Abstract
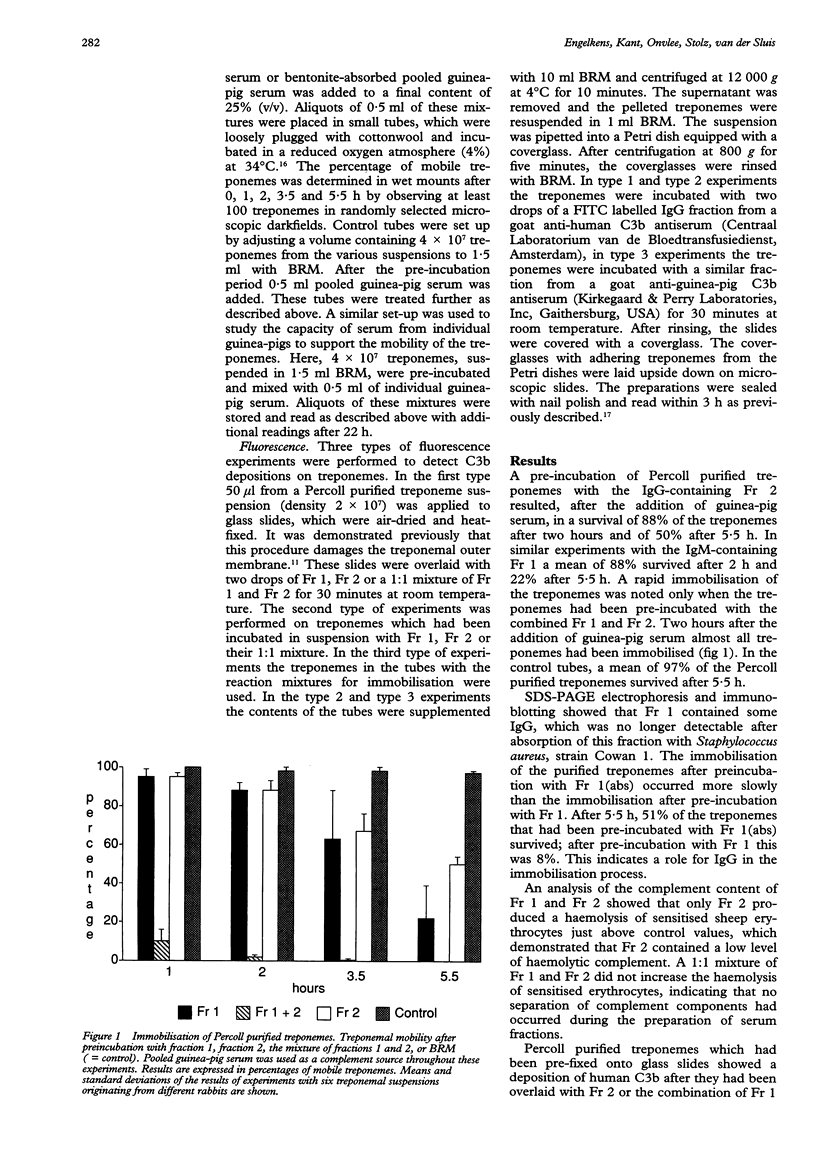
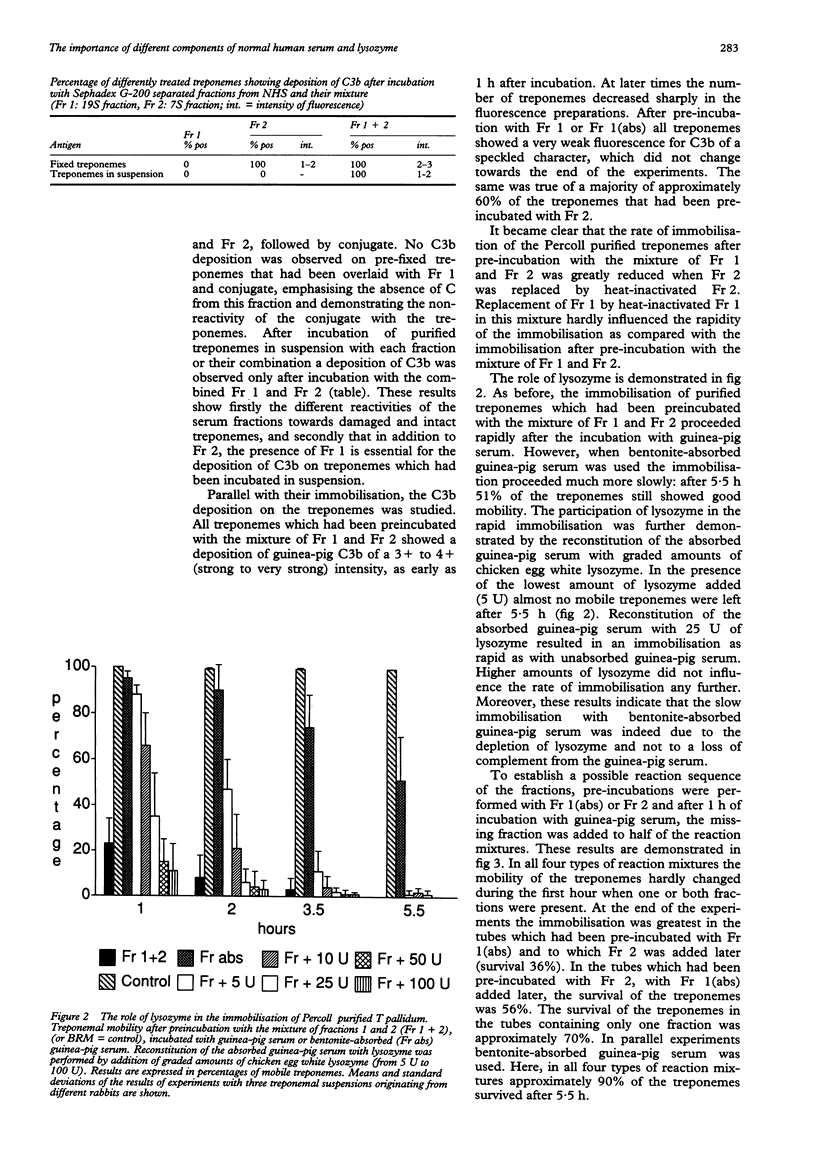
OBJECTIVES--To study the role of different components in normal human serum and the role of lysozyme in rapid immobilisation of Percoll purified T pallidum (Nichols). MATERIALS AND METHODS--The immobilisation of Percoll purified T pallidum was studied after pre-incubations with different serum fractions (Fr) of normal human serum (Fr 1, containing IgM; Fr 2, containing IgG and a low level of haemolytic complement, and Fr 1 (abs), depleted of IgG). A guinea-pig serum pool was used as a complement source in the immobilisation experiments. The influence was studied of removal of lysozyme from guinea-pig serum on the immobilisation reactions. Further experiments were performed, using a fluorescence technique, to detect C3b depositions on fixed treponemes and treponemes in suspension. RESULTS--Rapid immobilisation of Percoll-purified treponemes by the NHS serum fractions occurred only after preincubation with Fr 1 and Fr 2 simultaneously. This was largely dependent on the presence of a small amount of haemolytic C in Fr 2. Removal of lysozyme reduced this rapid rate of immobilisation. In fluorescence experiments it was demonstrated that C3b deposition on fixed (that is damaged) treponemes occurred upon their incubation with Fr 2 or the combination of Fr 1 and 2. However, on treponemes in suspension C3b deposition occurred only after incubation with the combination of Fr 1 and 2. CONCLUSION--The rapid immobilisation of Percoll purified treponemes by serum fractions from normal human serum requires antibodies of the IgM and IgG class, together with complement and lysozyme. Omission of one of these reactants slows immobilisation. Our experiments suggest that the reactants act in sequence: the loss of integrity of the outer membrane by an attack by IgM and C offers the opportunity for lysozyme to hydrolyse the peptidoglycan layer surrounding the cytoplasmic membrane of the treponemes, which then is accessible for attack by antibodies and C.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Baseman J. B. Surface-associated host proteins on virulent Treponema pallidum. Infect Immun. 1979 Dec;26(3):1048–1056. doi: 10.1128/iai.26.3.1048-1056.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco D. R., Walker E. M., Haake D. A., Champion C. I., Miller J. N., Lovett M. A. Complement activation limits the rate of in vitro treponemicidal activity and correlates with antibody-mediated aggregation of Treponema pallidum rare outer membrane protein. J Immunol. 1990 Mar 1;144(5):1914–1921. [PubMed] [Google Scholar]
- Cox D. L., Chang P., McDowall A. W., Radolf J. D. The outer membrane, not a coat of host proteins, limits antigenicity of virulent Treponema pallidum. Infect Immun. 1992 Mar;60(3):1076–1083. doi: 10.1128/iai.60.3.1076-1083.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelkens H. J., Kant M., Onvlee P. C., Stolz E., van der Sluis J. J. Rapid in vitro immobilisation of purified Treponema pallidum (Nichols strain), and protection by extraction fluids from rabbit testes. Genitourin Med. 1990 Oct;66(5):367–373. doi: 10.1136/sti.66.5.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelkens H. J., Kant M., Onvlee P. C., Stolz E., van der Sluis J. J. The influence of different sera on the in vitro immobilisation of Percoll purified Treponema pallidum, Nichols strain. Genitourin Med. 1992 Feb;68(1):20–25. doi: 10.1136/sti.68.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C. Surface mucopolysaccharides of Treponema pallidum. Infect Immun. 1979 Apr;24(1):244–251. doi: 10.1128/iai.24.1.244-251.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J. The Th1/Th2-like switch in syphilitic infection: is it detrimental? Infect Immun. 1992 Sep;60(9):3475–3479. doi: 10.1128/iai.60.9.3475-3479.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanff P. A., Norris S. J., Lovett M. A., Miller J. N. Purification of Treponema pallidum, Nichols strain, by Percoll density gradient centrifugation. Sex Transm Dis. 1984 Oct-Dec;11(4):275–286. doi: 10.1097/00007435-198410000-00003. [DOI] [PubMed] [Google Scholar]
- KENT J. F., DE WEERDT J. B. Enhancement by lysozyme of the sensitivity of Treponema pallidum immobilization tests. Br J Vener Dis. 1963 Mar;39:37–40. doi: 10.1136/sti.39.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- METZGER M., HARDY P. H., Jr, NELL E. E. Influence of lysozyme upon the treponeme immobilization reaction. Am J Hyg. 1961 Mar;73:236–244. doi: 10.1093/oxfordjournals.aje.a120182. [DOI] [PubMed] [Google Scholar]
- Müller F., Feddersen H., Segerling M. Studies on the action of lysozyme in immune immobilizaion of Treponema pallidium (Nichols strain). Immunology. 1973 Apr;24(4):711–719. [PMC free article] [PubMed] [Google Scholar]
- Radolf J. D., Norgard M. V., Schulz W. W. Outer membrane ultrastructure explains the limited antigenicity of virulent Treponema pallidum. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2051–2055. doi: 10.1073/pnas.86.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARDLAW A. C. The complement-dependent bacteriolytic activity of normal human serum. I. The effect of pH and ionic strength and the role of lysozyme. J Exp Med. 1962 Jun 1;115:1231–1249. doi: 10.1084/jem.115.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E. M., Zampighi G. A., Blanco D. R., Miller J. N., Lovett M. A. Demonstration of rare protein in the outer membrane of Treponema pallidum subsp. pallidum by freeze-fracture analysis. J Bacteriol. 1989 Sep;171(9):5005–5011. doi: 10.1128/jb.171.9.5005-5011.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigler J. A., Jones A. M., Jones R. H., Kubica K. M. Demonstration of extracellular material at the surface of pathogenic T. pallidum cells. Br J Vener Dis. 1976 Feb;52(1):1–8. doi: 10.1136/sti.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluis J. J., Kant M., Onvlee P. C., Stolz E. The inaccessibility of the outer membrane of adherent Treponema pallidum (Nichols strain) to anti-treponemal antibodies, a possible role of serum proteins. Genitourin Med. 1990 Jun;66(3):165–170. doi: 10.1136/sti.66.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluis J. J., Koehorst J. A., Boer A. M. Factors that inhibit adherence of Treponema pallidum (Nichols strain) to a human fibroblastic cell line: development in serum of patients with syphilis. Genitourin Med. 1987 Apr;63(2):71–76. doi: 10.1136/sti.63.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluis J. J., van Dijk G., Boer M., Stolz E., van Joost T. Mucopolysaccharides in suspensions of Treponema pallidum extracted from infected rabbit testes. Genitourin Med. 1985 Feb;61(1):7–12. doi: 10.1136/sti.61.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluis J. J., van Reede E. C., Boer M. Immunoglobulin G subclasses of fluorescent anti-Treponema pallidum antibodies: evidence for sequential development of specific anti-T. pallidum immunoglobulin G responses in patients with early syphilis. J Clin Microbiol. 1986 Sep;24(3):418–423. doi: 10.1128/jcm.24.3.418-423.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


