, 138° 94′ 12.4″ E |
3–11 |
Dredging and muddy substratum Platycephalus sp., Takifugu snyderi, |
|
Gnathia clementensis Schultz, 1966 [19] |
8.5 |
San Clemente Canyon, off southern San Clemente Island, California, the United States; 32° 44′ 00″ N, 118° 12′ 45′ W |
162 | Grab sample containing manganese nodules |
|
Gnathia coronadoensis Schultz, 1966 [19] |
3.5 |
Coronado Canyon, off San Diego, California, the United States; 32° 30′ 42″ N, 117° 21′ 37″ W |
182–812 | Green mud and grey mud with H2S smell |
|
Gnathia derzhavini Gurjanova, 1933 [20] |
5.1 | Peter the Great Bay, Sea of Japan, (south of Askold Island), Russia | 121–110 | Unknown |
|
Gnathia gurjanovae Golovan, 2006 [21] |
5 | Peter the Great Bay, Sea of Japan, Russia | 66 | Clay-like silt, silted sand |
|
Gnathia hirsuta Schultz, 1966 [19] |
4 |
Santa Cruz Canyon, southern coast of Santa Cruz Island, California, the United States; 33° 56′ 03″ N, 119° 52′ 03″ W |
218 | Rocks and some green sand |
|
Gnathia koreana Song & Ming, 2018 [22] |
4.3–4.6 |
Yeogaekseon terminal, Geomuno Island, Yeosu-si, Jeollanam-do, South Korea; 34° 01′ 37″ N, 127° 18′ 27″ E |
10 | Organic-rich muddy sand |
|
Gnathia mutsuensis Nunomura, 2004 [23] (see also [17]) |
2.1 | Asamushi, Aomori Prefecture, northern Honshu, Japan | Unknown | Intertidal shore |
|
Gnathia nasuta Nunomura, 1992 [24] (see also [17]) |
11.9–4.4 |
Off Tomioka, Reihoku-cho, Kumamoto Pref., Kyushu, western Japan; 32° 20′–22′ N, 130° 01′–03′ E |
8.5–412 | Sandy and muddy sediment |
|
Gnathia obtusispina Kim, Kim & Yoon, 2023 [3] |
3.2 |
Hongdo Island, Jeollanam-do (Province), southwestern South Korea; 34° 43′ 22.8″ N, 125° 11′ 59.5″ E |
10 | Rinsing bryozoans and macroalgae on the bedrock of sublittoral zones |
|
Gnathia productatridens Menzies & Barnard, 1959 [8] |
3.2 |
Off Summerland, Santa Barbara, California, the United States; 34° 14′ 50″ N, 119° 32′ 25″ W |
94 | Green silt |
|
Gnathia rectifrons Gurjanova, 1933 [20] |
6 | Peter the Great Bay, Sea of Japan, Russia | 80–88 | Unknown |
|
Gnathia sanrikuensis Nunomura, 1998 [25] (see also [17]) |
2.8–3 |
Otsuchi Bay, Iwate Prefecture, northern Japan; 39° 20′–21′ N, 141° 53′–58′ E |
42 | Sandy sediment |
|
Gnathia schmidti Gurjanova, 1933 [20] |
5.5 |
Vladimir Bay, Sea of Japan, Russia; 43° 56′ N, 135° 56′ E |
8 | Unknown |
|
Gnathia steveni Menzies, 1962 [26] |
2.3 |
Bahia de San Quintin, Baha California Mexico, c 30° 26′ 34.9″ N, 15° 57′ 27.2″ W |
Unknown | Intertidal rocks |
|
Gnathia tridens Menzies & Barnard, 1959 [8] |
3 |
Point Conception, San Diego, California, the United States; 34° 26′ 54″ N, 120° 28′ 17″ W |
11 | Kelp habitat; red algae |
|
Gnathia trilobata Schultz, 1966 [19] |
5 |
Coronado Canyon, off San Diego, California, the United States; 32° 30′ 42″ N, 117° 21′ 37″ W |
812–976 | Green sand and mud |
|
Gnathia trimaculata Coetzee, Smit, Grutter & Davies, 2009 [7] (see also [5]) |
4–5.4 |
Off Lizard Island, Australia; 14° 40′ 54.68″ S, 145° 26′ 53.72″ E |
Unknown |
Rocky shores First and second stage larvae: external surface of Enneapterygius etheostomus, E. miyakensis, and Springerichthys bapturus; Third stage larvae: gill filaments of Carcharinus melanopterus and C. amblyrhynchos |
|
Gnathia tuberculata Richardson, 1909 [27] |
3.5* |
Off Sudzii Misaki Light, east of Noto Peninsula, Japan; 37° 22′ 30″ N, 137° 47′ 00″ E |
1100 | Green mud and grey mud with H2S smell |
*Total length including mandibles, excluding antennae
Where available, additional descriptors such as the substratum where free-living adults have been collected, and hosts where parasitic larval stages were collected, are included (adapted from [3])
Gnathia tridens Menzies & Barnard, 1959 [8], was described more than 65 years ago from specimens collected off Point Conception, southern California (USA) within the TNP. In 1997, Wetzer and Brusca [9] provided a short description of G. tridens, as well as an illustration of the habitus which they labelled as the male holotype and a female paratype and a key to the Californian species of Gnathia. A considerable amount of interesting and valuable information on G. tridens was also captured in the newsletters of the Southern California Association of Marine Invertebrate Taxonomists (SCAMIT) [10], however, what they considered to be the male of G. tridens does not correspond to some critical aspects of the original description [8] or the notes provided by Wetzer and Brusca [9], specifically characters such as the cephalon (herein referred to as cephalosome) lacking setae and tuberculation. The most recent mention of G. tridens was in a review and guide to the isopods of the Southern California Bight by Stebbins and Wetzer [11], which included a key to Gnathiidae. In this review [11], the distribution of G. tridens extends from southern California northwards to British Columbia (Canada), however, it is not clear how the specimens from Canada (and elsewhere) were identified [12–15]. Although the species appears to be widespread, this cannot be confirmed as little or no taxonomic evidence was provided, and in several cases no specimens were retained. There is also no available data that corroborates an extended distribution beyond southern California for G. tridens. Currently, available data lack sufficient information on the key morphological characters needed for the consolidation of this species. Therefore, a comprehensive taxonomic redescription, integrated with molecular data, is essential. The molecular characterisation will also enable researchers to identify G. tridens males and distinguish this species from other, possibly undescribed, species as well to unambiguously identify females and parasitic larval stages of G. tridens when collected in the absence of males.
Materials and Methods
Field Methods
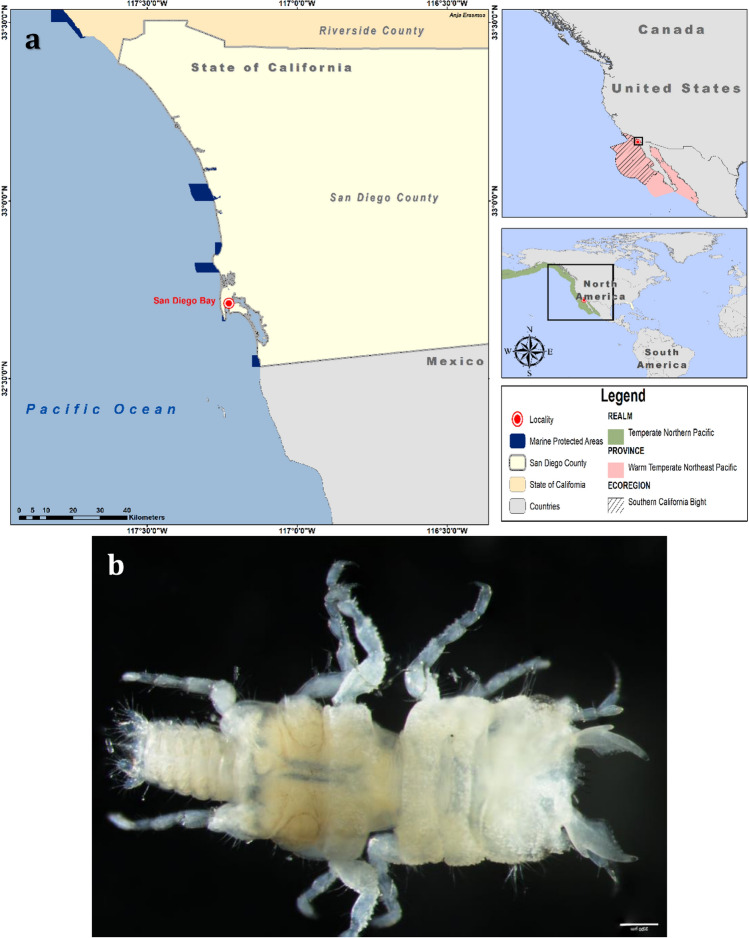
Specimens were collected from April 2016 through April 2018 at depths of 10–12 m as described by [28] during a trapping study off San Diego, California, USA (32° 42′ 44″ N, 117° 13′ 37″ W) (Fig. 1). Host fish were collected under permits issued by the California Department of Fish and Wildlife (SC-13350) and handled according to the approved SDSU IACUC protocol (APF 15-10-013A). Adult males were moulted from third-stage pranizae in the laboratory following [29], and preserved in varying grades of ethanol for morphological study and molecular sequencing, respectively. In addition, photographs of the type material (previously AHF Type No. 5711) deposited at the Natural History Museum of Los Angeles County [8], as well as photographs of Gnathia tridens specimens (SBMNH 669829; SBMNH 694194; SBMNH 697320; SBMNH 697452) collected during benthic trawling surveys in Santa Barbara County, California from 1982 to 1985 [9] provided by the Santa Barbara Museum of Natural History, were obtained for comparison.
Fig. 1.
a Study area map of San Diego Bay, California, indicating the realm, province and ecoregion within the Marine Ecoregions of the World (MEOW); b Gnathia tridens male voucher material (LACM 36831). Scale bar: 200 µm. Marine region spatial data was obtained via ArcGIS Hub based on the data from [6]
Identification Methods
The research conducted at North-West University was granted ethical clearance under the reference number NWU-00784-24-A5. Preserved specimens were cleaned and allocated for various identification techniques including light and scanning electron microscopy and gene sequencing. Morphological characterisation was done using lignin pink-stained specimens (whole and dissected) as described for light and scanning electron microscopy in [30]. This same protocol was followed for the illustrations. DELTA descriptions were made with terminology based on [31] and [32]. The total length of the habitus was measured from the frontal margin (including the processes and excluding the mandibles), mid-dorsally, to the midpoint of the pleotelson [32]. Voucher material is deposited in the Natural History Museum of Los Angeles County (LACM).
Molecular Characterisation
Mitochondrial DNA was isolated from two male specimens using the manufacturer’s protocol for animal tissue extraction of the NucleoSpin® Tissue Genomic DNA Tissue Kit (Macherey–Nagel, Düren, Germany). Amplification of COI mtDNA and ITS2 rDNA genes was done using specific polymerase chain reaction (PCR) protocols and primers (LCO1490 and HCO2198, and 3S-forward and ITS2.2-reverse, respectively) (Table 2). Polymerase chain reaction (PCR) protocols, reactions and conditions were followed as in [26]. Thereafter, sequences were trimmed, assembled and edited using bioinformatic software, Geneious R7.1.3 (RRID: SCR_010519) [33]. Sequences were compared to known sequences of Gnathia available on GenBank and BOLD and subsequently submitted to GenBank (COI: PV213449; PV213450 and ITS2: PV211460 and PV211461).
Table 2.
Gene regions of COI mtDNA and ITS2 rDNA, with selected PCR primers, used to amplify DNA for molecular characterisation of Gnathia tridens
| Gene region | Primers | Nucleotide sequence | References |
|---|---|---|---|
| COI mtDNA | LCO1490 | 5′-GGTCAACAAATCATAAAGATATTGG-3′ |
Folmer et al. (1994) [34] Shodipo et al. (2021) [35] |
| HCO2198 | 5′-TAAACTTCAGGGTGACCAAAAAATCA-3′ | ||
| ITS2 rDNA | 3S-Forward | 5′-GGTACCGGTGGATCACGTGGCTAGTG-3′ | Grutter et al. (2000) [36] |
| ITS2.2-Reverse | 5′-CCTGGTTAGTTTCTTTTCCTCCGC-3′ |
AHF Allan Hancock Foundation; DELTA DEscriptive Language for TAxonomy; LACM Natural History Museum of Los Angeles County; MEOW Marine Ecoregions of the World; NWU-WRG North-West University Water Research Group; SBMNH Santa Barbara Museum of Natural History; SEM scanning electron microscopy; TNP Temperate Northern Pacific
Results
Taxonomy
Suborder Cymothoida Wägele, 1989
Superfamily Cymothooidea Leach, 1814
Family Gnathiidae Leach, 1814
Gnathia Leach, 1814
Type species. Gnathia termitoides Leach, 1814 (= Gnathia maxillaris (Montagu, 1804) [37]); type by original designation [31].
Gnathia tridens Menzies & Barnard, 1959
Gnathia tridens Menzies and Barnard, 1959 [8]: 29, fig. 23.—Schultz, 1969 [38]: 229, fig. 365.—Wetzer et al. 1991 [39]: 36, 46.—Wetzer and Brusca, 1997 [9]: 47–49, figs 1.19, 1.20.—Spitzer et al. 2022 [28]: 69, fig. 2 photo.—Stebbins and Wetzer 2023 [11]: 5, 27, 29, 89–90, 164, fig. 12G.
Not Gnathia tridens.—Lissner et al. 1986 [12]: 29.—McLaughlin et al. 2005 [13]: 194.—Espinosa-Pérez et al. 2009 [14]: 229, table 1.—Macdonald et al. 2010 [15]: 20, table 2. [all = Gnathia sp.].
Excluded.—SCAMIT reports by [10] and [40]; Outer Continental Shelf (OCS) study by [41].
Material examined. 4 ♂ voucher specimens (2.4–3.1 mm TL) LACM 36831; LACM 36832, collected and reared as stated in [24], off San Diego, California, USA, 32° 42′ 44″ N, 117° 13′ 37″ W (LACM 36831; LACM 36832).
Other material: 3 ♂♂ (2.3–3.1 mm TL) dissected and stained with lignin pink used for light microscopy, same information as voucher. 1 ♂ (2.4 mm TL) prepared and used for SEM, same information as voucher. 2 ♂♂ (2.3–2.6 mm TL) used for genetic characterisation of COI mtDNA and ITS2 rDNA genes (GenBank numbers COI: PV213449; PV213450 and ITS2: PV211460 and PV211461), same information as voucher. 3 ♂♂ (2.3–4.1 mm TL) examined for variation in frontal margin, same information as voucher (in the collection of the NWU-WRG). Photos of the type material provided by LACM were examined for direct comparison.
Type locality. Point Conception, 34° 26′ 54″ N, 120° 28′ 17″ W, California [8].
Distribution. Confirmed records are Point Conception [8] and San Diego [28 and present material], in California, USA.
Habitat. Bottom of dead kelp fragments and red algae [8], rocky reef, corals, seagrass, sediment, and kelp forest [28] at depths of 10–12 m.
Host. Heterostichus rostratus (Giant kelpfish) [28].
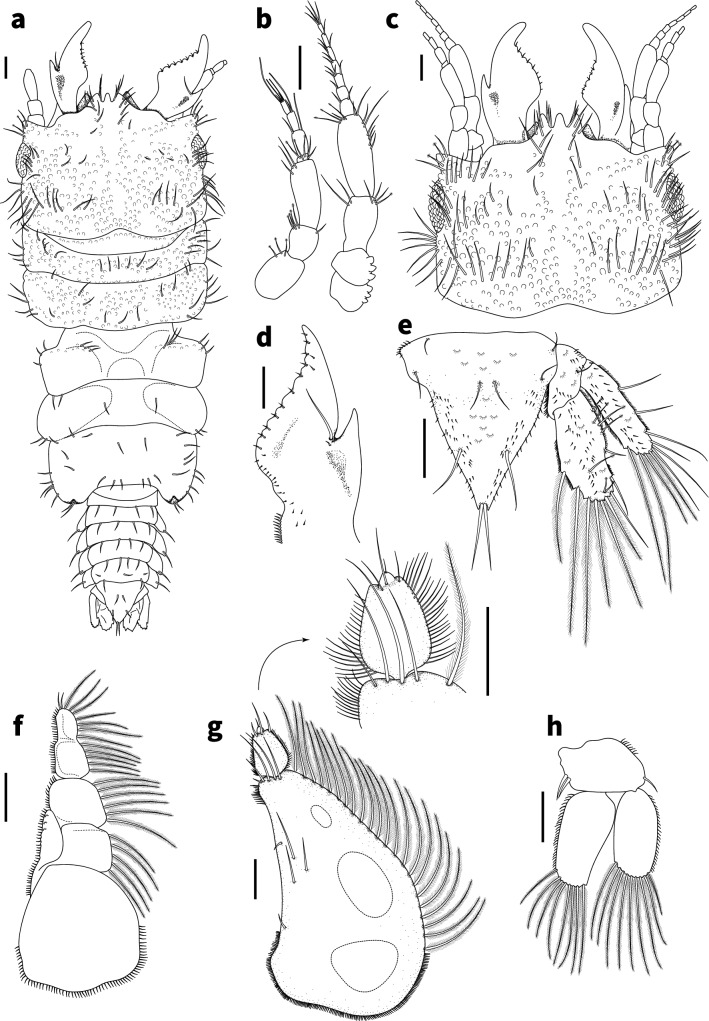
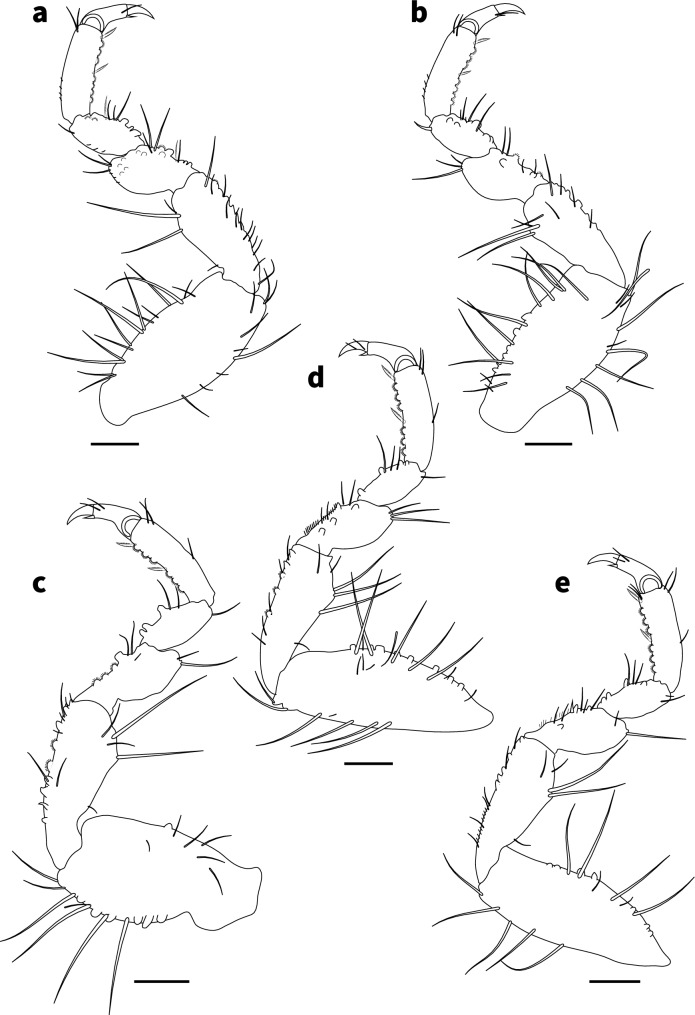
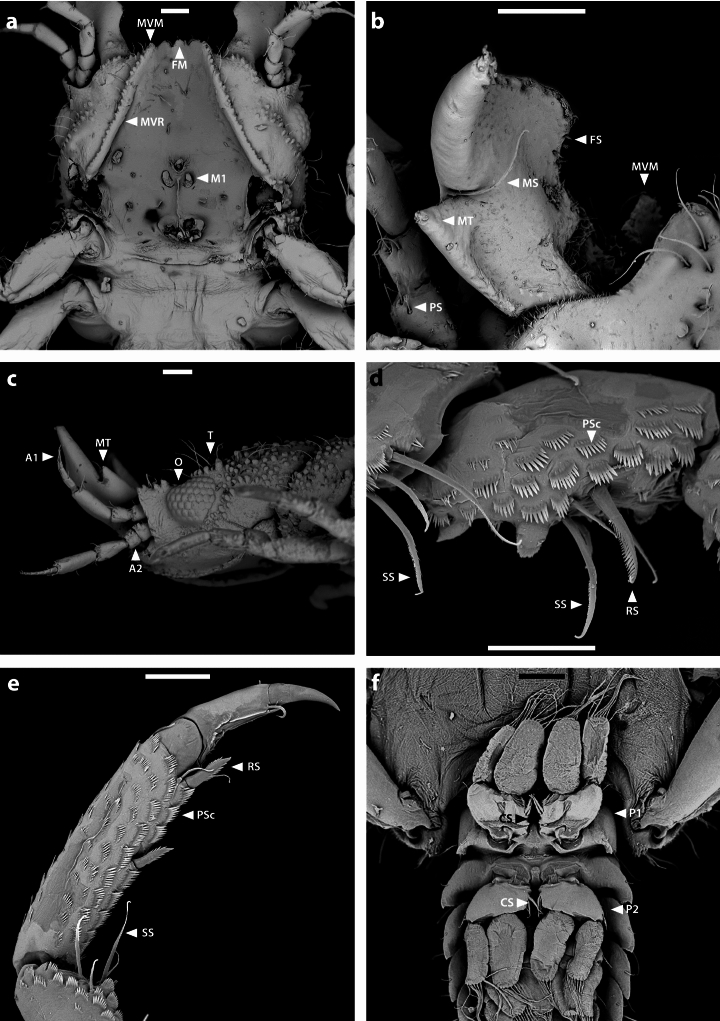
Description of adult male (Figs. 2, 3, 4):
Fig. 2.
Gnathia tridens, male (2.6 mm TL) a habitus dorsal view (LACM 36831) b antennae c dorsal view of cephalosome with frontal margin and mandibles d right mandible dorsal view e pleotelson and uropod f maxilliped g pylopod with detail of articles 2 and 3 h pleopod 2. Scale bars: 100 µm
Fig. 3.
Gnathia tridens, male (3.1 mm TL) a–e right pereopods 2–6, respectively. Scale bar: 100 μm
Fig. 4.
Gnathia tridens, male scanning electron microscope images. Cephalosome (a) ventral (b) dorsal (c) lateral, with three proximal tubercles on antenna peduncle article 1 and 2 d various types of setae on carpus of pereopod e ventral view of pereopod from propodus to unguis f ventral view of pleopod 1 and pleopod 2 with no appendix masculina. Scale bars: 100 μm (a–c, f); 50 μm (d, e). A2 antenna; A1 antennula; CS coupling seta/e; O eye ommatidia; FS fringe seta/e; FM frontal margin; MS mandibular seta/e; MT mandibular tooth; M1 maxilla 1; MVM mesioventral margin; MVR mesioventral ridge; PSc pectinate scale/s; P1 pleopod 1; P2 pleopod 2; RS robust seta/e; SS serrate seta/e; T tubercles
Body (Figs. 1b, 2a) 2.1 times as long as greatest width, widest at pereonite 2; dorsal surfaces with tubercules anteriorly and smooth posteriorly, sparsely setose, chromatophores not apparent in fixed specimens. Cephalosome (Figs. 2c, 4c) rectangular, 0.6 as long as wide, lateral margins parallel, posterior margin medially concave; dorsal surface with numerous granules and tubercles around eyes; dorsal sulcus wide, shallow, short; translucent region absent; paraocular ornamentation weakly developed and with several tubercles and setae, posterior median tubercle present; with lateral tubercles. Frontal margin (Figs. 2a, c, 4a) with processes. External scissura present, wide, shallow. Mediofrontal process present, strong, produced, equally trifid, with 3 simple setae on either side. Superior frontolateral process absent. Inferior frontolateral process absent. Mesioventral margin straight; granular and setose; anterior tip dorsally visible. Supraocular lobe pronounced, pointed, accessory supraocular lobe not pronounced. Eyes elongate, 0.3 as long as cephalosome length, contiguous with head surface, ommatidia arranged in rows, eye colour not apparent in fixed specimens.
Pereon (Figs. 1b, 2a) lateral margins sub-parallel, with few setae; anteriorly with numerous fine granules. Pereonite 1 not fused dorsally with cephalosome; dorsolateral margins fully obscured by cephalosome. Pereonites 2 and 3 wider than pereonite 1. Pereonite 4 with anterior constriction, median groove present. Areae laterales present on pereonite 5; dorsal sulcus wide. Pereonite 6 with weak lobi laterales; lobuii weak, notched. Pereonite 7 4 times longer than wide. Pleon epimera dorsally visible on all pleonites. Pleonite lateral margins with 1 pair of simple setae, with 2 pairs of simple setae medially.
Pleotelson (Figs. 1b, 2a, e, 4f) 1.1 times as long as anterior width, partially covered in pectinate scales and covered in fringe setae; lateral margins finely serrate, anterolateral margins weakly concave, with 2 pairs of submarginal setae; posterolateral margin distally weakly concave, with 1 pair of submarginal setae; mid-dorsal surface with 1 pair of sub-median setae, apex with 2 setae.
Antennula (Figs. 2b, 4c) 0.7 times shorter than antenna; article 2 0.6 times as long as article 1; article 3 1.8 times as long as article 2, 2.25 times as long as wide; flagellum 1.1 times as long as article 3, with 4 articles; article 1 with 3 penicillate setae and 2 simple setae; article 2 with 1 aesthetasc seta and 1 simple seta; article 3 with 1 aesthetasc seta and 1 simple seta; article 4 with 3 aesthetasc seta and 2 simple setae. Antenna (Figs. 2b, 4c) peduncle with 4 articles; article 1 and 2 each with three distinct proximal tubercles; article 3 1.5 times as long as wide, 1.5 times as long as article 2, with 1 penicillate seta, and 5 simple setae; article 4 1.6 times as long as article 3, 2.4 times as long as wide, with 3 penicillate setae, and with 9 simple setae; flagellum 1.2 times as long as article 4, flagellum 1.9 times as long as article 3, with 7 articles, terminating with 4 simple setae.
Mandible (Figs. 2d, 4b) 0.7 as long as wide, and as long as length of cephalosome, triangular, weakly curved; apex 21% of total length; mandibular seta present. Carina present, smooth along proximal half. Incisor elevated, standing clear of surface, distal denticulation absent. Blade present, dentate, unevenly convex, midventrally convex, dentate along 60% of margin, bearing 6–7 small teeth. Pseudoblade absent. Internal lobe absent. Dorsal lobe absent. Basal neck short. Erisma present. Lamina dentata absent.
Maxilliped (Fig. 2f) 5-articled; article 1 lateral margin with continuous marginal scale-setae; article 2 lateral margin with 5 plumose setae; article 3 lateral margin with 6 plumose setae; article 4 lateral margin with 5 plumose setae; article 5 lateral margin with 7 plumose setae, and 2 simple setae; endite extending to mid-margin of article 3; with no coupling setae.
Pylopod (Fig. 2g) article 1 1.6 times as long as wide; with 3 distinct areolae; without distolateral lobe; posterior and lateral margins forming rounded curve; lateral margin with 22–26 large plumose setae; mesial margin with scale-setae on distal part only; surface 5–6 simple setae present; distal margin with 5–6 simple setae; article 2 1.4 times as long as wide; with 3 simple setae; article 3 min and partially fused to article 2, with 1 seta.
Pereopods 2–6 (Fig. 3a–e) with long simple setae, and pectinate scales unevenly distributed along the inner margin of propodus, carpus, merus, and ischium; propodus distal robust setae (RS) slightly longer than proximal RS; inferior margins with weak tubercles, pereopod 2 with tubercles on ischium to carpus; pereopod 2 basis 2.3 times as long as greatest width, superior margin with 14 setae, inferior margin with 9 setae; ischium 1.5 times as long as basis, 2.3 as long as wide, superior margin with 4 setae, inferior margin with 12 setae; merus 0.5 times as long as ischium, 1.2 times as long as wide, superior margin with 3 setae; superior margin with bulbous protrusion; inferior margin with 5 setae; carpus 0.5 times as long as ischium, 1.7 times as long as wide, superior margin with 1 seta, inferior margin with 4 setae; propodus 0.7 times as long as ischium, 3.1 times as long as wide, superior margin with 2 simple setae, superior margin with 1 penicillate seta, inferior margin with 1 simple seta, no short setae, and 2 RS; dactylus 0.4 times as long as propodus; pereopods 3 and 4 similar to pereopod 2; pereopod 5 similar to pereopod 6; pereopod 6 with tubercles on basis to carpus; basis 2.7 times as long as greatest width, superior margin with 5 simple setae, and 2 penicillate setae, inferior margin with 5 setae; ischium 0.7 times as long as basis, 2.4 times as long as greatest width, superior margin with 5 setae, inferior margin with 5 setae; merus 0.6 times as long as ischium, 1.8 times as long as wide, superior margin with 4 setae, inferior margin with 6 setae, with dense patch of scale-setae; carpus 2 times as long as ischium, 1.3 times as long as wide, superior margin with 2 setae, inferior margin with 3 setae; propodus 1.3 times as long as ischium, 2.8 times as long as wide, superior margin with 3 setae, inferior margin with 2 simple setae, and 2 RS; dactylus 2.2 times as long as propodus.
Penes composed of non-prominent openings, almost flush with ventral surface of pereonite 6, penial process as long as basal width.
Pleopod 2 (Figs. 2h, 4f) exopod 2.3 as long as wide, distally broadly rounded, with 9 plumose setae; endopod 1.8 times as long as wide, distally narrowly rounded, with 8 plumose setae; appendix masculina absent; peduncle 1.5 times as wide as long, mesial margin with 2 coupling setae, lateral margin with 1 simple seta.
Uropod (Fig. 2e) rami extending to pleotelson apex, apices broadly rounded. Peduncle with no dorsal setae. Uropod endopod 1.7 times as long as greatest width, dorsally with 7 penicillate setae; lateral margin straight, with 3 simple setae; proximomesial margin weakly convex, with 6 long plumose setae. Uropod exopod not extending to end of endopod, 3.3 times as long as greatest width; lateral margin weakly sinuate, with 6 simple setae; proximomesial margin straight, with 4 long plumose setae.
Remarks
Gnathia tridens can be identified by the equally trifid processes on the mediofrontal process (with all three acute) that are longer than wide, the mandible with a large incisor (mandibular tooth), the mesioventral margin anterior tip dorsally visible, pereonite 4 with distinct visible anterior constriction separating pereonite 4 from pereonite 3, and the antenna peduncle articles 1 and 2 each with three proximal tubercles.
The material examined here agrees with the original description of G. tridens [8] based on the body size, trifid frons (herein referred to as frontal medial margin processes), third segment narrowed, inner face of the mandibles bearing 6–7 small teeth and the outer face bearing a large tooth (herein referred to as incisor). Additional distinguishing features not provided in the original description and subsequent notes on this species include: mesioventral margin anterior tip dorsally visible and antenna (A2) peduncle articles 1 and 2 each with three proximal tubercles.
Gnathia tridens may be identified and separated from all congeners within the TNP by the equally trifid frontal margin. In addition, Gnathia magdalenensis Müller, 1988 [42] from northern Colombia, Gnathia trilobata Schultz, 1966 [19] from the Coronado Canyon, Gnathia vellosa Müller, 1988 [42] from northern Colombia, and Gnathia virginalis Monod 1926 [43] from the Virgin Islands, may be distinguished by variations in their trifid frontal margin, none of which are equal in size. Gnathia magdalenensis can be distinguished by the subequal mediofrontal process and inner lobe on the mandible, while in G. trilobata the frontal margin is strongly produced with three mediofrontal processes. Gnathia vellosa appears closely related to G. virginalis, with both having granular body surfaces and three processes on the frontal margin. However, they differ in body size, and there is a distally notched carina on the mandible in G. vellosa, and a rounded mandibular carina in G. virginalis. Gnathia tridens can be further distinguished from G. virginalis by the presence of a mandibular seta, a 3-articled pylopod, and having 4 and 7 plumose marginal setae on the uropodal exopod and endopod, respectively.
In the original description by Menzies and Barnard [8], the authors mention that G. tridens is similar to Gnathia africana Barnard, 1914 [44], an intertidal species from the south and west coasts of South Africa. Gnathia africana was redescribed in 1999 [45], providing further morphological characters that clearly distinguish it from G. tridens. The two species can be distinguished by the produced equally trifid frontal margin present in G. tridens, the presence of frontolateral processes and inferior mediofrontal process that is divided in two in G. africana, supraocular ornamentation prominent in G. africana absent in G. tridens, and the three proximal tubercles of the antenna in G. tridens absent in G. africana.
Molecular Phylogeny
Two consensus sequences of Gnathia tridens males were successfully acquired for COI mtDNA and ITS2 rDNA genes, respectively. These are the first sequences obtained for Gnathia tridens, and for any Gnathia species from the TNP. The sequences ranged from 623–640 base pairs (bp) within a 0.2%–0.4% divergence for COI (see Table 3) and ITS2, respectively. Due to limited published COI sequences available for species of Gnathia on both GenBank and BOLD, with only one known species, Gnathia jimmybuffetti Erasmus et al. [30], with comparable sequences for ITS2 rDNA, phylogenetic trees were not constructed.
Table 3.
Genetic matrix for newly obtained COI sequences of Gnathia tridens, indicating the percentage similarity below the diagonal division and the nucleotide p-distances above the division
| COI sequences for Gnathia spp. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
Gnathia tridens |
0.000 | 0.318 | 0.264 | 0.322 | 0.335 | 0.287 | 0.282 | 0.328 | 0.284 | 0.327 | |
| 2 |
Gnathia tridens |
100 | 0.319 | 0.264 | 0.321 | 0.338 | 0.288 | 0.284 | 0.327 | 0.284 | 0.329 | |
| 3 |
Gnathia camuripenis |
68.2 | 68.1 | 0.313 | 0.318 | 0.313 | 0.305 | 0.325 | 0.320 | 0.272 | 0.313 | |
| 4 |
Gnathia jimmybuffetti |
73.6 | 73.6 | 68.7 | 0.307 | 0.342 | 0.301 | 0.270 | 0.330 | 0.292 | 0.332 | |
| 5 |
Gnathia limicola |
67.8 | 67.9 | 68.2 | 69.3 | 0.313 | 0.322 | 0.337 | 0.387 | 0.308 | 0.333 | |
| 6 |
Gnathia maculosa |
66.2 | 65.9 | 68.3 | 65.5 | 68.3 | 0.326 | 0.345 | 0.363 | 0.303 | 0.260 | |
| 7 |
Gnathia malaysiensis |
71.3 | 71.2 | 69.5 | 69.9 | 67.8 | 66.9 | 0.322 | 0.329 | 0.296 | 0.333 | |
| 8 |
Gnathia marleyi |
71.8 | 71.6 | 67.5 | 73 | 66.3 | 65.2 | 67.8 | 0.340 | 0.315 | 0.333 | |
| 9 |
PMACA067-17 Gnathia maxillaris |
67.2 | 67.3 | 68 | 67 | 61.3 | 63.4 | 67.1 | 66 | 0.350 | 0.334 | |
| 10 |
NOISO088-15 Gnathia oxyuraea |
71.6 | 71.6 | 72.8 | 70.8 | 69.2 | 69.3 | 70.4 | 68.5 | 65 | 0.298 | |
| 11 |
AB13956 Gnathia trimaculata |
67 | 66.7 | 68.3 | 66.5 | 66.3 | 74 | 66.2 | 66.3 | 66.2 | 69.8 | |
Column headings represent the corresponding species number in the first column of the row title. Novel sequences are indicated in bold
Discussion
Gnathia tridens is here comprehensively redescribed using an integrated approach of reliable morphological techniques and successfully sequenced using both COI mtDNA and ITS2 rDNA genes. There are several discrepancies between the illustration provided by Wetzer and Brusca ([9]: Fig. 1.19) and the holotype figures given by Menzies and Barnard ([8]: Fig. 23); specifically in that the body tapers in width posteriorly (vs not tapering, mesially constricted between pereonites 3 and 4 in [8]), the dorsal surface of the cephalosome is smooth (vs granular in [8]), and the spines on the anterior margin of the cephalosome are short, apically rounded, and appear to be five (vs three spines, apically acute in [8]). These differences suggest that two species may be included under the current use of the binomen Gnathia tridens. Alternately, the differences may be due to drawing perspective and misinterpretation of the acute distal point of the mesioventral ridge (see Fig. 4a). Through the present study, all the defining characteristics of G. tridens are described in detail and illustrated using both light and scanning electron microscopy, which will eliminate further misidentification of this species.
In addition to the identification of the male, the sequences provided can also aid in the identification of female and larval specimens of Gnathia tridens as proven in [30], and thereafter molecular techniques can be used to link the different life stages of parasitic larvae and free-living adults of Gnathia tridens. Haney [10] linked several males and females of Gnathia, from coastal California, based on the assumption that co-occurring specimens would likely be the same species, and perceived shared morphological characters that were not verified molecularly. Further studies are needed to clarify the correct female and larval stages by combining the morphological and molecular data available. More sequences are needed to confirm the phylogenetic relationship of species within the genus Gnathia, and even the family Gnathiidae.
The information provided by the present study has resolved the identity of G. tridens and will lead to resolving the actual distribution of the species, including its supposed status as a ubiquitous Pacific coast species. The combination of a comprehensive integrated morphological description with genetic sequences will aid in clarifying future reports of this species and as well as other related species.
Acknowledgements
We would like to acknowledge H Chaney of the Santa Barbara Museum of Natural History (SBMNH) for providing photographs of benthic macrofauna samples of G. tridens for comparative purposes. In addition, we would like to acknowledge the communication and efforts of Dr R Wetzer of the Natural History Museum of Los Angeles County (LACM) in providing photographs and information on specimens deposited at the museum. We thank C van Wyk (NWU-WRG) for assistance with molecular characterisation and W Landman (NWU) for assistance with scanning electron microscopy (SEM). Funding (for CS) was provided by a California State University Grant, the American Academy of Underwater Sciences, the California State University Council on Ocean Affairs Science and Technology, the American Museum of Natural History Lerner Gray Fund for Marine Research, the Western Society of Naturalists, and San Diego State University's Harold and June Grant Memorial Scholarship, Mabel Meyers Scholarship, and Elliot Family Fund Scholarship. Funding (for AE) was provided by the NWU Postdoctoral Programme. Funding for this project was provided by the US National Science Foundation (NSF OCE 2023420 and DEB 2231250, P Sikkel PI). This is contribution number #945 from the NWU-WRG.
Author Contributions
AE—Formal analysis, investigation, data curation, microscopy, illustrations, writing—original draft, writing—editing, visualization NJS—Conceptualisation, resources, investigation, writing—editing CS—Field collection, rearing and development of specimens, provision of specimens, writing—editing PCS—Supervision, field collection, rearing and development of specimens, funding, writing—editing NLB—Investigation, nomenclature, writing—editing KAH—Conceptualisation, supervision, resources, investigation, writing—editing.
Funding
Open access funding provided by North-West University. This article is funded by California State University Grant, American Academy of Underwater Sciences, California State University Council on Ocean Affairs Science and Technology, American Museum of Natural History Lerner Gray Fund for Marine Research, Western Society of Naturalists, San Diego State University's Harold and June Grant Memorial Scholarship, Mabel Meyers Scholarship, Elliot Family Fund Scholarship, NWU Postdoctoral Programme, US National Science Foundation, NSF OCE 2023420 and DEB 2231250.
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Leach WE (1814) Crustaceology. Brewster’s Edinburgh Encyclopaedia (7)1:383–437. Available online at https://www.biodiversitylibrary.org/page/37187640. Accessed 14 Dec 2024
- 2.Boyko CB, Bruce NL, Hadfield KA, Merrin KL, Ota Y, Poore GCB, Taiti S (eds) (2024) World Marine, Freshwater and Terrestrial Isopod Crustaceans database. Gnathia Leach, 1814. Accessed through: World Register of Marine Species at: https://www.marinespecies.org/aphia.php?p=taxdetails&id=118437 11 Nov 2024
- 3.Kim SH, Kim JG, Yoon SM (2023) Two new temporary ectoparasitic isopods (Cymothoida: Cymothooidea) from Korean waters with a note on geographical distributions of Rocinela Leach, 1818 and Gnathia Leach, 1814. PeerJ 11:e14593. 10.7717/peerj.14593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ota Y, Kohtsuka H, Tanaka K (2021) Description of a small-headed Gnathiid Isopod (Crustacea), Gnathia capitellum sp. nov., from Coastal Japan. Species Divers 26:207–216. 10.12782/specdiv.26.207 [Google Scholar]
- 5.Ota Y, Hoshino O, Hirose M, Tanaka K, Hirose E (2012) Third-stage larva shifts host fish from teleost to elasmobranch in the temporary parasitic isopod, Gnathia trimaculata (Crustacea; Gnathiidae). Mar Biol 159:2333–2347. 10.1007/s00227-012-2018-2 [Google Scholar]
- 6.Spalding MD, Fox HE, Allen GR, Davidson N, Ferdana ZA, Finlayson M, Halpern BS, Jorge MA, Lombana A, Lourie SA et al (2007) Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Biosci 57(7):573–583. 10.1641/B570707 [Google Scholar]
- 7.Coetzee ML, Smit NJ, Grutter AS, Davies AJ (2009) Gnathia trimaculata n. sp. (Crustacea: Isopoda: Gnathiidae), an ectoparasite found parasitising requiem sharks off Lizard Island, Great Barrier Reef, Australia. Syst Parasitol 72(2):97–112. 10.1007/s11230-008-9158-2 [DOI] [PubMed] [Google Scholar]
- 8.Menzies RJ, Barnard JL (1959) Marine Isopoda on coastal shelf bottoms of Southern California: systematics and ecology. Pacific Naturalist 1(11):3–35 [Google Scholar]
- 9.Wetzer R, Brusca RC (1997) Descriptions of the species of the suborders Anthuridea, Epicaridea, Flabellifera, Gnathiidea, and Valvifera. In: Blake JA, Scott PH (Eds) Taxonomic Atlas of the Benthic Fauna of the Santa Maria Basin and Western Santa Barbara Channel, Vol. 11: The Crustacea Part 2: The Isopoda, Cumacea and Tanaidacea, 9–58
- 10.Haney L (2006) Pairing Northeast Pacific gnathiid isopods. Which females go with what males? SCAMIT Newsletter, Supplement to Vol. 24(6):37. https://www.scamit.org/newsletters/2006-03.pdf. Accessed 19 Mar 2025
- 11.Stebbins TD, Wetzer R (2023) Review and guide to the isopods (Crustacea, Isopoda) of littoral and sublittoral marine habitats in the Southern California Bight. ZooKeys 1162:1. 10.3897/2Fzookeys.1162.100390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lissner A, Phillips C, Cadien D, Smith R, Bernstein B, Cimberg R, Kauwling T, Anikouchine W (1986) Assessment of long-term changes in biological communities in the Santa Maria Basin and Western Santa Barbara Channel – Phase I. Vol. II, Synthesis of Findings. Prepared for: United States Department of the Interior, Minerals Management Service, Pacific OCS Region, Los Angeles, p 789
- 13.McLaughlin PA, Camp DK, Angel MV, Bousfield EL, Brunel P, Brusca RC, Cadien D, Cohen AC, Conlan K, Eldredge LG, Felder DL, Goy JW, Haney T, Hann B, Heard RW, Hendrycks EA, Hobbs III HH, Holsinger JR, Kensley B, Laubitz DR, LeCroy SE, Lemaitre R, Maddocks RF, Martin JW, Mikkelsen P, Nelson E, Newman WA, Overstreet RM, Poly WJ, Price WW, Reid JW, Robertson A, Rogers DC, Ross A, Schotte M, Schram FR, Shih C-T, Watling L, Wilson GDF, Turgeon DD (2005) Common and Scientific Names of Aquatic Invertebrates from the United States and Canada: Crustaceans. American Fisheries Society Special Publication 31: [xiii +] 1–545 [+ CD-ROM]
- 14.Espinosa-Pérez MC, Hendrickx ME, Morrone JJ (2009) Identification of generalized tracks for the species of Isopoda (Peracarida) from the Eastern Pacific. J Crust Biol 29(2):224–231. 10.1651/08-3074R.1 [Google Scholar]
- 15.Macdonald TA, Burd BJ, Macdonald VI, van Roodselaar A (2010) Taxonomic and feeding guild classification for the marine benthic macroinvertebrates of the Strait of Georgia, British Columbia. Can Tech Rep Fish Aquat Sci 2874:63 [Google Scholar]
- 16.Nunomura N (1982) A new gnathiid isopod from Saeki Bay, Western Japan. Bull Toyam Sci Mus 4:17–21 [Google Scholar]
- 17.Ota Y (2013) Redescription of five gnathiid species from Japan (Crustacea: Isopoda). Zootaxa 3737(1):33–56. 10.11646/zootaxa.3737.1.3 [DOI] [PubMed] [Google Scholar]
- 18.Nunomura N, Honma Y (2004) Gnathia capillata, a new species of the genus Gnathia (Crustacea, Isopoda). Contrib Biol Lab Kyoto Univ 29:343–349 [Google Scholar]
- 19.Schultz GA (1966) Submarine canyons of southern California. Part 4. Systematics: Isopoda. Allan Hancock Pac Exped 27(1):1–56 [Google Scholar]
- 20.Gurjanova E (1933) Contribution to the Isopod-Fauna of the Pacific Ocean. II. New species of Gnathiidea and Asellota. Issledovaniia Fauny Morei: Akademiia Nauk SSSR 19:79–91 [Google Scholar]
- 21.Golovan OA (2006) Gnathia gurjanovae sp. n., a new species of gnathiids [sic] (Isopoda: Gnathiidae) from Peter the Great Bay, Sea of Japan. Russian J Mar Biol 32:28–36. 10.1134/S1063074006010044 [Google Scholar]
- 22.Song J-H, Min G-S (2018) First records of Gnathia Leach, 1814 and Tachaea Schioedte & Meinert, 1879 from South Korea, with descriptions of two new species (Isopoda, Cymothoida, Cymothooidea). ZooKeys 787:17–35. 10.3897/zookeys.787.26291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nunomura N (2004) Isopod crustaceans collected from Aomori Prefecture Northern Japan. Contrib Biol Lab Kyoto Univ 29:351–360 [Google Scholar]
- 24.Nunomura N (1992) Marine Isopoda from Amakusa, Kyushu (II). Amakusa Mar Biol Lab 11:59–71 [Google Scholar]
- 25.Nunomura N (1998) A new species of the gnathiid isopod crustacean from the sea off Sanriku, northern Japan. Bull Toyam Sci Mus 21:55–60 [Google Scholar]
- 26.Menzies RJ (1962) The marine isopod fauna of Bahia de San Quintin, Baja California, Mexico. Pac Nat 3(11):337–348 [Google Scholar]
- 27.Richardson H (1909) Isopods collected in the northwest Pacific by the U.S. Bureau of Fisheries steamer “Albatross” in 1906. Proc US Natl Mus 37:75–129 [Google Scholar]
- 28.Spitzer CA, Anderson TW, Sikkel PC (2022) Habitat associations and impacts on a juvenile fish host by a temperate gnathiid isopod. Int J Parasitol: Parasites Wildl 17:65–73. 10.1016/j.ijppaw.2021.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smit NJ, Basson L, Van As JG (2003) Life cycle of the temporary fish parasite, Gnathia africana (Crustacea: Isopoda: Gnathiidae). Folia Parasitol 50(2):135–142 [DOI] [PubMed] [Google Scholar]
- 30.Erasmus A, Hadfield KA, Sikkel PC, Smit NJ (2023) Morphological description and molecular characterization of Gnathia jimmybuffetti sp. nov. (Crustacea, Isopoda, Gnathiidae): the first new gnathiid in 100 years from the Floridian ecoregion. Bull Mar Sci 99(3):353–375. 10.5343/bms.2023.0040 [Google Scholar]
- 31.Cohen BF, Poore GCB (1994) Phylogeny and biogeography of the Gnathiidae (Crustacea Isopoda) with descriptions of new genera and species, most from south-eastern Australia. Mem Mus Vic 54(2):271–397. 10.24199/j.mmv.1994.54.13 [Google Scholar]
- 32.Smit NJ, Hadfield KA (2022) Gnathia pipinde sp. nov. (Crustacea, Isopoda, Gnathiidae), a temporary parasite of the pufferfish, Amblyrhynchotes honckenii, from temperate southern Africa. ZooKeys 1129:1–19. 10.3897/zookeys.1129.90986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. J Bioinform 28(12):1647–1649. 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299 [PubMed] [Google Scholar]
- 35.Shodipo MO, Sikkel PC, Smit NJ, Hadfield KA (2021) First record and molecular characterization of two Gnathia species (Crustacea, Isopoda, Gnathiidae) from Philippine coral reefs, including a summary of all Central-Indo Pacific Gnathia species. Int J Parasitol: Parasites Wildl 14:355–367. 10.1016/j.ijppaw.2021.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grutter AS, Morgan JAT, Adlard RD (2000) Characterising parasitic gnathiid isopod species and matching life stages with ribosomal DNA ITS2 sequences. Mar Biol 136:201–205. 10.1007/s002270050677 [Google Scholar]
- 37.Montagu G (1804) Description of several marine animals found on the south coast of Devonshire. Trans Linn Soc, London: 61–85, pls. 6–7. Available online at https://www.biodiversitylibrary.org/page/756268. Accessed 1 Nov 2024
- 38.Schultz GA (1969) How to know the marine isopod crustaceans. Wm. C Brown Company Publishers, Dubuque, p 359 [Google Scholar]
- 39.Wetzer R, Kuck HG, Baéz R, Brusca RC, Jurkevics LM (1991) Catalog of the isopod Crustacea type collection of the Natural History Museum of Los Angeles County. Technical Reports No. 3, Natural History Museum of Los Angeles County, Los Angeles, p 59
- 40.SCAMIT (2021) A Taxonomic Listing of Benthic Marco- and Megainvertebrates from Infaunal & Epifaunal Monitoring and Research Programs in the Southern California Bight, Edition 13. Cadien DB, Lovell LL, Barwick KL, Haggin BM, (eds) p 203
- 41.Henkel SK, Gilbane L, Phillips AJ, Gillett DJ (2020) Cross-shelf habitat suitability modeling for benthic macrofauna. Camarillo (CA): US Department of the Interior, Bureau of Ocean Energy Management. OCS Study BOEM 2020-008, p 71
- 42.Müller H-G (1988) The genus Gnathia Leach (Isopoda) from the Santa Marta area, northern Colombia, with a review of Gnathiidae from the Caribbean Sea and Gulf of Mexico. Bijd Dierkunde 58:88–104 [Google Scholar]
- 43.Monod T (1926) Les Gnathiidæ. Essai monographique (Morphologie, Biologie, Systématique). Mem Soc Sci Nat Maroc 13:1–668 [Google Scholar]
- 44.Barnard KH (1914) Contributions to the crustacean fauna of South Africa. 1. Additions to the marine Isopoda. Ann S Afr Mus 10(7):197–230 [Google Scholar]
- 45.Smit NJ, Van As JG, Basson L (1999) A redescription of the adult male and praniza of Gnathia africana Barnard, 1914 (Crustacea, Isopoda, Gnathiidae) from southern Africa. Folia Parasitol 46:229–240 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.